Convection Enhanced Delivery in the Setting of High-Grade Gliomas
Abstract
1. Introduction
1.1. Glioblastoma
1.2. Blood–Brain Barrier
1.3. Direct Delivery
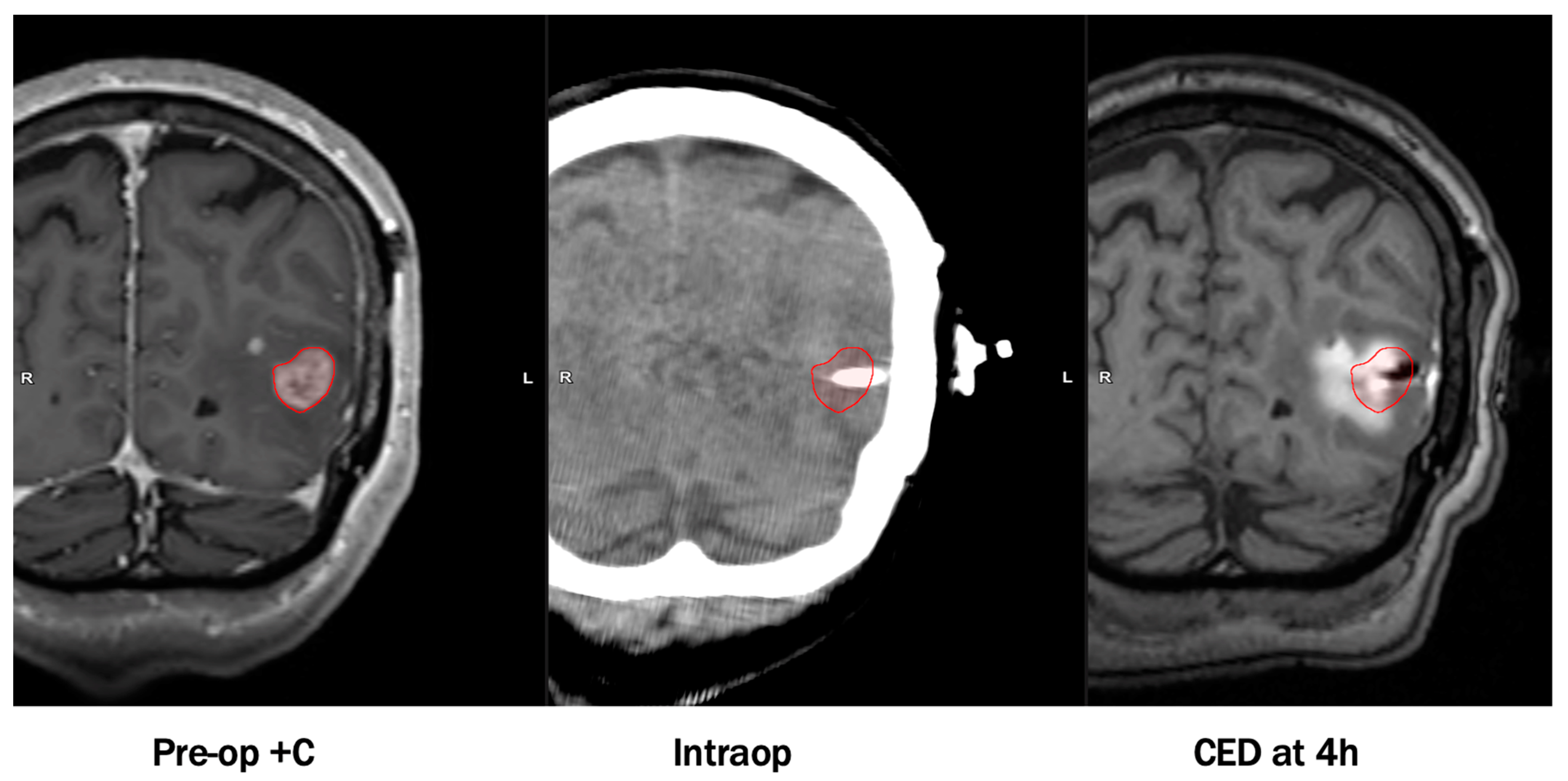
1.4. Convection Enhanced Delivery
2. Clinical Experiences with Convection Enhanced Delivery
2.1. Paclitaxel
2.2. Topotecan
2.3. Conjugated Toxins
2.3.1. Tf-CRM107
2.3.2. NB3001 (IL-4 PE, IL-4(38-37)-PE38KDEL)
2.3.3. TP-38
2.3.4. IL13–PE38QQR (Citredekin Besudotox)
2.4. Viruses
2.4.1. Reovirus
2.4.2. PVSRIPO
2.4.3. Delta-24-RGD
2.5. Liposomes
2.5.1. LIPO-HSV-1-tk
2.5.2. Liposomal Irinotecan (Onivyde)
2.6. Oligodeoxynucleotides
2.6.1. CpG-28
2.6.2. AP12009
2.7. Other Agents
131I-chTNT-1/B MAb (Cotara)
3. Limitations and Recent Developments in the Preclinical Setting
3.1. Drug Distribution
3.2. Hardware, Infusion Technique, and Long-Term Convection Enhanced Delivery
3.3. Therapeutic Agents
4. Discussion
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| Vd | volume of distribution |
| IFF | interstitial fluid flow |
| ECM | extracellular matrix |
| WM | white matter |
| OS | overall survival |
| PFS | progression-free survival |
| MTD | maximum tolerated dose |
| DLT | dose-limiting toxicity |
| DWI | diffusion-weighted imaging |
| FET | O-(2-[18F]fluoroethyl)-l-tyrosine |
| FDG | fluorodeoxyglucose |
| PET | positron emission tomography |
| CSF | cerebrospinal fluid |
| TGF-α | transforming growth factor-alpha |
| SPECT | single photon emission computed tomography |
| HAS | human serum albumin |
| TCID50 | tissue culture infectious dose 50 |
| ODN | oligodeoxynucleotide |
| TGFβ-2 | transforming growth factor beta-2 |
| Tf-CRM-107 | transferrin receptor-based diphtheria toxin |
| NBI-3001 | IL4-Pseudoamonas exotoxin |
| LIPO-HSV-1-tk | HSV-1-tk gene-bearing cationic liposomal vector |
| TCID50 | 50% tissue-culture infectious doses |
| 131I-chTNT-1/BMAb | cotara-131I-labeled chimeric monoclonal antibody |
| TP-38 | recombinant toxin targeting epidermal growth factor receptor (EGFR) |
| CpG-ODN | oligodeoxynucleotides containing CpG motifs |
| Delta-24-RGD | conditionally replication-competent adenovirus |
| Gd-DTPA | gadolinium-diethylene-triamine-pentaacetic acid |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| SPECT | single photon emission computed tomography |
| IT | intratumoral |
| IP | intraparenchymal |
| PFS6 | progression free survival at 6 months |
| mPFS | median progression free survival |
| rGBM | recurrent glioblastoma |
| HGG | high-grade glioma |
| HGG | high-grade glioma |
| IT | intratumoral |
| IP | intraparenchymal |
| AE | adverse event |
| DLT | dose limiting toxicity |
| TTP | time to progression |
| PFS | progression free survival |
| OS | overall survival |
| Vd | volume of distribution |
| Vi | volume infused |
| Agents: | |
| OS2966 | anti-CD29 mAb |
| PVSRIPO | polio-rhinovirus recombinant oncolytic virus |
| D2C7-IT | recombinant immunotoxin targeting EGFRwt and EGFRvIII |
| 2141-V11 | anti-CD40 mAb |
| Gd-DTPA | gadopentetic acid |
| EGFR-vIII CAR | chimeric antigen receptor (CAR) that recognizes epidermal growth factor receptor variant III |
| hrBMP4 | human-recombinant bone morphogenetic protein 4 |
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro. Oncol. 2020, 22, iv1–iv96. [Google Scholar]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar]
- Louis, D.N.; Wesseling, P.; Aldape, K.; Brat, D.J.; Capper, D.; Cree, I.A.; Eberhart, C.; Figarella-Branger, D.; Fouladi, M.; Fuller, G.N.; et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020, 30, 844–856. [Google Scholar] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood–Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 2020. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [PubMed]
- Stukel, J.M.; Caplan, M.R. Targeted drug delivery for treatment and imaging of glioblastoma multiforme. Expert Opin. Drug Deliv. 2009, 6, 705–718. [Google Scholar] [PubMed]
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; De Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [PubMed]
- Jain, K.K. Use of nanoparticles for drug delivery in glioblastoma multiforme. Expert Rev. Neurother. 2007, 7, 363–372. [Google Scholar] [PubMed]
- Konishi, Y.; Muragaki, Y.; Iseki, H.; Mitsuhashi, N.; Okada, Y. Patterns of Intracranial Glioblastoma Recurrence After Aggressive Surgical Resection and Adjuvant Management: Retrospective Analysis of 43 Cases. Neurol. Med. Chir. 2012, 52, 577–586. [Google Scholar] [CrossRef]
- Oppitz, U.; Maessen, D.; Zunterer, H.; Richter, S.; Flentje, M. 3D-recurrence-patterns of gliobastomas after CT-planned postoperative irradiation. Radiother. Oncol. 1999, 53, 53–57. [Google Scholar] [PubMed]
- Bashir, R.; Hochberg, F.; Oot, R. Regrowth Patterns of Glioblastoma Multiforme Related to Planning of Interstitial Brachytherapy Radiation Fields. Neurosurgery 1988, 23, 27–30. [Google Scholar]
- Hochberg, F.H.; Pruitt, A. Assumptions in the radiotherapy of glioblastoma. Neurology 1980, 30, 907–911. [Google Scholar] [PubMed]
- Hau, P.; Jachimczak, P.; Schlingensiepen, R.; Schulmeyer, F.; Jauch, T.; Steinbrecher, A.; Brawanski, A.; Proescholdt, M.; Schlaier, J.; Buchroithner, J.; et al. Inhibition of TGF-β2 with AP 12009 in Recurrent Malignant Gliomas: From Preclinical to Phase I/II Studies. Oligonucleotides 2007, 17, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.M.; Laske, D.W.; Morrison, P.F.; Bankiewicz, K.S.; Oldfield, E.H. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J. Neurosurg. 1995, 82, 1021–1029. [Google Scholar] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar]
- Lonser, R.R.; Schiffman, R.; Robison, R.A.; Butman, J.A.; Quezado, Z.; Walker, M.L.; Morrison, P.F.; Walbridge, S.; Murray, G.J.; Park, D.M.; et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology 2007, 68, 254–261. [Google Scholar] [PubMed]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar]
- Hunt Bobo, R.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar]
- Stine, C.A.; Munson, J.M. Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow. Front. Oncol. 2019, 9, 966. [Google Scholar] [PubMed]
- Kawakami, K.; Kawakami, M.; Kioi, M.; Husain, S.R.; Puri, R.K. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J. Neurosurg. 2004, 101, 1004–1011. [Google Scholar] [PubMed]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A Phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar]
- Tanner, P.G.; Holtmannspötter, M.; Tonn, J.-C.; Goldbrunner, R. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas. Neurosurgery 2007, 61, E880–E882. [Google Scholar] [CrossRef]
- Pöpperl, G.; Goldbrunner, R.; Gildehaus, F.J.; Kreth, F.W.; Tanner, P.; Holtmannspötter, M.; Tonn, J.C.; Tatsch, K. O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.N.; Fine, R.L.; Canoll, P.; Yun, J.; Kennedy, B.C.; Rosenfeld, S.S.; Sands, S.A.; Surapaneni, K.; Lai, R.; Yanes, C.L.; et al. Regression of Recurrent Malignant Gliomas With Convection-Enhanced Delivery of Topotecan. Neurosurgery 2011, 69, 1272–1280. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brewer, C.; Barnett, G.H.; Mohammadi, A.M.; Peereboom, D.M.; Ahluwalia, M.S.; Gao, S. First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: Results of pilot trial 1. J. Neurosurg. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Laske, D.W.; Youle, R.J.; Oldfield, E.H. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat. Med. 1997, 3, 1362–1368. [Google Scholar] [CrossRef]
- Weaver, M.; Laske, D.W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) therapy of malignant gliomas. J. Neurooncol. 2003, 65, 3–14. [Google Scholar]
- Weber, F.; Asher, A.; Bucholz, R.; Berger, M.; Prados, M.; Chang, S.; Bruce, J.; Hall, W.; Rainov, N.G.; Westphal, M.; et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J. Neurooncol. 2003, 64, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Berger, M.S.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Greer, K.; Herndon, J.E.; Kunwar, S.; et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro. Oncol. 2008, 10, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Sampson, J.H.; Kunwar, S.; Chang, S.M.; Shaffrey, M.; Asher, A.L.; Lang, F.F.; Croteau, D.; Parker, K.; Grahn, A.Y.; et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: Phase 1 study of final safety results. Neurosurgery 2007, 61, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Chang, S.; Prados, M.; Berger, M.; Sampson, J.; Croteau, D.; Sherman, J.; Grahn, A.; Shu, V.S.; Dul, J.L.; et al. Safety of intraparenchymal convection-enhanced delivery of cintredekin besudotox in early-phase studies. Neurosurg Focus 2006, 20, E15. [Google Scholar]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro. Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Kicielinski, K.P.; Chiocca, E.A.; Yu, J.S.; Gill, G.M.; Coffey, M.; Markert, J.M. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol. Ther. 2014, 22, 1056–1062. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Voges, J.; Reszka, R.; Gossmann, A.; Dittmar, C.; Richter, R.; Garlip, G.; Kracht, L.; Coenen, H.H.; Sturm, V.; Wienhard, K.; et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann. Neurol. 2003, 54, 479–487. [Google Scholar] [CrossRef]
- Kumar, K.; Chang, S.; Oberheim-Bush, N.A.; Clarke, J.; Taylor, J.; Page, M.; Chang, K.; Guthrie, L.; Lawrence, A.; Lindahl, C.; et al. ACTR-67. A phase I study of convection-enhanced delivery of liposomal-irinotecan (onivyde) using real-time imaging with gadolinium in patients with recurrent high grade gliomas: Results thus far. Neuro. Oncol. 2018, 20, vi26–vi27. [Google Scholar] [CrossRef][Green Version]
- Carpentier, A.; Laigle-Donadey, F.; Zohar, S.; Capelle, L.; Behin, A.; Tibi, A.; Martin-Duverneuil, N.; Sanson, M.; Lacomblez, L.; Taillibert, S.; et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent gliobastoma. Neuro. Oncol. 2006, 8, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Metellus, P.; Ursu, R.; Zohar, S.; Lafitte, F.; Barrie, M.; Meng, Y.; Richard, M.; Parizot, C.; Laigle-Donadey, F.; et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: A phase II study. Neuro. Oncol. 2010, 12, 401–408. [Google Scholar] [CrossRef]
- Patel, S.J.; Shapiro, W.R.; Laske, D.W.; Jensen, R.L.; Asher, A.L.; Wessels, B.W.; Carpenter, S.P.; Shan, J.S. Safety and Feasibility of Convection-enhanced Delivery of Cotara for the Treatment of Malignant Glioma: Initial Experience in 51 Patients. Neurosurgery 2005, 56, 1243–1253. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- eEML. Electronic Essential Medicines List. Available online: https://list.essentialmeds.org/ (accessed on 3 April 2021).
- Bruce, J.N.; Falavigna, A.; Johnson, J.P.; Hall, J.S.; Birch, B.D.; Yoon, J.T.; Wu, E.X.; Fine, R.L.; Parsa, A.T. Intracerebral Clysis in a Rat Glioma Model. Neurosurgery 2000, 46, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.G.; Parsa, A.T.; Fine, R.L.; Hall, J.S.; Chakrabarti, I.; Bruce, J.N. Tissue Distribution and Antitumor Activity of Topotecan Delivered by Intracerebral Clysis in a Rat Glioma Model. Neurosurgery 2000, 47, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-Enhanced Delivery for Diffuse Intrinsic Pontine Glioma Treatment. Curr. Neuropharmacol. 2017, 15, 116–128. [Google Scholar] [CrossRef]
- Johnson, V.G.; Wrobel, C.; Wilson, D.; Zovickian, J.; Greenfield, L.; Oldfield, E.H.; Youle, R. Improved tumor-specific immunotoxins in the treatment of CNS and leptomeningeal neoplasia. J. Neurosurg. 1989, 70, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Torp, S.H.; Helseth, E.; Ryan, L.; Stølan, S.; Dalen, A.; Unsgaard, G. Expression of the epidermal growth factor receptor gene in human brain metastases. APMIS 1992, 100, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Parney, I.F.; Kunwar, S.; McDermott, M.; Berger, M.; Prados, M.; Cha, S.; Croteau, D.; Puri, R.K.; Chang, S.M. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13—PE38QQR for recurrent malignant glioma. J. Neurosurg. 2005, 102, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A. Convection enhanced delivery for treating brain tumors and selected neurological disorders: Symposium review. J. Neuro-Oncol. 2007, 83, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Prados, M.D.; Chang, S.M.; Berger, M.S.; Lang, F.F.; Piepmeier, J.M.; Sampson, J.H.; Ram, Z.; Gutin, P.H.; Gibbons, R.D.; et al. Direct Intracerebral Delivery of Cintredekin Besudotox (IL13-PE38QQR) in Recurrent Malignant Glioma: A Report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007, 25, 837–844. [Google Scholar] [CrossRef]
- Mueller, S.; Polley, M.Y.; Lee, B.; Kunwar, S.; Pedain, C.; Wembacher-Schröder, E.; Mittermeyer, S.; Westphal, M.; Sampson, J.H.; Vogelbaum, M.A.; et al. Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J. Neurooncol. 2011, 101, 267–277. [Google Scholar] [CrossRef]
- Sampson, J.H.; Archer, G.; Pedain, C.; Wembacher-Schröder, E.; Westphal, M.; Kunwar, S.; Vogelbaum, M.A.; Coan, A.; Herndon, J.E.; Raghavan, R.; et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J. Neurosurg. 2010, 113, 301–309. [Google Scholar] [CrossRef]
- Sampson, J.H.; Brady, M.L.; Petry, N.A.; Croteau, D.; Friedman, A.H.; Friedman, H.S.; Wong, T.; Bigner, D.D.; Pastan, I.; Puri, R.K.; et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas. Oper. Neurosurg. 2007, 60, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, E.H.P.; Wembacher-Schröder, E.; Smits, M.; Dirven, C.M.F. Magnetic Resonance Imaging–Based Assessment of Gadolinium-Conjugated Diethylenetriamine Penta-Acetic Acid Test-Infusion in Detecting Dysfunction of Convection-Enhanced Delivery Catheters. World Neurosurg. 2016, 89, 272–279. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Berger, M.S.; Bankiewicz, K.S. The use of convection-enhanced delivery with liposomal toxins in neurooncology. Toxins 2011, 3, 369–397. [Google Scholar] [CrossRef] [PubMed]
- Reszka, R.C.; Jacobs, A.; Voges, J. Liposome-mediated suicide gene therapy in humans. Methods Enzymol. 2005, 391, 200–208. [Google Scholar]
- Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Papachristodoulou, A.; Silginer, M.; Weller, M.; Schneider, H.; Hasenbach, K.; Janicot, M.; Roth, P. Therapeutic Targeting of TGFb Ligands in Glioblastoma Using Novel Antisense Oligonucleotides Reduces the Growth of Experimental Gliomas. Clin. Cancer Res. 2019, 25, 7189–7201. [Google Scholar] [CrossRef]
- Lewis, O.; Woolley, M.; Johnson, D.; Rosser, A.; Barua, N.U.; Bienemann, A.S.; Gill, S.S.; Evans, S. Chronic, intermittent convection-enhanced delivery devices. J. Neurosci. Methods 2016, 259, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Mongelli, M.T.; Vidotto, M.; Madekurozwa, M.; Bernardini, A.; Overby, D.R.; De Momi, E.; Rodriguez y Baena, F.; Sherwood, J.M.; Dini, D. Infusion Mechanisms in Brain White Matter and its Dependence of Microstructure: An Experimental Study of Hydraulic Permeability. IEEE Trans. Biomed. Eng. 2020, 68, 1229–1237. [Google Scholar] [CrossRef]
- Zhan, W.; Rodriguez y Baena, F.; Dini, D. Effect of tissue permeability and drug diffusion anisotropy on convection-enhanced delivery. Drug Deliv. 2019, 26, 773–781. [Google Scholar] [CrossRef]
- Brady, M.; Raghavan, R.; Sampson, J. Determinants of Intraparenchymal Infusion Distributions: Modeling and Analyses of Human Glioblastoma Trials. Pharmaceutics 2020, 12, 895. [Google Scholar] [CrossRef]
- Chatterjee, K.; Atay, N.; Abler, D.; Bhargava, S.; Sahoo, P.; Rockne, R.C.; Munson, J.M. Utilizing Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) to Analyze Interstitial Fluid Flow and Transport in Glioblastoma and the Surrounding Parenchyma in Human Patients. Pharmaceutics 2021, 13, 212. [Google Scholar] [CrossRef]
- Vidotto, M.; Pederzani, M.; Castellano, A.; Pieri, V.; Falini, A.; Dini, D.; De Momi, E. Integrating Diffusion Tensor Imaging and Neurite Orientation Dispersion and Density Imaging to Improve the Predictive Capabilities of CED Models. Ann. Biomed. Eng. 2021, 49, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Chiarelli, P.A.; Revia, R.A.; Wang, K.; Kievit, F.; Dayringer, C.; Jeon, M.; Ellenbogen, R.; Zhang, M. Time-resolved MRI assessment of convection-enhanced delivery by targeted and nontargeted nanoparticles in a human glioblastoma mouse model. Cancer Res. 2019, 79, 4776–4786. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.J.; Zhang, Z.; Dillehay, D.; Stubbs, J. Assessment of a balloon-tipped catheter modified for intracerebral convection-enhanced delivery. J. Neurooncol. 2008, 89, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Odland, R.; Wilson, S.R.; Kroeger, K.M.; Liu, C.; Lowenstein, P.R.; Castro, M.G.; Hall, W.A.; Ohlfest, J.R. Improved distribution of small molecules and viral vectors in the murine brain using a hollow fiber catheter. J. Neurosurg. 2007, 107, 568–577. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Morgan, S.R.; Yun, J.; Yanagihara, T.; Mohajed, H.; Dashnaw, S.; Bruce, S.S.; Brown, T.; Romanov, A.; Sebastian, M.; et al. Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro. Oncol. 2011, 13, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Halle, B.; Mongelard, K.; Poulsen, F. Convection-enhanced drug delivery for glioblastoma: A systematic review focused on methodological differences in the use of the convection-enhanced delivery method. Asian J. Neurosurg. 2019, 14, 5. [Google Scholar] [CrossRef]
- Orozco, G.A.; Smith, J.H.; García, J.J. Three-dimensional nonlinear finite element model to estimate backflow during flow-controlled infusions into the brain. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2020, 234, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Grajales, G.A.O.; García, F.C.; Álvarez, J.J.G. Assessment of needle tip geometry during infusions into a brain phantom gel. Sci. Tech. 2020, 25, 616–620. [Google Scholar]
- Mehta, J.N.; McRoberts, G.R.; Rylander, C.G. Controlled Catheter Movement Affects Dye Dispersal Volume in Agarose Gel Brain Phantoms. Pharmaceutics 2020, 12, 753. [Google Scholar] [CrossRef]
- Quintero, J.E.; Zhang, R.; Pang, Q.; Xing, Y.; Hardy, P.; Fan, X.; Ai, Y.; Gash, D.M.; Gerhardt, G.A.; Grondin, R.; et al. Surgical methodology and protocols for preventing implanted cerebral catheters from becoming obstructed during and after neurosurgery. J. Neurosci. Methods 2021, 349, 109020. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Brady, M.L.; Rodríguez-Ponce, M.I.; Hartlep, A.; Pedain, C.; Sampson, J.H. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg. Focus 2006, 20, E12. [Google Scholar] [CrossRef] [PubMed]
- Antoine, L.H.; Koomullil, R.P.; Wick, T.M.; Nakhmani, A. Optimization of catheter placement for convection-enhanced delivery to brain tumors. F1000Research 2021, 10, 18. [Google Scholar] [CrossRef]
- Antoine, L.H.; Koomullil, R.P.; Wick, T.M.; Nabors, L.B.; Abdel Aal, A.K.; Bolding, M.S. Catheter placement selection for convection-enhanced delivery of therapeutic agents to brain tumors. F1000Research 2020, 9, 1415. [Google Scholar] [CrossRef]
- Faraji, A.H.; Jaquins-Gerstl, A.S.; Valenta, A.C.; Ou, Y.; Weber, S.G. Electrokinetic Convection-Enhanced Delivery of Solutes to the Brain. ACS Chem. Neurosci. 2020, 11, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Tosi, U.; Souweidane, M.M. Longitudinal Monitoring of Gd-DTPA Following Convection Enhanced Delivery in the Brainstem. World Neurosurg. 2020, 137, 38–42. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Neira, J.A.; Yun, J.; Alexiades, N.G.; Banu, M.; Englander, Z.K.; Kennedy, B.C.; Ung, T.H.; Rothrock, R.J.; Romanov, A.; et al. Validation of an effective implantable pump-infusion system for chronic convection-enhanced delivery of intracerebral topotecan in a large animal model. J. Neurosurg. 2020, 133, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Bander, E.D.; Ramos, A.D.; Wembacher-Schroeder, E.; Ivasyk, I.; Thomson, R.; Morgenstern, P.F.; Souweidane, M.M. Repeat convection-enhanced delivery for diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2020, 26, 661–666. [Google Scholar] [CrossRef]
- Rechberger, J.S.; Power, E.A.; Lu, V.M.; Zhang, L.; Sarkaria, J.N.; Daniels, D.J. Evaluating infusate parameters for direct drug delivery to the brainstem: A comparative study of convection-enhanced delivery versus osmotic pump delivery. Neurosurg. Focus 2020, 48, E2. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W. Convection enhanced delivery of anti-angiogenic and cytotoxic agents in combination therapy against brain tumour. Eur. J. Pharm. Sci. 2020, 141, 105094. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Kanamori, M.; Sonoda, Y.; Yamashita, Y.; Nagamatsu, K.; Murata, T.; Mugikura, S.; Kumabe, T.; Wembacher-Schröder, E.; Thomson, R.; et al. Phase I trial of convection-enhanced delivery of nimustine hydrochloride (ACNU) for brainstem recurrent glioma. Neuro-Oncol. Adv. 2020, 2, vdaa033. [Google Scholar]
- Wang, J.L.; Barth, R.F.; Cavaliere, R.; Puduvalli, V.K.; Giglio, P.; Lonser, R.R.; Elder, J.B. Phase I trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas. PLoS ONE 2020, 15, e0244383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Control. Release 2017, 263, 112–119. [Google Scholar] [CrossRef]
- Shi, M.; Fortin, D.; Paquette, B.; Sanche, L. Convection-enhancement delivery of liposomal formulation of oxaliplatin shows less toxicity than oxaliplatin yet maintains a similar median survival time in F98 glioma-bearing rat model. Investig. New Drugs 2016, 34, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Anantha, M.; Wehbe, M.; Bally, M.B.; Fortin, D.; Roy, L.O.; Charest, G.; Richer, M.; Paquette, B.; Sanche, L. Liposomal formulations of carboplatin injected by convection-enhanced delivery increases the median survival time of F98 glioma bearing rats. J. Nanobiotechnol. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Park, J.H. Convection-enhanced delivery of liposomal drugs for effective treatment of glioblastoma multiforme. Drug Deliv. Transl. Res. 2020, 10, 1876–1887. [Google Scholar] [CrossRef]
- Rousseau, J.; Barth, R.F.; Fernandez, M.; Adam, J.F.; Balosso, J.; Estève, F.; Elleaume, H. Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J. Neurooncol. 2010, 98, 287–295. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lonser, R.R.; Morrison, P.F.; Governale, L.S.; Oldfield, E.H. Variables affecting convection-enhanced delivery to the striatum: A systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J. Neurosurg. 1999, 90, 315–320. [Google Scholar] [CrossRef]
- Nwagwu, C.D.; Immidisetti, A.V.; Bukanowska, G.; Vogelbaum, M.A.; Carbonell, A.-M. Convection-Enhanced Delivery of a First-in-Class Anti-β1 Integrin Antibody for the Treatment of High-Grade Glioma Utilizing Real-Time Imaging. Pharmaceutics 2020, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Barua, N.U.; Lowis, S.P.; Woolley, M.; O’Sullivan, S.; Harrison, R.; Gill, S.S. Robot-guided convection-enhanced delivery of carboplatin for advanced brainstem glioma. Acta Neurochir. (Wien) 2013, 155, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
| Reference | Therapeutic Agent | Population | Dose | N | Catheter | Catheter Size | Infusion Rate | Volume Infused | Duration | Adverse Events | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lidar et al. [23] | Paclitaxel | Recurrent malignant glioma | 0.55, 1.2 mg/mL | 15 | Medtronic VP shunt catheter | - | 0.3 mL/h | 6–6.6 mL/day | 2–5 days | Chemical meningitis | Median survival = 7.5 months |
| Tanner et al. [24] | Paclitaxel | rGBM | 0.5, 0.25 mg/mL | 8 | Medtronic VP shunt catheter | 1.5 mm inner, 3 mm outer diameter | 0.3 mL/h | 26 mL | 120 h | Edema, neurological deterioration, skin necrosis | Survival 4–15 months |
| Popperl et al. [25] | Paclitaxel | rGBM | 0.5, 0.25 mg/mL | 8 | Silicone catheter | - | 0.3 mL/h | - | 120 h | Edema, skin necrosis | Survival 5–16 months |
| Bruce et al. [26] | Topotecan | Recurrent malignant glioma | 0.02–0.133 mg/mL | 16 | 2 Silastic CSF-peritoneal catheters | 2.5 mm outer diameter | 200 μL/h | 40 mL | 100 h | Parietal syndrome, dysmetria, hemineglect, weakness | Median survival = 60 weeks, PFS6 = 44%, mPFS = 23 weeks |
| Vogelbaum et al. [27] | Topotecan | Recurrent HGG | 0.0067 mg/mL | 3 | 2 Cleveland Multiport catheters | 2.5 mm central shaft, 0.38 mm outer diameter per micro-catheter | 0.396 mL/h (6.6 μL/min) | 38 mL | 96 h | - | - |
| Laske et al. [28] | Tf-CRM107 | Recurrent malignant brain tumors | 0.1–3.2 μg/mL | 18 | 1–3 CSF-peritoneal catheters | 2.5 mm outer diameter | 0.5–10 μL/min | 5–180 mL | 2–16 days | Edema, seizures | Decrease in tumor volume, 13% complete response |
| Weaver and Laske [29] | Tf-CRM107 | rGBM, AA | 0.67 μg/mL | 44 | 2 catheters | 2.5 mm outer diameter | Up to 0.2 mL/h | 40 mL | 4–5 days | Edema, seizures | Median survival = 37 weeks |
| Weber et al. [30] | NBI-3001 | Recurrent malignant glioma | 6–15 μg/mL | 31 | Up to 3 infusion catheters | - | - | 40 mL, 100 mL | 96 ±4 h | Seizures, headache, weakness, edema | Median survival = 8.2 months, 5.8 for glioblastoma |
| Sampson et al. [31] | TP-38 | Recurrent malignant brain tumors | 25–100 ng/mL | 20 | Barium-impregnated CSF-ventricular catheters (2) | Outer diameter 2.1 mm | 0.4 mL/h | 40 mL | 50 h | Hemiparesis, fatigue, subdural hygroma, hemorrhage | Median survival = 28 weeks |
| Vogelbaum et al. [32] | IL13-PE38QQR † | Newly diagnosed malignant glioma | 0.25 ug/mL, 0.5 μg/mL | 22 | Barium-impregnated silicone catheters (2–4) | 1mm inner, 2 mm outer diameter | 0.75 mL/h * | - | 96 h | Seizure, aphasia, confusion, fatigue, gait disturbance, nystagmus | Survival range 5–113 weeks, PFS range 5–78+ weeks |
| Kunwar et al. [33,34] | IL13-PE38QQR | Recurrent malignant glioma | IT:0.25–2 μg/mLIP: 0.25–1 μg/mL | 51 | Barium-impregnated silicone catheters | Inner diameter 1–1.3 mm, outer diameter 2–2.5 mm | IT: 0.4 or 0.54 mL/h * IP: 0.75 mL/h * | IT: 19.2–51.8 mL IP: 72–108 mL | IT: 48–96 h IP: 96 h–6 days | Hemiparesis, convulsions, headache | Median survival = 45.9 weeks (95% CI, 37.4–59.3) |
| Kunwar [34] | IL13-PE38QQR | rGBM | 0.5 μg/mL | 183 | Barium-impregnated silicone catheters | - | 0.75 mL/h | - | 96 h | Pulmonary embolism | Median survival = 36.4 weeks |
| Kicielinski et al. [35] | Reovirus | Recurrent malignant glioma | 108–1010 TIDC | 15 | 1–4 Phoenix Biomedical CED catheters | - | 400 μL/hour * | 72 h | Catheter clogging, convulsions | Median survival = 140 days, mTTP = 61 days | |
| Desjardins et al. [36] | PVSRIPO | rGBM | 107–1010 TIDC | 61 | 1 Vygon catheter | - | 500 μL/h | 3.25 mL | 6.5 h | Hemorrhage, headache, hemiparesis, seizure | Median survival = 12.5 months |
| Voges [37] | LIPO-HSV-1-tk | rGBM | - | 8 | 1–2 silicon catheters | - | 0.025–0.6 mL/h | 3.5 mL | 2 h | Transient worsening of neurological condition, elevated body temperature | Median survival = 28.1 ± 3 weeks, Median TTP = 8 ± 6 weeks |
| Kumar et al. [38] | Liposomal irinotecan (Onivyde) | Recurrent HGG | 20, 40 mg/mL | 10 | Up to 4 catheters | - | - | 2–17 mL | <5 h | - | Survival >1 year in 7 patients |
| Carpentier et al. [39] | CpG-28 | rGBM | 0.5–20 mg | 24 | 1–2 catheters | - | 0.2 mL/h | 1 mL | 6 h | Lymphopenia, worsening of neurological condition | Median survival = 7.2 months, PFS6 = 4.5% |
| Carpentier et al. [40] | CpG-28 | rGBM | 20 mg | 31 | 2 catheters | - | 4 mg/h | 2 mL * | 6 h | Transient neurological worsening, fever, hemorrhage at catheter site SAEs- infection, penumoencephaly, hemorrhage | PFS6 = 19% mPFS = 9.1 weeks Median survival = 28 weeks |
| Hau et al. [15] | AP-12009 | Recurrent HGG | 2.5–80 μM | 24 | 1 catheter | - | 4–8 μL/min | 23.04–80.6 mL | 4 days, 7 days | Brain edema | Overall survival = GBM: 44 weeksAA:146.6 weeks |
| Patel et al. [41] | Cotara | Malignant glioma | 0.5–3 mCi 131I/cm3, 1 mg/mL | 51 | Barium-impregnated cardiac/peritoneal catheters | - | 0.18 mL/h | 4.5–18 mL | 1–2 days | Edema, hemiparesis, headache | Median survival (GBM subset, n = 12): 37.9 weeks |
| Trial Registration | Therapeutic Agent | Phase | N | Status | Estimated Completion | Population | Study Design | Endpoints |
|---|---|---|---|---|---|---|---|---|
| NCT04608812 [92] * | OS2966 | I | 24 | Active, recruiting | March 2022 | Recurrent/progressive HGG | Treat-resect-treat (IT and IP infusions), Gadoteridol co-infusion | Safety (AEs, DLTs), optimal dose Distribution of infusion, tumor response rate, TTP |
| NCT01491893 | PVSRIPO | I | 61 | Active, not recruiting | June 2021 | Grade IV malignant glioma | IT infusion | MTD (DLTs), median OS, median PFS |
| NCT04479241 (LUMINOS-101) | PVSRIPO, pembrolizumab | II | 30 | Active, recruiting | March 2023 | Recurrent glioblastoma | IT infusion | Objective response rate, duration of response, durable radiographic response, AEs, biomarker identification |
| NCT02303678 | D2C7-IT | I | 115 | Active, recruiting | December 2022 | Recurrent malignant glioma | IT infusion | MTD (DLTs), OS, association between EGFRvIII and EGFRwt expression and PFS/OS |
| NCT04547777 | D2C7-IT, 2141-V11 | I | 30 | Active, not yet recruiting | December 2025 | Recurrent HGG | IT infusion | Safety (AEs, DLTs) |
| NCT04160494 | D2C7-IT With Atezolizumab | I | 18 | Active, recruiting | January 2026 | Recurrent grade IV glioma | IT infusion | Safety (AEs, DLTs) |
| NCT03927274 | Topotecan | I | 5 | Active, recruiting | April 2022 | Recurrent/progressive HGG | IT, Gd-DTPAco-infusion | Drug distribution, AEs, extent of backflow |
| NCT03283631 | EGFR-vIII CAR-T Cells | I | 24 | Suspended pending protocol amendment | December 2022 | Recurrent glioblastoma | IT infusion | MTD, change in T cell trafficking in tumor and systemically, median survival, median PFS |
| NCT03154996 | Topotecan | I | 5 | Active, not recruiting | September 2021 | Recurrent HGG | Chronic IT and IP infusion for 32 days via subcutaneous pump | Clinical toxicity rate, tumor response, PFS |
| NCT02869243 | hrBMP4 | Ib | 15 | Active, not recruiting | March 2021 | Progressive/multiple recurrent glioblastoma | Treat-resect-treat (IT and IP infusions), Gd-DTPA co-infusion | MTD (DLTs), tumor response, distribution |
| NCT02022644 | Liposomal irinotecan | I | 18 | Active, not recruiting | November 2021 | Recurrent HGG | IT, gadolinium co-infusion | MTD (DLTs), tumor response rate, TTP, OS, infusion modeling and imaging, Vd/Vi, tumor histology |
| NCT01906385 | 186RNL (ReSPECT) | I/II | 55 | Active, recruiting | January 2025 | Recurrent glioma | IT | MTD (DLTs), dose distribution on SPECT imaging, response rate, survival |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwagwu, C.D.; Immidisetti, A.V.; Jiang, M.Y.; Adeagbo, O.; Adamson, D.C.; Carbonell, A.-M. Convection Enhanced Delivery in the Setting of High-Grade Gliomas. Pharmaceutics 2021, 13, 561. https://doi.org/10.3390/pharmaceutics13040561
Nwagwu CD, Immidisetti AV, Jiang MY, Adeagbo O, Adamson DC, Carbonell A-M. Convection Enhanced Delivery in the Setting of High-Grade Gliomas. Pharmaceutics. 2021; 13(4):561. https://doi.org/10.3390/pharmaceutics13040561
Chicago/Turabian StyleNwagwu, Chibueze D., Amanda V. Immidisetti, Michael Y. Jiang, Oluwasegun Adeagbo, David C. Adamson, and Anne-Marie Carbonell. 2021. "Convection Enhanced Delivery in the Setting of High-Grade Gliomas" Pharmaceutics 13, no. 4: 561. https://doi.org/10.3390/pharmaceutics13040561
APA StyleNwagwu, C. D., Immidisetti, A. V., Jiang, M. Y., Adeagbo, O., Adamson, D. C., & Carbonell, A.-M. (2021). Convection Enhanced Delivery in the Setting of High-Grade Gliomas. Pharmaceutics, 13(4), 561. https://doi.org/10.3390/pharmaceutics13040561






