Non-Chloride in Situ Preparation of Nano-Cuprous Oxide and Its Effect on Heat Resistance and Combustion Properties of Calcium Alginate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
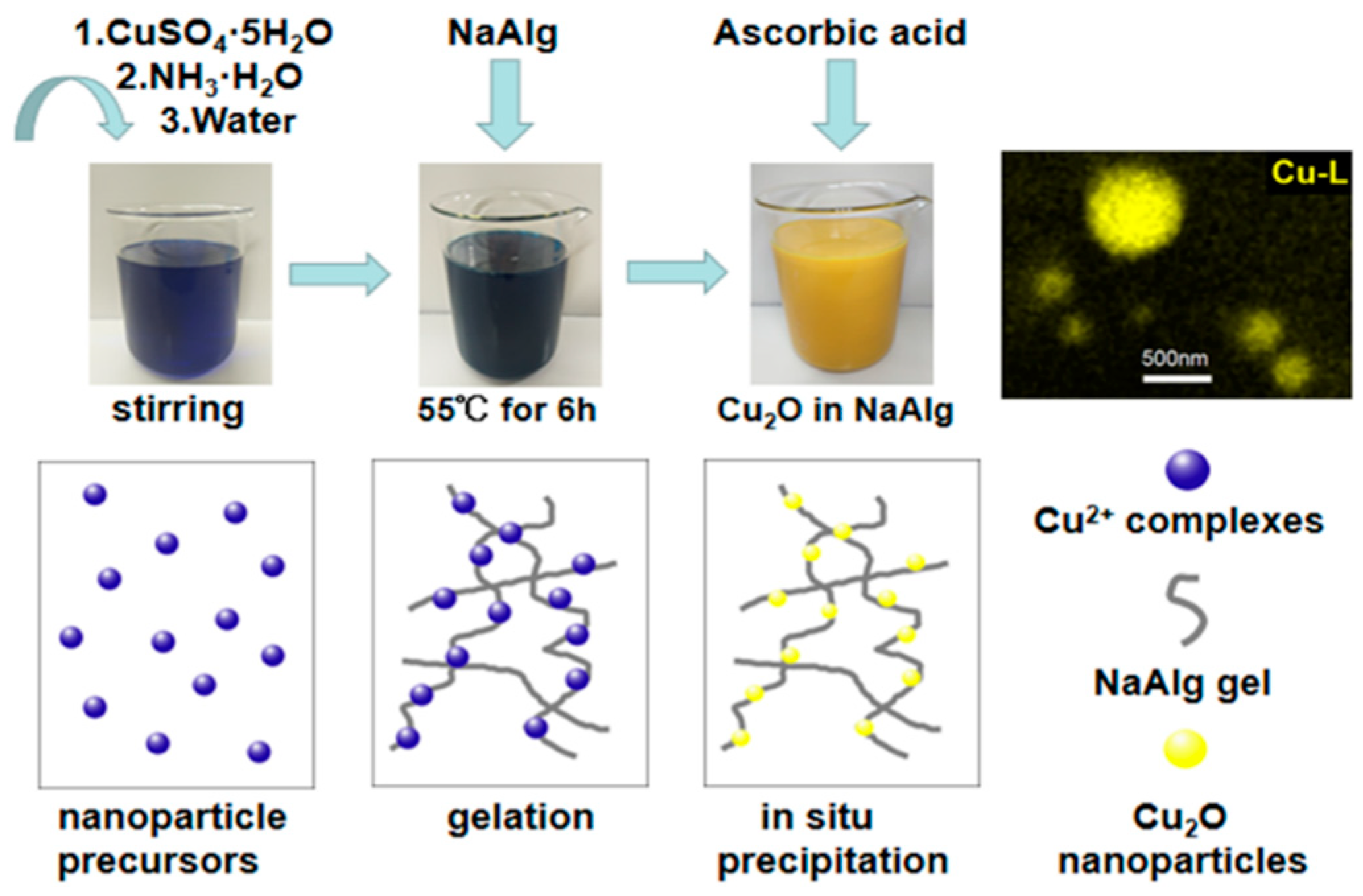
2.2. Preparation of Nano-Cu2O In Situ
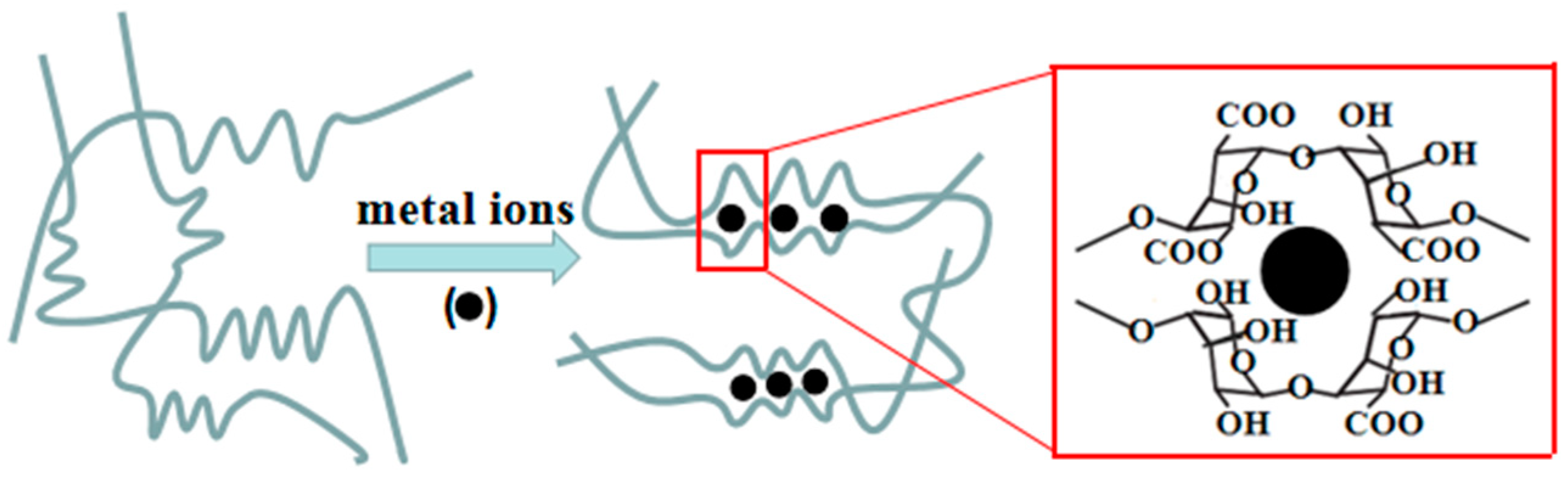
2.3. Preparation of Calcium Alginate/Nano-Cuprous Oxide Hybrid Materials
2.4. Measurements and Characterizations
2.4.1. Density
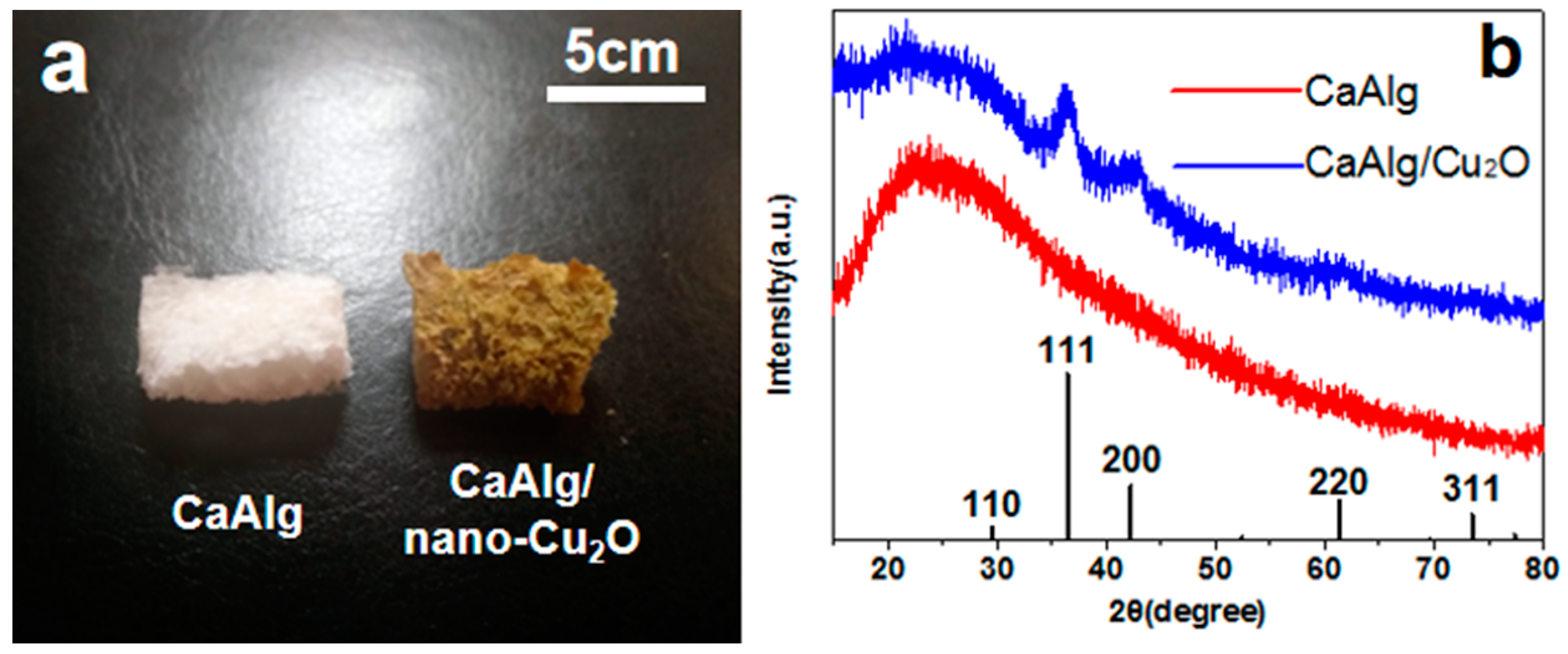
2.4.2. X-ray Diffraction (XRD)
2.4.3. Field Emission Scanning Electron Microscopy (FESEM)
2.4.4. Synchronous Thermal Analyzer (STA)
2.4.5. Cone Calorimeter (CONE)
2.4.6. Limiting Oxygen Index (LOI)
2.4.7. Vertical Burning (UL-94)
2.4.8. Microscale Combustion Calorimeter (MCC)
3. Results and Discussion
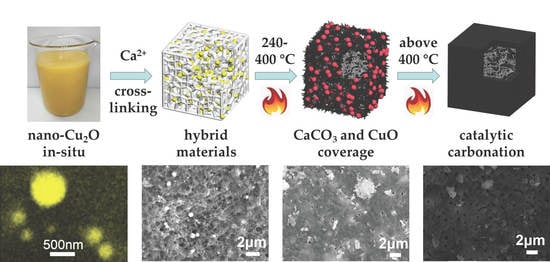
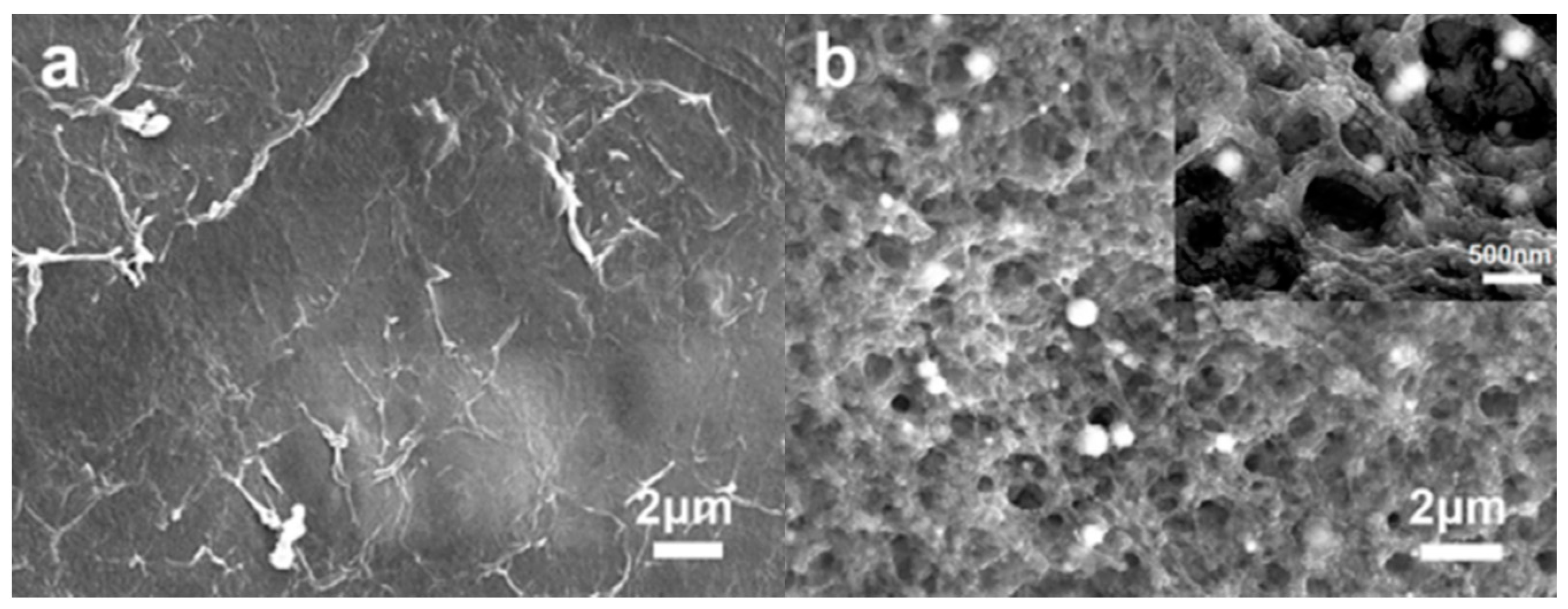
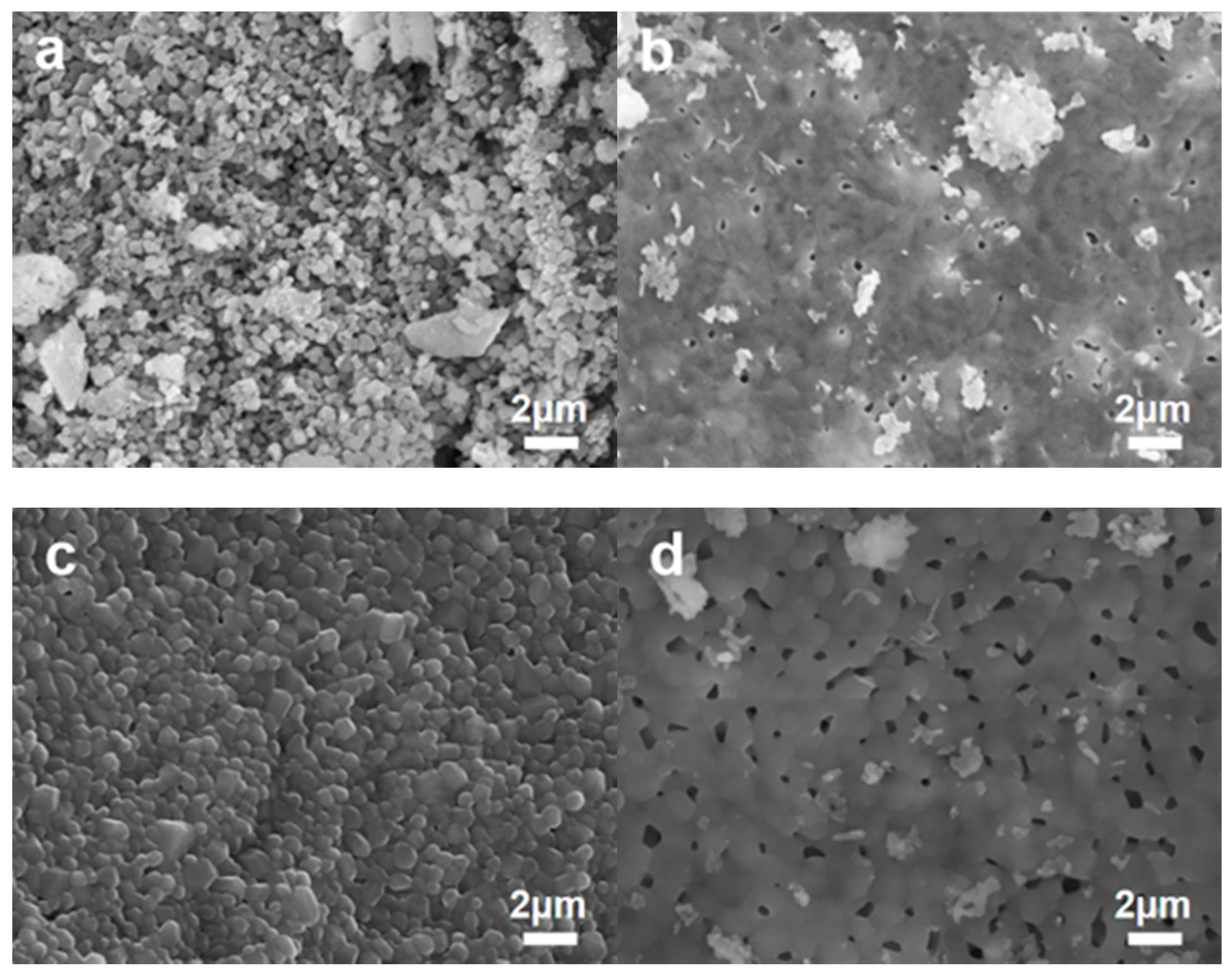
3.1. Morphology of Materials
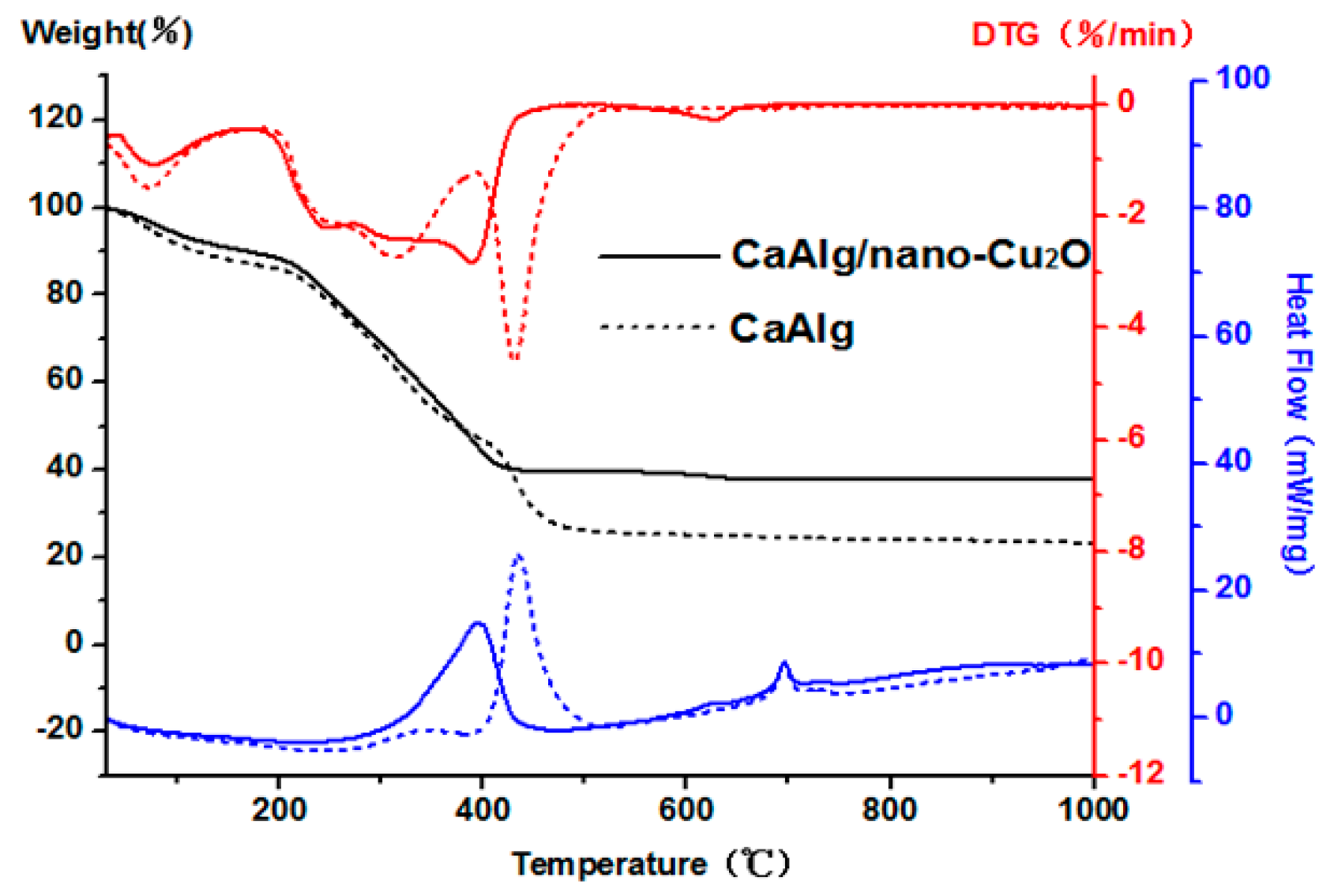
3.2. Thermal Stability
3.3. Combustion Behavior
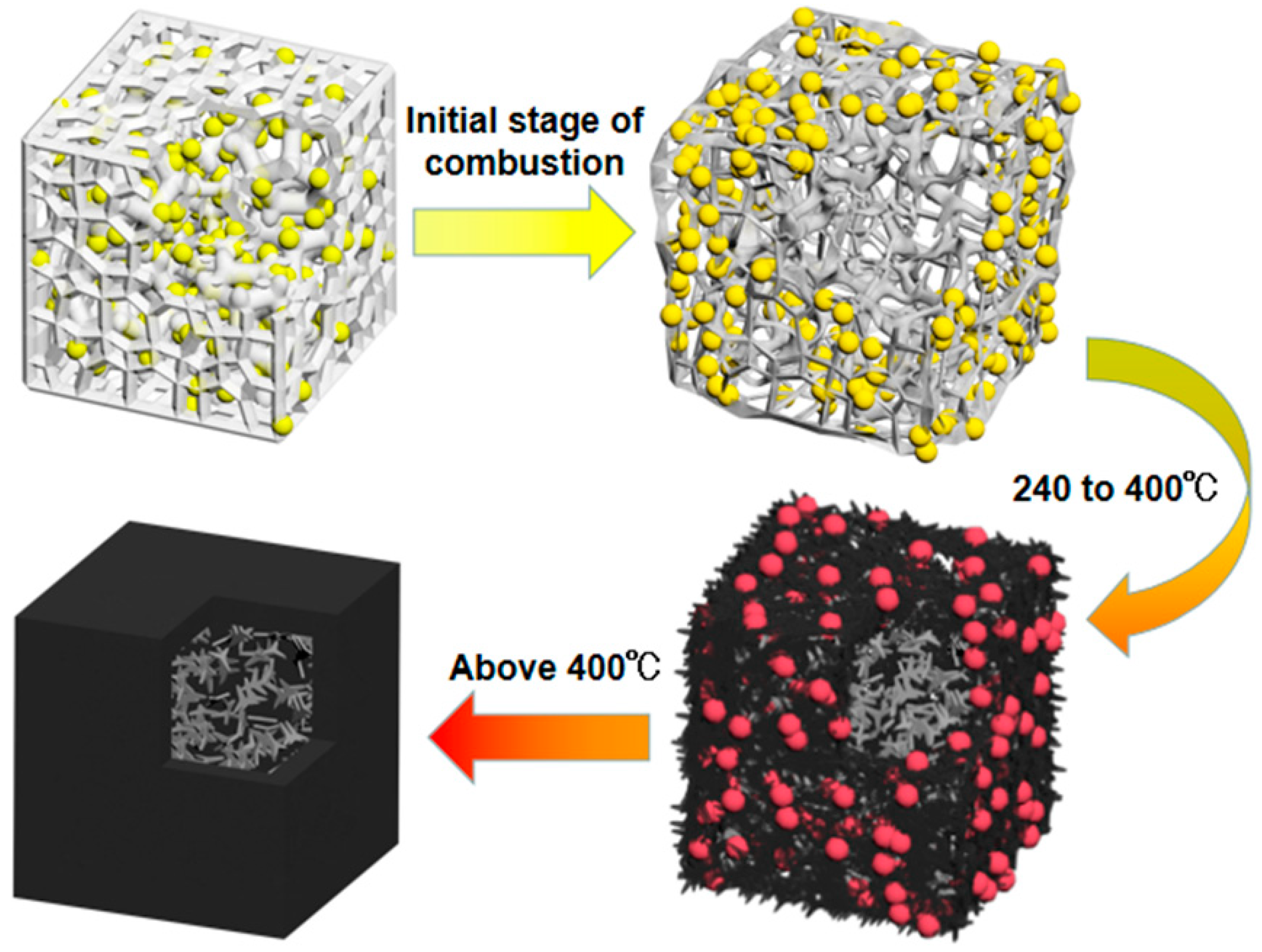
3.4. Flame-Retardant Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, S.; Zhang, X.; Yang, Q.; Liang, S.; Zhang, X.; Yang, Z. Cuprous oxide (Cu2O) crystals with tailored architectures: A comprehensive review on synthesis, fundamental properties, functional modifications and applications. Prog. Mater. Sci. 2018, 96, 111–173. [Google Scholar] [CrossRef]
- Qiu, S.L.; Xing, W.Y.; Feng, X.M.; Yu, B.; Mu, X.W.; Yuen, R.K.K.; Hu, Y. Self-standing cuprous oxide nanoparticles on silica@polyphosphazene nanospheres: 3D nanostructure for enhancing the flame retardancy and toxic effluents elimination of epoxy resins via synergistic catalytic effect. Chem. Eng. J. 2017, 309, 802–814. [Google Scholar] [CrossRef]
- Aguilar, M.S.; Rosas, G. A new synthesis of Cu2O spherical particles for the degradation of methylene blue dye. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100195. [Google Scholar] [CrossRef]
- Su, R.; Li, Q.; Chen, Y.; Gao, B.; Yue, Q.; Zhou, W. One-step synthesis of Cu2O@carbon nanocapsules composites using sodium alginate as template and characterization of their visible light photocatalytic properties. J. Clean. Prod. 2019, 209, 20–29. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K.; Bhorolua, D.; Das, J.; Kumar, A.; Hazarika, A. Facile synthesis of CuO nanowires and Cu2O nanospheres grown on rGO surface and exploiting its photocatalytic, antibacterial and supercapacitive properties. Phys. B 2019, 558, 74–81. [Google Scholar] [CrossRef]
- Kociolek-Balawejder, E.; Stanislawska, E.; Dworniczek, E.; Seniuk, A.; Jacukowicz-Sobala, I.; Winiarska, K. Cu2O doped gel-type anion exchanger obtained by reduction of brochantite deposit and its antimicrobial activity. React. Funct. Polym. 2019, 141, 42–49. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, X.; Chen, S.; Ma, Z.; Wang, W.; Wang, C.; Yue, L.; Sun, H.; Shao, Q.; Murugadoss, V.; et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 533, 13–23. [Google Scholar] [CrossRef]
- Wang, D.; Kan, Y.; Yu, X.; Liu, J.; Song, L.; Hu, Y. In situ loading ultra-small Cu2O nanoparticles on 2D hierarchical TiO2-graphene oxide dual-nanosheets: Towards reducing fire hazards of unsaturated polyester resin. J. Hazard. Mater. 2016, 320, 504–512. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, M.M.; Zhu, Z.X.; Chen, Y.Q.; He, H.; Qin, T.H. Chitosan modified Cu2O nanoparticles with high catalytic activity for p-nitrophenol reduction. Appl. Surf. Sci. 2019, 480, 601–610. [Google Scholar] [CrossRef]
- Jing, G.J.; Zhang, X.J.; Zhang, A.A.; Li, M.; Zeng, S.H.; Xu, C.J.; Su, H.Q. CeO2-CuO/Cu2O/ Cu monolithic catalysts with three-kind morphologies Cu2O layers for preferential CO oxidation. Appl. Surf. Sci. 2018, 434, 445–451. [Google Scholar] [CrossRef]
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.T.; Wang, Y.Z.; Schiraldi, D.A. Nonflammable alginate nanocomposite aerogels prepared by a simple freeze-drying and post-cross-linking method. ACS Appl. Mater. Interfaces 2016, 8, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aime, C.; Coradin, T. Nanocomposites from biopolymer hydrogels: Blueprints for white biotechnology and green materials chemistry. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 669–680. [Google Scholar] [CrossRef]
- Chen, H.B.; Shen, P.; Chen, M.J.; Zhao, H.B.; Schiraldi, D.A. Highly efficient flame retardant polyurethane foam with alginate/clay aerogel coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef]
- Safaei, M.; Taran, M.; Imani, M.M. Preparation, structural characterization, thermal properties and antifungal activity of alginate-CuO bionanocomposite. Mater. Sci. Eng. C 2019, 101, 323–329. [Google Scholar] [CrossRef]
- Zhai, Z.; Ren, B.; Xu, Y.; Wang, S.; Zhang, L.; Liu, Z. Green and facile fabrication of Cu-doped carbon aerogels from sodium alginate for supercapacitors. Org. Electron. 2019, 70, 246–251. [Google Scholar] [CrossRef]
- Carneiro-Da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalhoc, S.; Quintas, M.A.C.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Aroca, A.; Ruiz-Pividal, J.F.; Llorens-Gamez, M. Enhancement of water diffusion and compression performance of crosslinked alginate films with a minuscule amount of graphene oxide. Sci. Rep. 2017, 7, 11684. [Google Scholar] [CrossRef]
- Das, D.; Bang, S.; Zhang, S.M.; Noh, I. Bioactive Molecules release and cellular responses of alginate-tricalcium phosphate particles hybrid gel. Nanomaterials 2017, 7, 389. [Google Scholar] [CrossRef]
- Matricardi, P.; Pontoriero, M.; Coviello, T.; Casadei, M.A.; Alhaique, F. In situ cross-linkable novel alginate-dextran methacrylate IPN hydrogels for biomedical applications: Mechanical characterization and drug delivery properties. Biomacromolecules 2008, 9, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, P.; Siqueira, E.; de Lima, A.E.; Siqueira, G.; Pinzon-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-dimensional stable alginate-nanocellulose gels for biomedical applications: Towards tunable mechanical properties and cell growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.R.; Peng, Y.L.; Wang, D.; Yang, L.W.; Peng, H.; Zhu, P.; Wang, D.Y. Effect of reactive time on flame retardancy and thermal degradation behavior of bio-based zinc alginate film. Polym. Degrad. Stab. 2016, 127, 20–31. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.J.; Zhao, J.C.; Guo, Y.; Zhu, P.; Wang, D.Y. Bio-based barium alginate film: Preparation, flame retardancy and thermal degradation behavior. Carbohydr. Polym. 2016, 139, 106–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ji, Q.; Wang, F.; Tan, L.; Xia, Y. Effects of divalent metal ions on the flame retardancy and pyrolysis products of alginate fibers. Polym. Degrad. Stab. 2012, 97, 1034–1040. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.F.; Wang, J.S.; Zhu, P.; Zhao, J.C.; Zhang, C.J.; Guo, Y.; Jin, X. Thermal degradation and pyrolysis behavior of aluminum alginate investigated by TG-FTIR-MS and Py-GC-MS. Polym. Degrad. Stab. 2015, 118, 59–68. [Google Scholar] [CrossRef]
- Ma, X.M.; Li, R.; Zhao, X.H.; Ji, Q.; Xing, Y.C.; Sunarso, J.; Xia, Y.Z. Biopolymer composite fibres composed of calcium alginate reinforced with nanocrystalline cellulose. Compos. Part A Appl. Sci. Manuf. 2017, 96, 155–163. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, Z.; Yang, Y.X.; Zhang, Y.L.; Wen, X.; Li, N.; Fu, C.; Jian, R.K.; Li, L.J.; Wang, D.Y. A geometry effect of carbon nanomaterials on flame retardancy and mechanical properties of ethylene-vinyl acetate/magnesium hydroxide composites. Polymers 2018, 10, 1028. [Google Scholar] [CrossRef]
- Wu, N.J.; Niu, F.K.; Lang, W.C.; Xia, M.F. Highly efficient flame-retardant and low-smoke-toxicity poly (vinyl alcohol)/alginate/montmorillonite composite aerogels by two-step crosslinking strategy. Carbohydr. Polym. 2019, 221, 221–230. [Google Scholar] [CrossRef]
- Lewin, M. Some comments on the modes of action of nanocomposites in the flame retardancy of polymers. Fire Mater. 2003, 27, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Zhao, X.; Deng, Y.; Xue, Y.; Li, Q. Flame retardancy and thermal degradation mechanism of calcium alginate/CaCO3 composites prepared via in situ method. J. Therm. Anal. Calorim. 2017, 131, 2167–2177. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhao, X.; Zhang, L.; Li, Q. Highly efficient flame retardant hybrid composites based on calcium alginate/nano-calcium borate. Polymers 2018, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Shao, P.Y.; Zhang, Q.; Cheng, W.; Li, Z.C.; Li, Q. A novel inherently flame-retardant composite based on zinc alginate/nano-Cu2O. Polymers 2019, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qi, Y.; Wang, Y.; Xue, Y.; Xu, P.; Li, Z.; Li, Q. Morphology and thermal properties of calcium alginate/reduced graphere oxide composites. Polymers 2018, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Zhao, X.; Li, Z.; Li, Q. Surface coating for flame retardancy and pyrolysis behavior of polyester fabric based on calcium alginate nanocomposites. Nanomaterials 2018, 8, 875. [Google Scholar] [CrossRef]
- Zhang, C.J.; Liu, Y.H.; Wang, L.; Zhu, P. Properties of Cu2+ modified calcium alginate fiber. Text. Aux. 2011, 1, 4. [Google Scholar]
- Zhang, H.Y.; Liu, C.J.; Chen, L.; Dai, B. Control of ice crystal growth and its effect on porous structure of chitosan cryogels. Chem. Eng. Sci. 2019, 201, 50–57. [Google Scholar] [CrossRef]
- Yang, J.R.; Li, Y.Q.; Liu, Y.B.; Li, D.X.; Zhang, L.; Wang, Q.G.; Xiao, Y.M.; Zhang, X.D. Influence of hydrogel network microstructures on mesenchymal stem cell chondrogenesis in vitro and in vivo. Acta Biomater. 2019, 91, 159–172. [Google Scholar] [CrossRef]
- Hou, X.; Xue, Z.; Xia, Y.; Qin, Y.; Zhang, G.; Liu, H.; Li, K. Effect of SiO2 nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. Int. J. Biol. Macromol. 2019, 125, 1289–1298. [Google Scholar] [CrossRef]
- Hou, X.B.; Xue, Z.X.; Xia, Y.Z. Preparation of a novel agar/sodium alginate fire-retardancy film. Mater. Lett. 2018, 233, 274–277. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.M.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, Y.; Liu, Q.; Huiquan, G.; Liu, P.; Zegaoui, A.; Wang, J. Synthesis and thermal behavior of Cu2O flower-like, Cu2O-C60 and Al/Cu2O-C60 as catalysts on the thermal decomposition of ammonium perchlorate. Vacuum 2018, 153, 277–290. [Google Scholar] [CrossRef]
- Shi, R.; Tan, L.; Zong, L.; Ji, Q.; Li, X.; Zhang, K.; Cheng, L.; Xia, Y. Influence of Na (+) and Ca (+) on flame retardancy, thermal degradation, and pyrolysis behavior of cellulose fibers. Carbohydr. Polym. 2017, 157, 1594–1603. [Google Scholar] [CrossRef]
- Dong, Q.X.; Liu, M.M.; Ding, Y.F.; Wang, F.; Gao, C.; Liu, P.; Wen, B.; Zhang, S.M.; Yang, M.S. Synergistic effect of DOPO immobilized silica nanoparticles in the intumescent flame retarded polypropylene composites. Polym. Adv. Technol. 2013, 24, 732–739. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q.L.; Lyon, R.E.; Hu, Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym. Degrad. Stab. 2010, 95, 108–115. [Google Scholar] [CrossRef]

| Samples | TTI (s) | HRR (kW/m2) | THR (MJ/m2) | PHRR (kW/m2) | EHC (MJ/kg) | Mean SEA (m2/kg) |
|---|---|---|---|---|---|---|
| CaAlg | 11 | 39.73 | 6.40 | 93.59 | 10.87 | 41.46 |
| CaAlg/nano-Cu2O | 6 | 16.09 | 2.02 | 67.30 | 9.14 | 21.43 |
| Samples | LOI (%) | UL-94 | HRC (J/g/K) | PHRR (W/g) | THR (kJ/g) | Residues (%) |
|---|---|---|---|---|---|---|
| CaAlg | 49.2 | V-0 | 18 | 16.19 | 4.3 | 55.65 |
| CaAlg/nano-Cu2O | 54.0 | V-0 | 16 | 14.01 | 4 | 60.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, P.; Xu, P.; Zhang, L.; Xue, Y.; Zhao, X.; Li, Z.; Li, Q. Non-Chloride in Situ Preparation of Nano-Cuprous Oxide and Its Effect on Heat Resistance and Combustion Properties of Calcium Alginate. Polymers 2019, 11, 1760. https://doi.org/10.3390/polym11111760
Shao P, Xu P, Zhang L, Xue Y, Zhao X, Li Z, Li Q. Non-Chloride in Situ Preparation of Nano-Cuprous Oxide and Its Effect on Heat Resistance and Combustion Properties of Calcium Alginate. Polymers. 2019; 11(11):1760. https://doi.org/10.3390/polym11111760
Chicago/Turabian StyleShao, Peiyuan, Peng Xu, Lei Zhang, Yun Xue, Xihui Zhao, Zichao Li, and Qun Li. 2019. "Non-Chloride in Situ Preparation of Nano-Cuprous Oxide and Its Effect on Heat Resistance and Combustion Properties of Calcium Alginate" Polymers 11, no. 11: 1760. https://doi.org/10.3390/polym11111760