Study Protocol of a Multicenter Randomized Controlled Trial to Tackle Obesity through a Mediterranean Diet vs. a Traditional Low-Fat Diet in Adolescents: The MED4Youth Study
Abstract
:1. Introduction
2. Materials and Methods
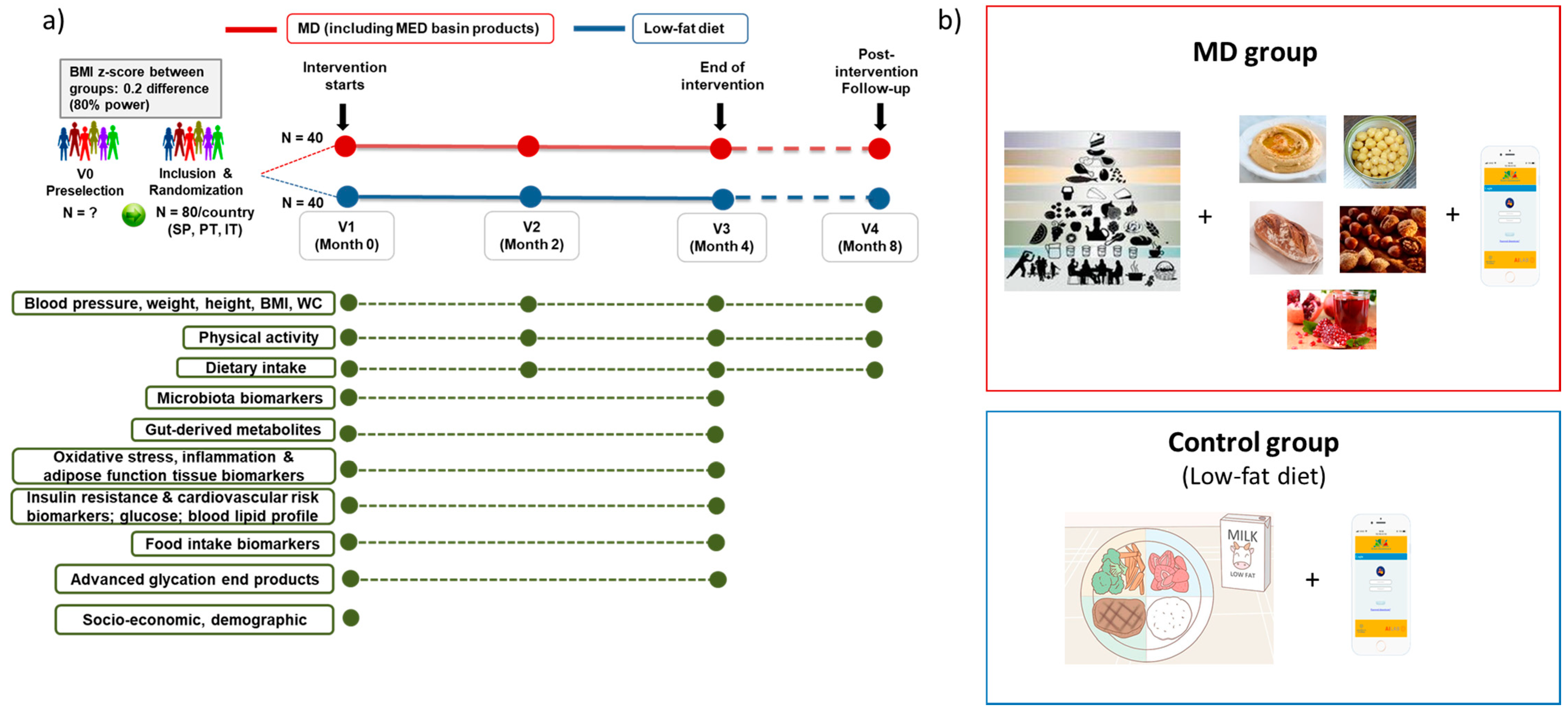
2.1. Study Design and Setting
2.2. Selection Criteria
- Boys and girls aged 13–17 years,
- Having obesity, defined as an age- and sex-specific BMI in the 95th percentile or greater (1), or great overweight (age- and sex-specific BMI in the ≥90th to <95th percentile),
- If they and their parents (or holders of parental responsibility over the child) provide written informed consent (signed by parents and by the adolescent), and
- Having easily access to internet (e.g., via mobile phone, tablet, pc).
- Having diabetes and other metabolic, endocrine, and chronic disorders,
- Intake of antibiotics, drug, probiotics, or nutritional supplements in the last month,
- Prescribed medicine to control hypertension, inflammation, or dyslipidaemia,
- Following a prescribed diet for any reason, including weight loss, in the last 3 months,
- Following a religion-restricted diet, and
- Having allergies or food intolerances to nuts, pomegranate, bread, and/or chickpeas.
2.3. Dietary Interventions
- (1)
- The MD will be based on a high consumption of unsaturated fat from vegetable sources (extra virgin olive oil and nuts) and minimally processed plant foods (vegetables, fruits, nuts, whole grains and legumes), low consumption of meat (especially red and processed meats) and sweets, and moderate consumption of fish and dairy products (mainly yogurt and cheese) [6,43]. Accordingly, this diet will provide a high amount of mono and polyunsaturated fatty acids, fiber, and phenolic compounds [44]. The MD will be based on the one proposed as MD in the PREDIMED study [45], providing 40–45% of energy from carbohydrate, 35–40% from fat, and 15–20% from protein. Special attention will be paid to the benefits of whole and minimally processed foods, traditional food vs. fast food, and seasonal products. Within the MD pattern, the intervention group (MD) will consume a sourdough bread and 3 specific foods from the Mediterranean basin with proven healthy effects (pomegranate juice, chickpeas/hummus, and mixed nuts) (Table 1).
- (2)
- The low-fat diet (control diet) consists of a restricted consumption of fats. This diet will be based on the one proposed as low-fat diet in the PREDIMED study [45], and it will provide 55% of energy from carbohydrate, 25–30% from fat, and 15–20% from protein. The low-fat diet is the most used diet for obesity treatment in adolescents [42]. This group will not receive any additional specific food by the researchers.
2.4. Implementation of the Motivational Interview Technique
2.5. Educational Web-Application
2.6. Manufacturing and Distribution of the Mediterranean Basin Foods
2.7. Outcomes
- MD adherence, using the KidMed questionnaire [19]
- Physical activity, using Physical Activity Questionnaire for Adolescents (PAQ-A) [52]
- Dietary intake and habits:
- Nutritional knowledge, using the NK Helena questionnaire [54]
- Quality of life, using the kidscreen-27 Index [55]
- Sociodemographic data, using the Health Behavior in School Age Children (HBSC) socio-demographic questionnaire [56]
- Anthropometric data: weight, height, BMI (weight/height2), body composition using bioimpedance, waist circumference (WC), and waist–hip ratio (WHR)
- Blood pressure
- Biochemical variables, gut microbiota composition, and metabolomics in urine, blood, and feces
2.8. Sample Size
2.9. Statistical Analyses
2.9.1. Univariate Data Analysis
2.9.2. Multivariate Data Analysis
2.9.3. Data Integration: Systems Biology Approach
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. W.H.O. Childhood Overweight and Obesity; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Commission on Ending Childhood Obesity. Ending Childhood Obesity. 2020. Available online: https://www.who.int/end-childhood-obesity/en/ (accessed on 12 April 2021).
- OECD. The Heavy Burden of Obesity: The Economics of Prevention; OECD Health Policy Studies; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M.; Casini, A.; Paul, G.; et al. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, M.; Boqué, N.; del Bas, J.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra-Majem, L.; Tomaino, L.; Dernini, S.; Berry, E.M.; Lairon, D.; de la Cruz, J.N.; Bach-Faig, A.; Donini, L.M.; Medina, F.X.; Belahsen, R.; et al. Updating the mediterranean diet pyramid towards sustainability: Focus on environmental concerns. Int. J. Environ. Res. Public Health 2020, 17, 8758. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Raheem, D.; Ramos, F.; Carrascosa, C.; Saraiva, A.; Raposo, A. Highlights of current dietary guidelines in five continents. Int. J. Environ. Res. Public Health 2021, 18, 2814. [Google Scholar] [CrossRef]
- Guasch-ferré, M.; Babio, N.; Mart, M.A.; Corella, D.; Ros, E.; Mart, S.; Estruch, R.; Ar, F.; Enrique, G.; Fiol, M. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease 1. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salvadó, J.S.; Covas, M.; Corella, D.; Arós, F.; Gracia, E.G.; Gutiérrez, V.R.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e3. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Kastorini, C.-M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and metabolic syndrome: An updated systematic review. Rev. Endocr. Metab. Disord. 2013, 14, 255–263. [Google Scholar] [CrossRef]
- Esposito, K.; Kastorini, C.-M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean Diet and Weight Loss: Meta-Analysis of Randomized Controlled Trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: One-year results of the PREDIMED-Plus trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Dinu, M.; Pagliai, G.; Angelino, D.; Rosi, A.; Dall’Asta, M.; Bresciani, L.; Ferraris, C.; Guglielmetti, M.; Godos, J.; Cristian Del Bo’, C.; et al. Effects of Popular Diets on Anthropometric and Cardiometabolic Parameters: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 815–833. [Google Scholar] [CrossRef]
- Schröder, H.; Mendez, M.A.; Ribas-Barba, L.; Covas, M.I.; Serra-Majem, L. Mediterranean diet and waist circumference in a representative national sample of young Spaniards. Int. J. Pediatr. Obes. 2010. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, A.; Marventano, S.; Antoci, M.; Cagnetti, A.; Giogianni, G.; Nolfo, F.; Rametta, S.; Pecora, G.; Marranzano, M. Mediterranean diet adherence and body composition among Southern Italian adolescents. Obes. Res. Clin. Pract. 2017, 11, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, S.G.; Fernández, N.H.; Hernández, C.R.; Nissensohn, M.; Román-Viña, B.; Serra-Majem, L. Test KIDMED; prevalencia de la Baja Adhesión a la Dieta Mediterránea en Niños y Adolescentes; Revisión Sistemática. Nutr. Hosp. 2015, 32, 2390–2399. [Google Scholar] [CrossRef]
- Iaccarino Idelson, P.; Scalfi, L.; Valerio, G. Adherence to the Mediterranean Diet in children and adolescents: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Rosi, A.; Paolella, G.; Biasini, B.; Scazzina, F. Dietary habits of adolescents living in North America, Europe or Oceania: A review on fruit, vegetable and legume consumption, sodium intake, and adherence to the Mediterranean Diet. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Llauradó, E.; Tarro, L.; Moriña, D.; Queral, R.; Giralt, M.; Solà, R. EdAl-2 (Educació en Alimentació) programme: Reproducibility of a cluster randomised, interventional, primaryschool-based study to induce healthier ifestyle activities in children. BMJ Open 2014, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wärnberg, J.; Carlos, J.; Felipe, S.; Labayen, I.; Zapico, A.G.; Gusi, N.; Aznar, S.; Alcaraz, P.E.; Gonz, M.; Serra-majem, L.; et al. Screen Time and Parents’ Education Level Are Associated with Poor Adherence to the Mediterranean Diet in Spanish Children and Adolescents: The PASOS Study. J. Clin. Med. 2021, 10, 795. [Google Scholar] [CrossRef]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 13, 928. [Google Scholar] [CrossRef] [Green Version]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean diet and health: Food effects on gut microbiota and disease control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef]
- Lopez-Legarrea, P.; Fuller, N.R.; Zulet, M.A.; Martinez, J.A.; Caterson, I.D. The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac. J. Clin. Nutr. 2014, 23, 360–368. [Google Scholar]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Bailey, M.; Holscher, H. Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [Green Version]
- Kerimi, A.; Nyambe-Silavwe, H.; Gauer, J.S.; Tomás-Barberán, F.A.; Williamson, G. Pomegranate juice, but not an extract, confers a lower glycemic response on a high–glycemic index food: Randomized, crossover, controlled trials in healthy subjects. Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A.; Makahleh, S.M.; El-Akawi, Z.; Al-Fashtaki, R.A.; Khabour, O.F.; Gharibeh, M.Y.; Saadah, N.A.; Al-Hashimi, F.H.; Al-Khasieb, N.J. Fresh pomegranate juice ameliorates insulin resistance, enhances β-cell function, and decreases fasting serum glucose in type 2 diabetic patients. Nutr. Res. 2014, 34, 862–867. [Google Scholar] [CrossRef]
- Reister, E.J.; Belote, L.N.; Leidy, H.J. The Benefits of Including Hummus and Hummus Ingredients into the American Diet to Promote Diet Quality and Health: A Comprehensive Review. Nutrients 2020, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Gil-cardoso, K.; Saldan, G.; Luengo, E.; Pastor, J.; Virto, R.; Alcaide-hidalgo, J.M.; Bas, J.M.; Caimari, A. Consumption of Sourdough Breads Improves Postprandial Glucose Response and Produces Sourdough-Speci fi c E ff ects on Biochemical and In fl ammatory Parameters and Mineral Absorption. J. Agric. Food Chem. 2021, 69, 3044–3059. [Google Scholar] [CrossRef] [PubMed]
- Wilfley, D.E.; Tibbs, T.L.; Van Buren, D.J.; Reach, K.P.; Walker, M.S.; Epstein, L.H. Lifestyle Interventions in the Treatment of Childhood Overweight: A Meta-Analytic Review of Randomized Controlled Trials. Heal. Psychol. 2007, 26, 521. [Google Scholar] [CrossRef] [Green Version]
- Hofsteenge, G.H.; Chinapaw, M.J.M.; Delemarre-van De Waal, H.A.; Weijs, P.J.M. Validation of predictive equations for resting energy expenditure in obese adolescents. Am. J. Clin. Nutr. 2010, 91, 1244–1254. [Google Scholar] [CrossRef] [Green Version]
- Truby, H.; Baxter, K.A.; Barrett, P.; Ware, R.S.; Cardinal, J.C.; Davies, P.S.; Daniels, L.A.; Batch, J.A. The eat smart study: A randomised controlled trial of a reduced carbohydrate versus a low fat diet for weight loss in obese adolescents. BMC Public Health 2010, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Seiquer, I.; Mesías, M.; Navarro, M.P.; Muñoz Hoyos, A.; Galdó, G. A Mediterranean Dietary Style Improves Calcium Utilization in Healthy Male Adolescents. J. Am. Coll. Nutr. 2008, 27, 454–462. [Google Scholar] [CrossRef]
- Bean, M.K.; Powell, P.; Quinoy, A.; Ingersoll, K.; Wickham, E.P.; Mazzeo, S.E. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: Results from the MI Values randomized controlled trial. Pediatr. Obes. 2015, 10, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañedo-Argüelles, C.A.; de la Fuente García, A.; Díaz, J.; García Rebollar, C.; Lorente García-Mauriño, A.M.; Martínez García, M.S.; Ruiz Chércoles, E.; Padilla Esteban, M.L. Alimentación y Actividad Física en el Niño Mayor y el Adolescente; AMPap: Madrid, Spain, 2014; ISBN 978-84-695-3798-5. [Google Scholar]
- Canani, S.B.; Censi, L.; Cialfa, E.; D’Amicis, A.; Gennaro, L.; Ghiselli, A.; Leclercq, C.; Quaglia, G.B.; Rossi, L.; Scognamiglio, U.; et al. Linee Guida per una Sana Alimentazione—Revisione 2018; CREA: Rome, Italy, 2019; ISBN 9788833850375. [Google Scholar]
- Resnicow, K.; Davis, R.; Rollnick, S. Motivational interviewing for pediatric obesity: Conceptual issues and evidence review. J. Am. Diet. Assoc. 2006, 106, 2024–2033. [Google Scholar] [CrossRef]
- Rosi, A.; Brighenti, F.; Finistrella, V.; Ingrosso, L.; Monti, G.; Vanelli, M.; Vitale, M.; Volta, E.; Scazzina, F. Giocampus school: A “learning through playing” approach to deliver nutritional education to children. Int. J. Food Sci. Nutr. 2016, 67, 207–215. [Google Scholar] [CrossRef]
- Grossman, D.C.; Bibbins-Domingo, K.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for obesity in children and adolescents us preventive services task force recommendation statement. JAMA J. Am. Med. Assoc. 2017, 317, 2417–2426. [Google Scholar] [CrossRef]
- Baker, J.L.; Olsen, L.W.; Sørensen, T.I.A. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 2007, 357, 2329–2337. [Google Scholar] [CrossRef]
- The IPAQ Group. International Physical Activity Questionnaire Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms; Revised on November 2005. Available online: http://www.ipaq.ki.se (accessed on 12 April 2021).
- Moreno, L.A.; Kersting, M.; de Henauw, S.; González-Gross, M.; Sichert-Hellert, W.; Matthys, C.; Mesana, M.I.; Ross, N. How to measure dietary intake and food habits in adolescence: The European perspective. Int. J. Obes. 2005, 29, S66–S77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersting, M.; Sichert-Hellert, W.; Vereecken, C.A.; Diehl, J.; Béghin, L.; De Henauw, S.; Grammatikaki, E.; Manios, Y.; Mesana, M.I.; Papadaki, A.; et al. Food and nutrient intake, nutritional knowledge and diet-related attitudes in European adolescents. Int. J. Obes. 2008, 32, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Ravens-Sieberer, U. KIDSCREEN Instruments Health-Related Quality of Life Questionnaire for Children and Young People; Pabst Science Publishers: Lengerich, Germany, 2004. [Google Scholar]
- Currie, C.; Griebler, R.; Inchley, J.; Theunissen, A.; Molcho, M.; Samdal, O.; Dür, W. Health Behaviour in School-Aged Children (HBSC) Study Protocol: Background, Methodology and Mandatory Items for the 2009/10 Survey; CAHRU & Vienna, LBIHPR: Edinburgh, UK, 2010. [Google Scholar]
- Gual-Grau, A.; Guirro, M.; Mayneris-Perxachs, J.; Arola, L.; Boqué, N. Impact of different hypercaloric diets on obesity features in rats: A metagenomics and metabolomics integrative approach. J. Nutr. Biochem. 2019, 71, 122–131. [Google Scholar] [CrossRef]
- Singh, A.; Gautier, B.; Shannon, C.P.; Vacher, M.; Rohart, F.; Tebutt, S.J.; Cao, K.-A. Le DIABLO—An integrative, multi-omics, multivariate method for multi-group classification. Bioinformatics 2019, 35, 3055–3062. [Google Scholar] [CrossRef]
- Arias, M.; Cobo, M.; Jaime-Sánchez, P.; Pastor, J.; Marijuan, P.; Pardo, J.; Rezusta, A.; Del Campo, R. Gut microbiota and systemic inflammation changes after bread consumption: The ingredients and the processing influence. J. Funct. Foods 2017, 32, 98–105. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Abbondio, M.; Palomba, A.; Tanca, A.; Fraumene, C.; Pagnozzi, D.; Serra, M.; Marongiu, F.; Laconi, E.; Uzzau, S. Fecal metaproteomic analysis reveals unique changes of the gut microbiome functions after consumption of sourdough Carasau bread. Front. Microbiol. 2019, 10, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamataki, N.S.; Yanni, A.E.; Karathanos, V.T. Bread making technology influences postprandial glucose response: A review of the clinical evidence. Br. J. Nutr. 2017, 117, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The nutritional value and health benefits of chickpeas and hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, A.; Hill, A.; Tan, S. Nuts and Cardiovascular Disease Prevention. Curr. Atheroscler. Rep. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Cabré-Vila, J.J.; Salas-Salvadó, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [Green Version]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelishadi, R.; Growth, C.; Jazi, M.H.; Poursafa, P. Acute and long term effects of grape and pomegranate juice consumption on endothelial dysfunction in pediatric metabolic syndrome. J. Res. Med. Sci. 2011, 16, 245–253. [Google Scholar]
- Pine, K.; Fletcher, B. (C) Time to shift brain channels to bring about effective changes in health behaviour. Perspect. Public Health 2014, 134, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.C.; Hanson, J.; Page, N.; Pine, K. FIT—Do something different: A new behavioral programfor sustainedweight loss. Swiss J. Psychol. 2011, 70, 25–34. [Google Scholar] [CrossRef]
| Specific Food | Frequency of Portions |
|---|---|
| Pomegranate | It is recommended at least 2 servings/day of fruit: 14–21 servings/week In addition to the fresh whole fruit, 4 servings of pomegranate juice (200 mL) will be consumed during the week. |
| Hummus and chickpeas | It is recommended 3 servings/week of pulses (150–200 g (50–80 g dry)). Two servings/week must be cooked chickpeas, minimum one of them as hummus, and another serving may be chickpeas or different pulses. The portion size of hummus is 200 g. |
| Nuts (almonds, walnuts, and hazelnuts) | It is recommended 3 servings/week: 30 g of mixed nuts/serving. Four servings will be consumed to properly combine the 4 food items included in the MD along the week. Mixed nuts will be walnuts, almonds, and hazelnuts. |
| Sourdough bread | It is recommended 4–6 servings/day of cereals. Two of these must be a serving of sourdough bread (1 serving of bread is approximately 50–60 g [46,47]). |
| Variables | Tool | Timeline |
|---|---|---|
| Blood pressure, weight, height, BMI, body composition, WC, WHR | Dietitians/Nutritionists | V1, V2, V3, V4 |
| Physical activity | PAQ-A * | V1, V2, V3, V4 |
| Dietary intake | 3-day dietary record * | V1, V2, V3, V4 |
| Food habits | FFQ Helena questionnaire * | V1, V2, V3, V4 |
| MD adherence | KidMed questionnaire * | V1, V2, V3, V4 |
| Quality of life | Kidscreen-27 questionnaire * | V1, V2, V3, V4 |
| Sociodemographic data | HBSC questionnaire * | V1 |
| Knowledge on food and nutrition | NK Helena questionnaire * | V1, V3, V4 |
| Microbiota (feces) | 16sRNA sequencing | V1 and V3 |
| Gut-derived metabolites (LPS, SCFAs, lactate, bile acids) (feces and/or plasma) | NMR/GC-MS | V1 and V3 |
| Biomarkers of oxidative stress (8-OHdG, F2-isoprostanes) (urine) | ELISA | V1 and V3 |
| Biomarkers of inflammation (IL-6, CRP, TNFα, MCP1, IL-8) (plasma) | Magnetic bead-based multiplex assays Homeostatic Model Assessment for insulin resistance | V1 and V3 V1 and V3 |
| Biomarkers of adipose tissue function (adiponectin, leptin, resistin) (plasma) | ||
| Biomarkers of insulin resistance (plasma) | ||
| Biomarkers of cardiovascular risk (TMAO) (plasma and urine) | UHPLC‑MS | |
| Circulating levels of glucose and blood lipid profile | Enzymatic assays | V1 and V3 |
| Key food intake biomarkers and phenolic metabolites (urine) | Targeted metabolomics (UHPLC‑MS) | V1 and V3 |
| Advanced glycation end products (AGEs) related analyses (plasma or erythrocytes) | ELISA / enzymatic assays | V1 and V3 |
| Visit | Type of Visit | Description |
|---|---|---|
| 0 | pre-screening visit before randomization | To check inclusion/exclusion criteria in the study |
| 1 | after randomization, inclusion visit | Feces, urine, and blood samples will be collected, anthropometric and blood pressure will be measured, and questionnaires (food, knowledge and physical activity) and dietary records will be completed. |
| 2 | after 2 months | Anthropometric and blood pressure will be measured, and questionnaires (food, knowledge and physical activity) and dietary records will be completed. |
| 3 | after 4 months, final study visit | Feces, urine, and blood samples will be collected, anthropometric and blood pressure will be measured, and questionnaires (food, knowledge and physical activity) and dietary records will be completed. |
| 4 | visit 4 months after the end of the intervention | Anthropometric and blood pressure will be measured, and questionnaires (food, knowledge and physical activity) and dietary records will be completed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boqué, N.; Tarro, L.; Rosi, A.; Torrell, H.; Saldaña, G.; Luengo, E.; Rachman, Z.; Pires, A.; Tavares, N.T.; Pires, A.S.; et al. Study Protocol of a Multicenter Randomized Controlled Trial to Tackle Obesity through a Mediterranean Diet vs. a Traditional Low-Fat Diet in Adolescents: The MED4Youth Study. Int. J. Environ. Res. Public Health 2021, 18, 4841. https://doi.org/10.3390/ijerph18094841
Boqué N, Tarro L, Rosi A, Torrell H, Saldaña G, Luengo E, Rachman Z, Pires A, Tavares NT, Pires AS, et al. Study Protocol of a Multicenter Randomized Controlled Trial to Tackle Obesity through a Mediterranean Diet vs. a Traditional Low-Fat Diet in Adolescents: The MED4Youth Study. International Journal of Environmental Research and Public Health. 2021; 18(9):4841. https://doi.org/10.3390/ijerph18094841
Chicago/Turabian StyleBoqué, Noemi, Lucía Tarro, Alice Rosi, Helena Torrell, Guillermo Saldaña, Elisa Luengo, Zeev Rachman, António Pires, Nuno Tiago Tavares, Ana Salomé Pires, and et al. 2021. "Study Protocol of a Multicenter Randomized Controlled Trial to Tackle Obesity through a Mediterranean Diet vs. a Traditional Low-Fat Diet in Adolescents: The MED4Youth Study" International Journal of Environmental Research and Public Health 18, no. 9: 4841. https://doi.org/10.3390/ijerph18094841









