A Systematic Review and Meta-Analysis on the Impact of Statin Treatment in HIV Patients on Antiretroviral Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Registration
2.2. Search Strategy and Information Sources
2.3. Eligibility Criteria and Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis and Analysis
3. Results
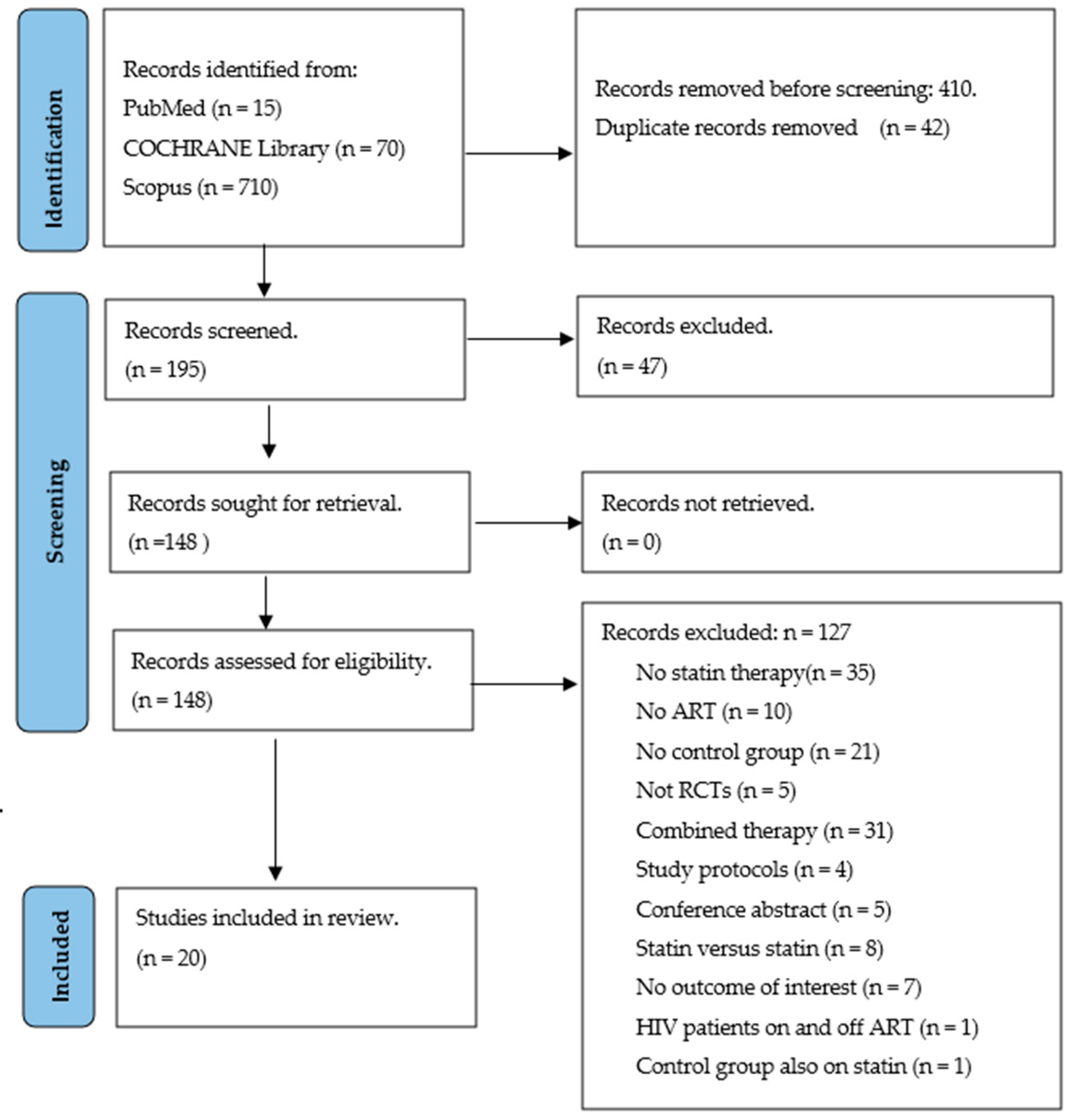
3.1. Search, General Characteristics and Quality of Included Trials
3.2. Effect of Statin on Markers of HIV Infection
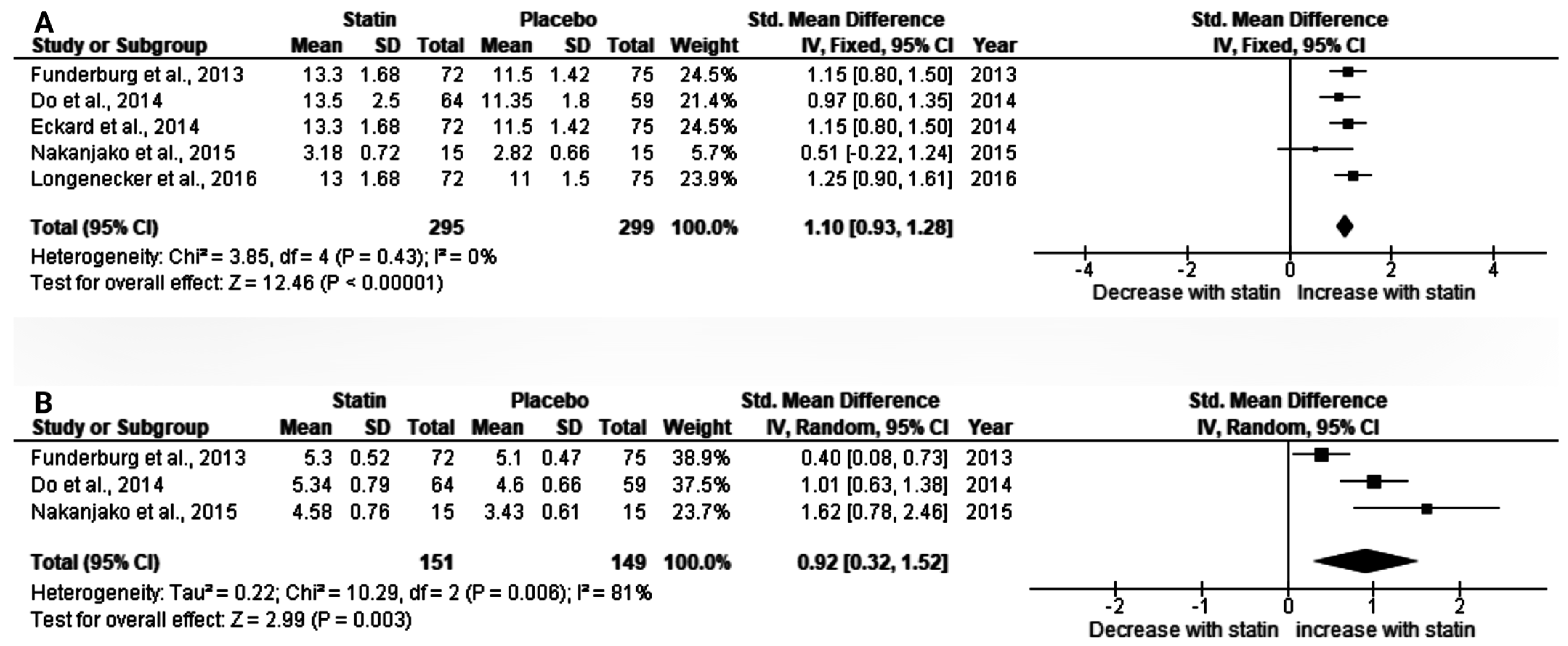
3.2.1. The Effect of Statin on Baseline CD4 T-Cell Count in HIV Patients on Antiretroviral Therapy (ART)
3.2.2. Effect of Statin on CD8+, and CD4+CD38+HLA-DR+ Cells
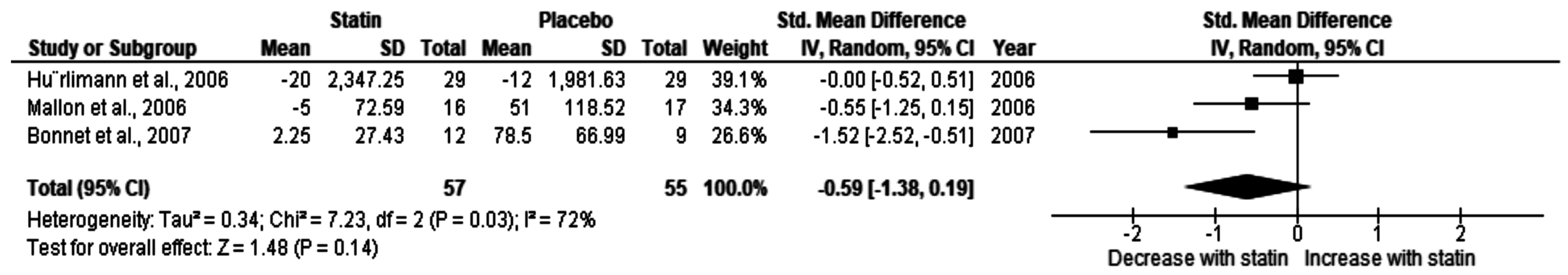
3.2.3. Effect of Statin on Undetectable HIV Load (<50 HIV RNA Copy)
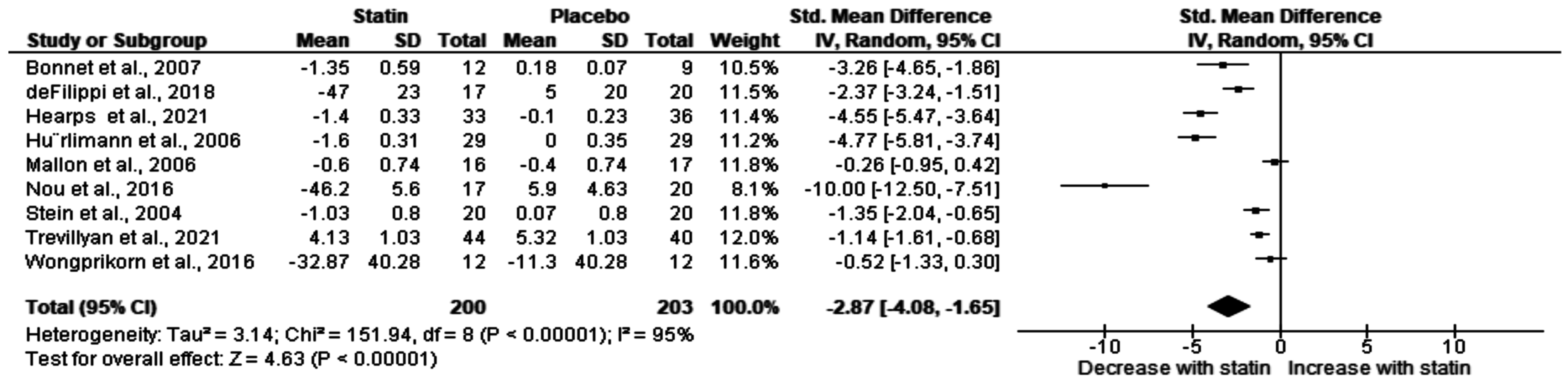
3.3. Effect of Statin on Total Cholesterol in HIV Patients on ART
3.4. Test for Sub-Group Differences
3.5. Sensitivity Analysis
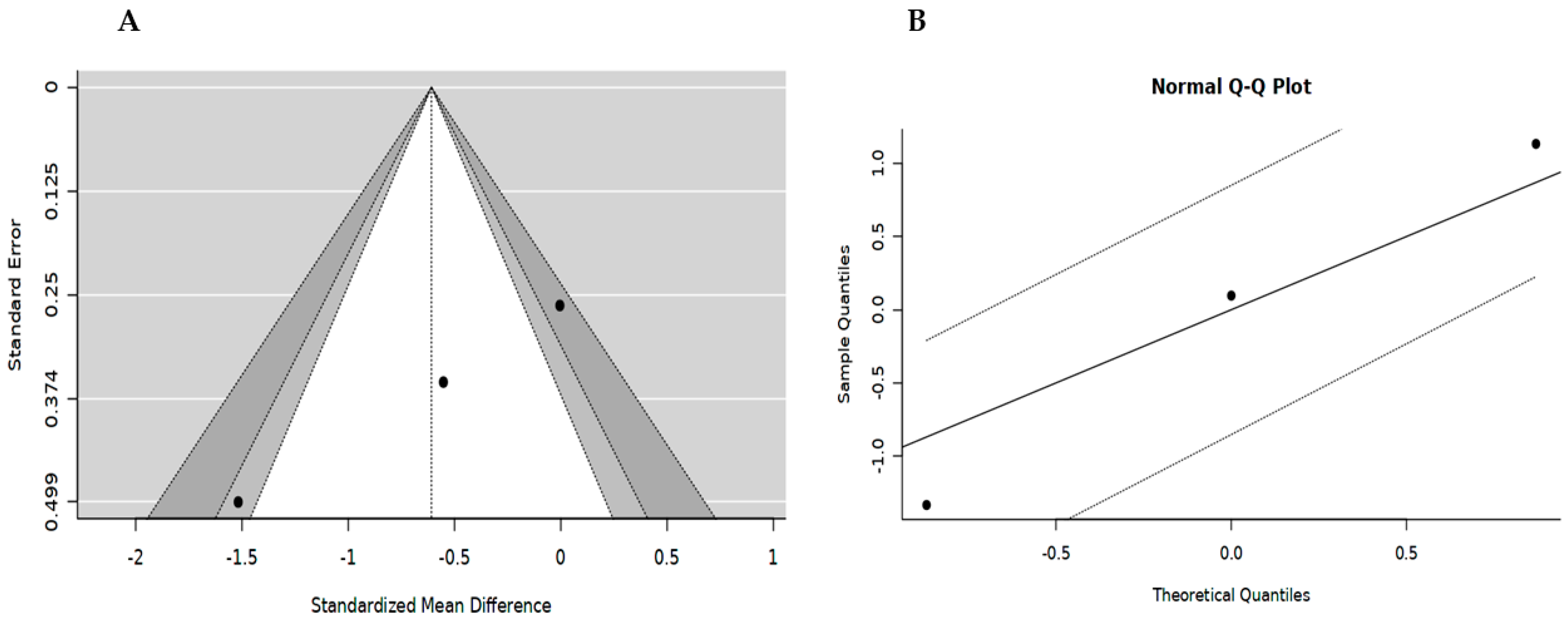
3.6. Publication Bias
4. Discussion
5. Strength and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global HIV Statistics. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 23 January 2023).
- UNAIDS HIV and AIDS Estimates. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 24 January 2023).
- Vijayan, K.V.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Jensen, B.E.O.; Knops, E.; Cords, L.; Lübke, N.; Salgado, M.; Busman-Sahay, K.; Estes, J.D.; Huyveneers, L.E.P.; Perdomo-Celis, F.; Wittner, M.; et al. In-Depth Virological and Immunological Characterization of HIV-1 Cure after CCR5Δ32/Δ32 Allogeneic Hematopoietic Stem Cell Transplantation. Nat. Med. 2023, 615, 13–14. [Google Scholar] [CrossRef]
- Schramm, B.; Temfack, E.; Descamps, D.; Nicholas, S.; Peytavin, G.; Bitilinyu-Bangoh, J.E.; Storto, A.; Lê, M.P.; Abdi, B.; Ousley, J.; et al. Viral Suppression and HIV-1 Drug Resistance 1 Year after Pragmatic Transitioning to Dolutegravir First-Line Therapy in Malawi: A Prospective Cohort Study. Lancet HIV 2022, 9, e544–e553. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, K.; Modjadji, P.; Madiba, S. The Nutritional Status of Adult Antiretroviral Therapy Recipients with a Recent Hiv Diagnosis; a Cross-Sectional Study in Primary Health Facilities in Gauteng, South Africa. Healthcare 2020, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Hyle, E.P.; Mayosi, B.M.; Middelkoop, K.; Mosepele, M.; Martey, E.B.; Walensky, R.P.; Bekker, L.G.; Triant, V.A. The Association between HIV and Atherosclerotic Cardiovascular Disease in Sub-Saharan Africa: A Systematic Review. BMC Public Health 2017, 17, 954. [Google Scholar] [CrossRef] [PubMed]
- Kishabongo, A.S.; Shabani, C.U.; Bisangamo, C.K.; Shindano, T.A.; Takaisi-Kikuni, N.B. Changes of Lipid Profile and Other Biological Parameters in People Living with Human Immunodeficiency Virus on Highly Active Antiretroviral Therapy in the General Referral Provincial Hospital of Bukavu, Eastern of the Democratic Republic of Congo. J. HIV AIDS 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Schaefer, R.; Amparo da Costa Leite, P.H.; Silva, R.; Abdool Karim, Q.; Akolo, C.; Cáceres, C.F.; Dourado, I.; Green, K.; Hettema, A.; Hoornenborg, E.; et al. Kidney Function in Tenofovir Disoproxil Fumarate-Based Oral Pre-Exposure Prophylaxis Users: A Systematic Review and Meta-Analysis of Published Literature and a Multi-Country Meta-Analysis of Individual Participant Data. Lancet HIV 2022, 9, e242–e253. [Google Scholar] [CrossRef]
- Myerson, M.; Malvestutto, C.; Aberg, J.A. Management of Lipid Disorders in Patients Living with HIV. J. Clin. Pharmacol. 2015, 55, 957–974. [Google Scholar] [CrossRef]
- Rodriguez, B.; Valdez, H.; Mijch, A.; Watson, K.; Lederman, M.M.; McComsey, G.A.; Loupa, C.V.; Woolley, I. Statins Blunt HAART-Induced CD4 T-Cell Gains but Have No Long-Term Effect on Virologic Response to HAART. J. Int. Assoc. Physicians. AIDS Care 2007, 6, 198–202. [Google Scholar] [CrossRef]
- Mallon, P.W.G.; Miller, J.; Kovacic, J.C.; Kent-Hughes, J.; Norris, R.; Samaras, K.; Feneley, M.P.; Cooper, D.A.; Carr, A. Effect of Pravastatin on Body Composition and Markers of Cardiovascular Disease in HIV-Infected Men-a Randomized, Placebo-Controlled Study. AIDS 2006, 20, 1003–10101. [Google Scholar] [CrossRef]
- Hileman, C.O.; Turner, R.; Funderburg, N.T.; Semba, R.D.; McComsey, G.A. Changes in Oxidized Lipids Drive the Improvement in Monocyte Activation and Vascular Disease after Statin Therapy in HIV. AIDS 2016, 30, 65–73. [Google Scholar] [CrossRef]
- El Kamari, V.; Hileman, C.O.; Gholam, P.M.; Kulkarni, M.; Funderburg, N.; McComsey, G.A. Statin Therapy Does Not Reduce Liver Fat Scores in Patients Receiving Antiretroviral Therapy for HIV Infection. Clin. Gastroenterol. Hepatol. 2019, 17, 536–542.e1. [Google Scholar] [CrossRef]
- Calza, L.; Colangeli, V.; Magistrelli, E.; Contadini, I.; Bon, I.; Re, M.C.; Conti, M.; Mancini, R.; Viale, P. Significant Decrease in Plasma Levels of D-Dimer, Interleukin-8, and Interleukin-12 after a 12-Month Treatment with Rosuvastatin in HIV-Infected Patients under Antiretroviral Therapy. AIDS Res. Hum. Retrovir. 2017, 33, 126–132. [Google Scholar] [CrossRef]
- Hearps, A.C.; Angelovich, T.A.; Trevillyan, J.M.; Wong, M.E.; Calmy, A.; Hoy, J.F.; Jaworowski, A. Effect of Rosuvastatin Therapy on Biomarkers of Inflammation and Immune Activation in People with Human Immunodeficiency Virus at Intermediate Cardiovascular Risk. J. Infect. Dis. 2021, 224, 667–672. [Google Scholar] [CrossRef]
- Bittar, R.; Giral, P.; Aslangul, E.; Assoumou, L.; Valantin, M.A.; Kalmykova, O.; Federspiel, M.C.; Cherfils, C.; Costagliola, D.; Bonnefont-Rousselot, D. Effects of Rosuvastatin versus Pravastatin on Low-Density Lipoprotein Diameter in HIV-1-Infected Patients Receiving Ritonavir-Boosted Protease Inhibitor. AIDS 2012, 26, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Bedimo, R.J.; Mar, H.; Bosch, R.J.; Drechsler, H.; Cyktor, J.C.; Macatangay, B.J.C.; Lalama, C.; Rinaldo, C.; Collier, A.; Godfrey, C.; et al. No Evidence for an Association Between Statin Use and Lower Biomarkers of HIV Persistence or Immune Activation/Inflammation During Effective ART. J. Acquir. Immune. Defic. Syndr. 2019, 82, 27–31. [Google Scholar] [CrossRef]
- Wongprikorn, A.; Sukasem, C.; Puangpetch, A.; Numthavej, P.; Thakkinstian, A.; Kiertiburanakul, S. Effects of Pitavastatin on Lipid Profiles in HIV-Infected Patients with Dyslipidemia and Receiving Atazanavir/Ritonavir: A Randomized, Double-Blind, Crossover Study. PLoS ONE 2016, 11, e0157531. [Google Scholar] [CrossRef] [PubMed]
- Hürlimann, D.; Chenevard, R.; Ruschitzka, F.; Flepp, M.; Enseleit, F.; Béchir, M.; Kobza, R.; Muntwyler, J.; Ledergerber, B.; Lüscher, T.F.; et al. Effects of Statins on Endothelial Function and Lipid Profile in HIV Infected Persons Receiving Protease Inhibitor-Containing Anti-Retroviral Combination Therapy: A Randomised Double Blind Crossover Trial. Heart 2006, 92, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, C.T.; Sattar, A.; Gilkeson, R.; Mccomsey, G.A. Rosuvastatin Slows Progression of Subclinical Atherosclerosis in Patients with Treated HIV Infection. AIDS 2016, 30, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.T.; Jiang, Y.; Debanne, S.M.; Storer, N.; Labbato, D.; Clagett, B.; Robinson, J.; Lederman, M.M.; McComsey, G.A. Rosuvastatin Treatment Reduces Markers of Monocyte Activation in HIV-Infected Subjects on Antiretroviral Therapy. Clin. Infect. Dis. 2014, 58, 588–595. [Google Scholar] [CrossRef]
- Eckard, A.R.; Jiang, Y.; Debanne, S.M.; Funderburg, N.T.; Mccomsey, G.A. Effect of 24 Weeks of Statin Therapy on Systemic and Vascular Inflammation in HIV-Infected Subjects Receiving Antiretroviral Therapy. J. Infect. Dis. 2014, 209, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Cromarty, R.; Archary, D. Inflammation, HIV, and Immune Quiescence: Leveraging on Immunomodulatory Products to Reduce HIV Susceptibility. AIDS Res. Treat. 2020, 2020, 8672850. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Belmonti, S.; Moschese, D.; Fabbiani, M.; Borghetti, A.; Ciccullo, A.; Visconti, E.; di Giambenedetto, S. Inflammation Markers in Virologically Suppressed HIV-Infected Patients after Switching to Dolutegravir plus Lamivudine vs Continuing Triple Therapy: 48-Week Results in Real-Life Setting. HIV Res. Clin. Pract. 2022, 23, 28–36. [Google Scholar] [CrossRef]
- Werida, R.; Khairat, I.; Khedr, N.F. Effect of Atorvastatin versus Rosuvastatin on Inflammatory Biomarkers and LV Function in Type 2 Diabetic Patients with Dyslipidemia. Biomed. Pharmacother. 2021, 135, 111179. [Google Scholar] [CrossRef]
- Nachega, J.B.; Hsu, A.J.; Uthman, O.A.; Spinewine, A.; Pham, P.A. Antiretroviral Therapy Adherence and Drug-Drug Interactions in the Aging HIV Population. AIDS 2012, 26, S39–S53. [Google Scholar] [CrossRef]
- Pontelo, B.M.; Greco, D.B.; Guimarães, N.S.; Rotsen, N.; Braga, V.A.R.; Pimentel, P.H.N.; Barbosa, H.; Barroso, T.M.; Tupinambás, U. Profile of Drug–Drug Interactions and Impact on the Effectiveness of Antiretroviral Therapy among Patients Living with HIV Followed at an Infectious Diseases Referral Center in Belo Horizonte, Brazil. Braz. J. Infect. Dis. 2020, 24, 104–109. [Google Scholar] [CrossRef]
- Gili, S.; Marra, W.G.; D’Ascenzo, F.; Lonni, E.; Calcagno, A.; Cannillo, M.; Ballocca, F.; Cerrato, E.; Pianelli, M.; Barbero, U.; et al. Comparative Safety and Efficacy of Statins for Primary Prevention in Human Immunodeficiency Virus-Positive Patients: A Systematic Review and Meta-Analysis. Eur. Heart J. 2016, 37, 3600–3609. [Google Scholar] [CrossRef]
- Banach, M.; Dinca, M.; Ursoniu, S.; Serban, M.C.; Howard, G.; Mikhailidis, D.P.; Nicholls, S.; Lip, G.Y.H.; Glasser, S.; Martin, S.S.; et al. A PRISMA-Compliant Systematic Review and Meta-Analysis of Randomized Controlled Trials Investigating the Effects of Statin Therapy on Plasma Lipid Concentrations in HIV-Infected Patients. Pharmacol. Res. 2016, 111, 343–356. [Google Scholar] [CrossRef]
- Vigny, N.N.; Bonsu, K.O.; Kadirvelu, A. Effectiveness and Safety of Statins on Outcomes in Patients with HIV Infection: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 18121. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 18121. [Google Scholar] [CrossRef]
- Jadad, A.R.; Andrew Moore, R.; Carroll, D.; Jenkinson, C.; John Reynolds, D.M.; Gavaghan, D.J.; McQuay DM, H.J. Assessing the Quality of Reports of Randomised Clinical Trials: Is Blinding Necessary? Control. Clin Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hassani, H.; Ghodsi, M.; Howell, G. A Note on Standard Deviation and Standard Error. Teach. Math. Its Appl. 2010, 29, 108–112. [Google Scholar] [CrossRef]
- Cochrane Handbook 6.1.3.2 Imputing Standard Deviations for Changes from Baseline. Available online: https://handbook-5-1.cochrane.org/chapter_16/16_1_3_2_imputing_standard_deviations_for_changes_from_baseline.htm (accessed on 28 January 2023).
- Yagiz, G.; Akaras, E.; Kubis, H.P.; Owen, J.A. The Effects of Resistance Training on Architecture and Volume of the Upper Extremity Muscles: A Systematic Review of Randomised Controlled Trials and Meta-Analyses. Appl. Sci. 2022, 12, 1593. [Google Scholar] [CrossRef]
- Bowden, J.; Tierney, J.F.; Copas, A.J.; Burdett, S. Quantifying, Displaying and Accounting for Heterogeneity in the Meta-Analysis of RCTs Using Standard and Generalised Q Statistics. BMC Med. Res. Methodol. 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Meta-Analyses: Heterogeneity and Subgroup Analysis. BMJ 2013, 346, f4040. [Google Scholar] [CrossRef]
- Mathur, M.B.; VanderWeele, T.J. Sensitivity Analysis for Publication Bias in Meta-Analyses. J. R Stat. Soc. Ser. C Appl. Stat. 2020, 69, 1091–1119. [Google Scholar] [CrossRef]
- Bonnet, F.; Aurillac-Lavignolle, V.; Breilh, D.; Thiébaut, R.; Peuchant, E.; Bernard, N.; Lacoste, D.; Dabis, F.; Beylot, J.; Chêne, G.; et al. Pravastatin in HIV-Infected Patients Treated with Protease Inhibitors: A Placebo-Controlled Randomised Study. HIV Clin. Trials 2007, 8, 53–60. [Google Scholar] [CrossRef]
- Stein, J.H.; Merwood, M.A.; Bellehumeur, J.L.; Aeschlimann, S.E.; Korcarz, C.E.; Underbakke, G.L.; Mays, M.E.; Sosman, J.M. Effects of Pravastatin on Lipoproteins and Endothelial Function in Patients Receiving Human Immunodeficiency Virus Protease Inhibitors. Am. Heart J. 2004, 147, 713–717. [Google Scholar] [CrossRef]
- Hileman, C.O.; McComsey, G.A. Short Communication: The Effect of Rosuvastatin on Vascular Disease Differs by Smoking Status in Treated HIV Infection. AIDS Res. Hum. Retrovir. 2018, 34, 282–285. [Google Scholar] [CrossRef]
- Trevillyan, J.M.; Dart, A.; Paul, E.; Cavassini, M.; Fehr, J.; Staehelin, C.; Dewar, E.M.; Hoy, J.F.; Calmy, A. Impact of Rosuvastatin on Atherosclerosis in People with HIV at Moderate Cardiovascular Risk: A Randomised, Controlled Trial. AIDS 2021, 35, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.E.; Bosch, R.J.; Chan, E.S.; Funderburg, N.T.; Hodder, S.; Lake, J.E.; Lederman, M.M.; Klingman, K.L.; Aberg, J.A.; Bergstrom, K.; et al. Effects of Atorvastatin on Biomarkers of Immune Activation, Inflammation, and Lipids in Virologically Suppressed, Human Immunodeficiency Virus-1–Infected Individuals with Low-Density Lipoprotein Cholesterol <130 Mg/DL (AIDS Clinical Trials Group Study A5275). J. Clin. Lipidol. 2017, 11, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.T.; Longenecker, C.T.; Mittelsteadt, A.; Jiang, Y.; Debanne, S.M.; McComsey, G.A. Effect of Rosuvastatin on Plasma Coenzyme Q10 in HIV-Infected Individuals on Antiretroviral Therapy. HIV Clin. Trials 2016, 17, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Nou, E.; Lu, M.T.; Looby, S.E.; Fitch, K.V.; Kim, E.A.; Lee, H.; Hoffmann, U.; Grinspoon, S.K.; Lo, J. Serum Oxidized Low-Density Lipoprotein Decreases in Response to Statin Therapy and Relates Independently to Reductions in Coronary Plaque in Patients with HIV. AIDS 2016, 30, 583–590. [Google Scholar] [CrossRef]
- Nakanjako, D.; Ssinabulya, I.; Nabatanzi, R.; Bayigga, L.; Kiragga, A.; Joloba, M.; Kaleebu, P.; Kambugu, A.D.; Kamya, M.R.; Sekaly, R.; et al. Atorvastatin Reduces T-Cell Activation and Exhaustion among HIV-Infected CART-Treated Suboptimal Immune Responders in Uganda: A Randomised Crossover Placebo-Controlled Trial. Trop. Med. Int. Health 2015, 20, 380–390. [Google Scholar] [CrossRef]
- Hileman, C.O.; Tangpricha, V.; Sattar, A.; McComsey, G.A. Baseline Vitamin D Deficiency Decreases the Effectiveness of Statins in HIV-Infected Adults on Antiretroviral Therapy. J. Acquired. Defic. Syndr. 2017, 74, 539–547. [Google Scholar] [CrossRef]
- Defilippi, C.; Christenson, R.; Joyce, J.; Park, E.A.; Wu, A.; Fitch, K.V.; Looby, S.E.; Lu, M.T.; Hoffmann, U.; Grinspoon, S.K.; et al. Statin Effects on Myocardial Fibrosis Markers in People Living With HIV. J. Acquir. Immune. Defic. Syndr. 2018, 78, 105–110. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Jiang, Y.; Debanne, S.M.; McComsey, G.A. Rosuvastatin Worsens Insulin Resistance in HIV-Infected Adults on Antiretroviral Therapy. Clin. Infect. Dis. 2015, 61, 1566–1572. [Google Scholar] [CrossRef]
- Dirajlal-Fargo, S.; Kinley, B.; Jiang, Y.; Longenecker, C.T.; Hileman, C.O.; Debanne, S.; McComsey, G.A. Statin Therapy Decreases N-Terminal pro-B-Type Natriuretic Peptide in HIV: Randomized Placebo-Controlled Trial. AIDS 2014, 29, 313–321. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Jiang, Y.; Debanne, S.M.; Mccomsey, G.A. Effects of 96 Weeks of Rosuvastatin on Bone, Muscle, and Fat in HIV-Infected Adults on Effective Antiretroviral Therapy. AIDS Res. Hum. Retrovir. 2016, 32, 311–316. [Google Scholar] [CrossRef]
- Lok, J.J.; Bosch, R.J.; Benson, C.A.; Collier, A.C.; Robbins, G.K.; Shafer, R.W.; Hughes, M.D. Long-Term Increase in CD4+ T-Cell Counts during Combination Antiretroviral Therapy for HIV-1 Infection. AIDS 2010, 24, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Weijma, R.G.M.; Vos, E.R.A.; Oever, J.T.; Van Schilfgaarde, M.; Dijksman, L.M.; Van Der Ven, A.; Van Den Berk, G.E.L.; Brinkman, K.; Frissen, J.P.H.J.; Leyte, A.; et al. The Effect of Rosuvastatin on Markers of Immune Activation in Treatment-Naive Human Immunodeficiency Virus-Patients. Open Forum. Infect Dis. 2016, 3, ofv201. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.C.; Maisa, A.; Cheng, W.J.; Angelovich, T.A.; Lichtfuss, G.F.; Palmer, C.S.; Landay, A.L.; Jaworowski, A.; Crowe, S.M. HIV Infection Induces Age-Related Changes to Monocytes and Innate Immune Activation in Young Men That Persist despite Combination Antiretroviral Therapy. AIDS 2012, 26, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, M.F.; Marsland, A.; Flory, J.D.; Rabin, B.S.; Whiteside, T.L.; Manuck, S.B. Immune System Differences in Men with Hypo- or Hypercholesterolemia. Clin. Immunol. Immunopathol. 1997, 84, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Motkowski, R.; Alifier, M.; Abramowicz, P.; Konstantynowicz, J.; Mikołuć, B.; Stasiak-Barmuta, A. Innate and Acquired Cellular Immunity in Children with Familial Hypercholesterolemia Treated with Simvastatin. J. Clin. Med. 2022, 11, 2924. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Levert, A.; Yeung, J.; Starr, M.; Cameron, J.; Williams, R.; Rismanto, N.; Stark, T.; Druery, D.; Prasad, S.; et al. HIV-1 Viral Blips Are Associated with Repeated and Increasingly High Levels of Cell-Associated HIV-1 RNA Transcriptional Activity. AIDS 2021, 35, 2095–2103. [Google Scholar] [CrossRef]
- Sörstedt, E.; Nilsson, S.; Blaxhult, A.; Gisslén, M.; Flamholc, L.; Sönnerborg, A.; Yilmaz, A. Viral Blips during Suppressive Antiretroviral Treatment Are Associated with High Baseline HIV-1 RNA Levels. BMC Infect Dis. 2016, 16, 305. [Google Scholar] [CrossRef]
- Corbeau, P.; Reynes, J. Immune Reconstitution under Antiretroviral Therapy: The New Challenge in HIV-1 Infection. Blood 2011, 117, 5582–5590. [Google Scholar] [CrossRef]
- Bourgeois, C.; Gorwood, J.; Olivo, A.; Le Pelletier, L.; Capeau, J.; Lambotte, O.; Béréziat, V.; Lagathu, C. Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment. Front. Immunol. 2021, 12, 2222. [Google Scholar] [CrossRef]
- Overton, E.T.; Sterrett, S.; Westfall, A.O.; Kahan, S.M.; Burkholder, G.; Zajac, A.J.; Goepfert, P.A.; Bansal, A. Effects of Atorvastatin and Pravastatin on Immune Activation and T-Cell Function in Antiretroviral Therapy-Suppressed HIV-1-Infected Patients. AIDS 2014, 28, 2627–2631. [Google Scholar] [CrossRef]
- Cheung, R.; Ravyn, V.; Wang, L.; Ptasznik, A.; Collman, R.G. Signaling Mechanism of HIV-1 Gp120 and Virion-Induced IL-1 Release in Primary Human Macrophages 1. J. Immunol. 2008, 180, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Yao, X.D.; Rosenthal, K.L. HIV-1 Structural Proteins Serve as PAMPs for TLR2 Heterodimers Significantly Increasing Infection and Innate Immune Activation. Front. Immunol. 2015, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Psomas, C.; Reynes, J.; Corbeau, P. Immune Activation in the Course of HIV-1 Infection: Causes, Phenotypes and Persistence under Therapy. HIV Med. 2016, 17, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Crum-Cianflone, N.; Higgins, J.; Qin, J.; Rehm, C.; Metcalf, J.; Brandt, C.; Vita, J.; Decker, C.F.; Sklar, P.; et al. High Dose Atorvastatin Decreases Cellular Markers of Immune Activation without Affecting HIV-1 RNA Levels: Results of a Double-Blind Randomized Placebo Controlled Clinical Trial. J. Infect. Dis. 2011, 203, 756–764. [Google Scholar] [CrossRef]
- Massaro, M.; Zampolli, A.; Scoditti, E.; Carluccio, M.A.; Storelli, C.; Distante, A.; De Caterina, R. Statins Inhibit Cyclooxygenase-2 and Matrix Metalloproteinase-9 in Human Endothelial Cells: Anti-Angiogenic Actions Possibly Contributing to Plaque Stability. Cardiovasc. Res. 2010, 86, 311–320. [Google Scholar] [CrossRef]
- Hussain, S.K.; Golozar, A.; Widney, D.P.; Rappocciolo, G.; Penugonda, S.; Bream, J.H.; Martínez-Maza, O.; Jacobson, L.P. Effect of Statin Use on Inflammation and Immune Activation Biomarkers in HIV-Infected Persons on Effective Antiretroviral Therapy. AIDS Res. Hum. Retrovir. 2021, 37, 357–367. [Google Scholar] [CrossRef]
- Chastain, D.B.; Stover, K.R.; Riche, D.M. Evidence-Based Review of Statin Use in Patients with HIV on Antiretroviral Therapy. J. Clin. Transl. Endocrinol. 2017, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.V.; Fulda, E.S.; Grinspoon, S.K. Statins for Primary Cardiovascular Disease Prevention among People with HIV: Emergent Directions. Curr. Opin. HIV AIDS 2022, 17, 293–300. [Google Scholar] [CrossRef]
- Baldini, F.; di Giambenedetto, S.; Cingolani, A.; Murri, R.; Ammassari, A.; Luca, A. de Efficacy and Tolerability of Pravastatin for the Treatment of HIV-1 Protease Inhibitor-Associated Hyperlipidaemia: A Pilot Study. AIDS 2000, 14, 1660–1662. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Manfredi, R.; Chiodo, F. Statins and Fibrates for the Treatment of Hyperlipidaemia in HIV-Infected Patients Receiving HAART. AIDS 2003, 17, 851–859. [Google Scholar] [CrossRef]
- Calza, L.; Manfredi, R.; Colangeli, V.; Trapani, F.F.; Salvadori, C.; Magistrelli, E.; Danese, I.; Verucchi, G.; Serra, C.; Viale, P. Two-Year Treatment with Rosuvastatin Reduces Carotid Intima-Media Thickness in HIV Type 1-Infected Patients Receiving Highly Active Antiretroviral Therapy with Asymptomatic Atherosclerosis and Moderate Cardiovascular Risk. AIDS Res. Hum. Retrovir. 2013, 29, 547–556. [Google Scholar] [CrossRef]
- Grandi, A.M.; Nicolini, E.; Rizzi, L.; Caputo, S.; Annoni, F.; Cremona, A.M.; Marchesi, C.; Guasti, L.; Maresca, A.M.; Grossi, P. Dyslipidemia in HIV-Positive Patients: A Randomized, Controlled, Prospective Study on Ezetimibe+fenofibrate versus Pravastatin Monotherapy. J. Int. AIDS Soc. 2014, 17, 19004. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.V.; Huppler Hullsiek, K.; Prosser, R.; Duprez, D.; Grimm, R.; Tracy, R.P.; Rhame, F.; Henry, K.; Neaton, J.D. Angiotensin Converting Enzyme Inhibitor and HMG-CoA Reductase Inhibitor as Adjunct Treatment for Persons with HIV Infection: A Feasibility Randomised Trial. PLoS ONE 2012, 7, e46894. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Buhaescu, I.; Izzedine, H. Mevalonate Pathway: A Review of Clinical and Therapeutical Implications. Clin. Biochem. 2007, 40, 575–584. [Google Scholar] [CrossRef]
| Study, Year, Country | Study Design | Population Status | Duration of HIV (Years/Months) | Duration of ART (Years/Months) | Sample Size Statin Group, n | Form of Statin, Dosage, and Duration | Age (Years) | Gender, Male (%) |
|---|---|---|---|---|---|---|---|---|
| Hearps et al., 2021 [16] Switzerland | Post hoc analysis of the RCT. | Sixty-nine virologically suppressed patients. | 19.1 ± 3.65 | NR | 33 | 20 mg rosuvastatin for 96 weeks. | 54 ± 6.5 | 32 (97) |
| Trevillyan et al., 2021 [44] Australia | Post hoc analysis of the RCT. | Eighty-eight controlled PLHIV on ART. | 17.2 ± 8.5 | NR | 44 | 20 mg rosuvastatin or 10 mg on antiretroviral therapy for 96 weeks. | 53.9 ± 5.9 | 42 (95.5) |
| Kamari et al., 2019 [14] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on ART. | NR | 7.0 ± 5.3 | 72 | 10 mg rosuvastatin for 96 weeks. | 45 ± 9.0 | 58 (81) |
| deFillippi et al., 2018 [50] USA | Post hoc analysis of the RCT. | Forty PLHIV on stable ART. | 16.8 ± 5.1 | 12.4 ± 3.7 | 19 | 20 mg to 40 mg atorvastatin for three months. | 52.2 ± 3.8 | 15 (79) |
| Hileman et al., 2017 [49] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on ART. | 11.71 ± 6.6 | 7.0 ± 5.3 | 72 | 10 mg rosuvastatin for 96 weeks. | 45.37 ± 9.13 | 58 (81) |
| Nixon et al., 2017 [45] USA | Post hoc analysis of the RCT. | Ninety-four PLHIV on boosted PI-based ART. | 6 ± 1.3 | NR | 94 | 10 mg of atorvastatin for 4 weeks, followed by 20 mg for 16 weeks. | 48 ± 2.3 | 64 (68) |
| Hileman et al., 2016 [13] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on stable ART. | NR | NR | 72 | 10 mg rosuvastatin for 48 weeks. | 45.4 ± 9.1 | 58 (81) |
| Longenecker et al., 2016 [21] Australia | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on stable ART. | 11 ± 1.83 | 5.2 ± 1.13 | 72 | 10 mg rosuvastatin for 96 weeks. | 45 ± 1.7 | 58 (81) |
| Morrison et al., 2016 [46] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on ART. | 11 ± 1.83 | 3.9 ± 1.23 | 72 | 10 mg rosuvastatin for 24 weeks. | 45 ± 1.67 | 58 (81) |
| Nou et al., 2016 [47] USA | Post hoc analysis of the RCT. | Forty PLHIV with sub-clinical coronary atherosclerosis on stable ART. | 16.8 ± 5.1 | 12.4 ± 3.7 | 19 | 40 atorvastatin for 12 months. | 52.2 ± 3.8 | 15 (79) |
| Wongprikorn et al., 2016 [19] China | Post hoc analysis of the RCT. | Twenty-four PLHIV on ART. | NR | 42 ± 6.93 | 12 | 2 mg pitavastatin for 12 weeks. | 49.6 ± 10.6 | 8 (66.7) |
| Do et al., 2014 [52] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on stable ART. | 122 ± 31.5 | 56 ± 21.25 | 64 | 10 mg rosuvastatin for 96 weeks | 46 ± 2.5 | 53 (83) |
| Erlandson et al., 2015 [51] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on stable ART. | NR | 63 ± 13.67 | 72 | 10 mg of rosuvastatin for 96 weeks. | 45.6 ± 1.72 | 58 (81) |
| Nakanjako et al., 2015 [48] Uganda | Post hoc analysis of the RCT. | Thirty PLHIV on a suppressive cART. | NR | NR | 15 | 80 mg of rosuvastatin for 12 weeks. | 43 ± 8.54 | 8 (53) |
| Eckard et al., 2014 [23] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on stable ART. | 133 ± 20.7 | 63 ± 13.7 | 72 | 10 mg rosuvastatin 24 weeks | 45.6 ± 1.72 | 58 (81) |
| Funderburg et al., 2013 [22] USA | Post hoc analysis of the RCT. | One hundred and forty-seven PLHIV on ART. | 133 ± 20.7 | 63 ± 13.7 | 72 | 10 mg rosuvastatin for 24 weeks. | 45.6 ± 1.71 | 58 (81) |
| Bonnet et al., 2007 [41] France | Post hoc analysis of the RCT. | Twenty PLHIV on stable ART. | NR | NR | 12 | 40 mg of pravastatin for three months. | 42.5 ± 2.31 | 11 (92) |
| Hürlimann et al., 2006 [20] Switzerland | Post hoc analysis of the RCT. | Twenty-nine PLHIV on a PI-containing cART. | NR | NR | 29 | 40 mg pravastatin for 8 weeks. | 43 | 23 (79) |
| Mallon et al., 2006 [12] Australia | Post hoc analysis of the RCT. | Thirty-one PLHIV with hypercholesterolaemia on PI-containing therapy. | 13.5 ± 4.5 | NR | 16 | 40 mg pravastatin for 12 weeks. | 52 ± 12 | 16 (100) |
| Stein et al., 2004 [42] USA | Post hoc analysis of the RCT. | Twenty PLHIV on HIV PI-based ART. | 11.8 ± 0.5 | 8 ± 3.7 | 20 | 40 mg pravastatin for 8 weeks. | 44.1 ± 1.6 | 18 (90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokgalaboni, K.; Phoswa, W.N.; Yates, S.; Lebelo, S.L.; Madiba, S.; Modjadji, P. A Systematic Review and Meta-Analysis on the Impact of Statin Treatment in HIV Patients on Antiretroviral Therapy. Int. J. Environ. Res. Public Health 2023, 20, 5668. https://doi.org/10.3390/ijerph20095668
Mokgalaboni K, Phoswa WN, Yates S, Lebelo SL, Madiba S, Modjadji P. A Systematic Review and Meta-Analysis on the Impact of Statin Treatment in HIV Patients on Antiretroviral Therapy. International Journal of Environmental Research and Public Health. 2023; 20(9):5668. https://doi.org/10.3390/ijerph20095668
Chicago/Turabian StyleMokgalaboni, Kabelo, Wendy Nokhwezi Phoswa, Samantha Yates, Sogolo Lucky Lebelo, Sphiwe Madiba, and Perpetua Modjadji. 2023. "A Systematic Review and Meta-Analysis on the Impact of Statin Treatment in HIV Patients on Antiretroviral Therapy" International Journal of Environmental Research and Public Health 20, no. 9: 5668. https://doi.org/10.3390/ijerph20095668






