Methodological Approach of the Iron and Muscular Damage: Female Metabolism and Menstrual Cycle during Exercise Project (IronFEMME Study)
Abstract
:Abstract
Trial registration
1. Background
2. Methods/Design
2.1. Study Design
2.2. Participants
2.3. Inclusion and Exclusion Criteria
2.4. Recruitment
2.5. Sample Size Estimation
2.6. Randomization
2.7. Menstrual Cycle Monitoring and Phase Determination
2.7.1. Calendar-Based Counting
2.7.2. Urinary LH Measurement
2.7.3. Serum Hormone Analysis
2.8. Diet and Exercise Restrictions and Recommendations
2.9. Ethical Issues
2.10. Study Interventions
2.10.1. Screening Protocol
2.10.2. Blood Count and Hormonal Measurements
2.10.3. Dual Energy X-Ray Absorptiometry (DXA)
2.10.4. Study I: Endurance Protocol
Maximal Aerobic Test
Testing Procedure Day
Baseline Measurements
Interval Running Protocol
Post-Testing Measurements
2.10.5. Study II: Resistance Protocol
RM Estimation
Testing Procedure Day
Baseline Measurements
Delayed Onset Muscle Soreness
Circumferences
Range of Movement
Counter Movement Jump Test
Eccentric-Based Resistance Exercise Protocol
Post-Testing Measurement
2.11. Blood Sampling Collection and Analysis
2.12. Genetic Testing
2.13. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1RM | One repetition maximum |
| APP | Active pill phase |
| CMJ | Counter movement jump test |
| CRP | C reactive protein |
| DOMS | Perceived delayed onset muscle soreness |
| DXA | Dual-energy X-ray |
| EFP | Early-follicular phase |
| EIMD | Exercise-induced muscle damage |
| FSH | Follicle stimulating hormone |
| HR | Heart rate |
| IL-6 | Interleukin-6 |
| IronFEMME | Iron and muscular damage: FEmale Metabolism and Menstrual cycle during Exercise |
| ISAK | International Society for the Advancement of Kinanthropometry |
| LDH | Lactate dehydrogenase |
| LFP | Late-follicular phase |
| Mb | Myoglobin |
| MLP | Mid-luteal phase |
| OC | Oral contraceptive |
| RER | Respiratory exchange ratio |
| ROM | Range of movement |
| SNPs | Genetic single nucleotide polymorphisms |
| STRAW | Stages of Reproductive Aging Workshop |
| TNF-α | Tumor necrosis factor α |
| TSH | Thyroid-stimulating hormone |
| VAS | Visual analogue scale |
| VCO2 | Carbon dioxide production |
| VE | Pulmonary ventilation |
| VO2 | Oxygen uptake |
| VO2peak | Peak oxygen uptake |
| vVO2peak | Maximal aerobic speed |
| WP | Withdrawal phase |
References
- Williams, T.J.; Krahenbuhl, G.S. Menstrual cycle phase and running economy. Med. Sci. Sports Exerc. 1997, 29, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, X.A.K.J.; Thompson, M.W.; Chuter, V.; Silk, L.N.; Thom, J.M. Exercise Performance over the Menstrual Cycle in Temperate and Hot, Humid Conditions. Med. Sci. Sports Exerc. 2012, 44, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Pivarnik, J.M.; Marichal, C.J.; Spillman, T.; Morrow, J.R. Menstrual cycle phase affects temperature regulation during endurance exercise. J. Appl. Physiol. 1992, 72, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Matzuk, M.M. The Menstrual Cycle. Ann. N. Y. Acad. Sci. 2008, 1135, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.M.; McKenzie, D.C.; Prior, J.C.; Taunton, J.E. Effects of menstrual cycle phase on athletic performance. Med. Sci. Sports Exerc. 1995, 27, 437–444. [Google Scholar] [CrossRef]
- Hall, J.E. Female physiology before pregnancy and female hormones. In Guyton and Hall Textbook of Medical Physiology; Hall, J.E., Ed.; Saunders Elsevier: Philadelphia, PA, USA, 2011; pp. 987–1000. [Google Scholar]
- Davis, H.C.; Hackney, A.C. The Hypothalamic–Pituitary–Ovarian Axis and Oral Contraceptives: Regulation and Function. In Sex Hormones, Exercise and Women; Hackney, A.C., Ed.; Springer: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar]
- Constantini, N.W.; Dubnov, G.; Lebrun, C.M. The Menstrual Cycle and Sport Performance. Clin. Sports Med. 2005, 24, e51–e82. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; Smith, S.; Bacon, J.; Clayton, D.; McPhilimey, M.; Goutianos, G.; Hampson, J.; Sale, C. Examining the role of oral contraceptive users as an experimental and/or control group in athletic performance studies. Contraception 2013, 88, 408–412. [Google Scholar] [CrossRef]
- Rechichi, C.; Dawson, B.; Goodman, C. Athletic Performance and the Oral Contraceptive. Int. J. Sports Physiol. Perform. 2009, 4, 151–162. [Google Scholar] [CrossRef]
- Al-Azzawi, F.; Palacios, S. Hormonal changes during menopause. Maturitas 2009, 63, 135–137. [Google Scholar] [CrossRef]
- Delamater, L.; Santoro, N. Management of the Perimenopause. Clin. Obstet. Gynecol. 2018, 61, 419–432. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, S.; Wang, L.; Li, J.; Qu, G.; He, J.; Rong, H.; Ji, H.; Liu, S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tajima, S.; Izawa-Ishizawa, Y.; Kihira, Y.; Ishizawa, K.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Estrogen Regulates Hepcidin Expression via GPR30-BMP6-Dependent Signaling in Hepatocytes. PLoS ONE 2012, 7, e40465. [Google Scholar] [CrossRef] [PubMed]
- Lehtihet, M.; Bonde, Y.; Beckman, L.; Berinder, K.; Hoybye, C.; Rudling, M.; Sloan, J.H.; Konrad, R.J.; Angelin, B. Circulating Hepcidin-25 Is Reduced by Endogenous Estrogen in Humans. PLoS ONE 2016, 11, e0148802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Rhee, D.K.; Malhotra, R.; Mayeur, C.; Hurst, L.A.; Ager, E.; Shelton, G.; Kramer, Y.; McCulloh, D.; Keefe, D.L.; et al. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J. Clin. Investig. 2015, 126, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17β-Estradiol Inhibits Iron Hormone Hepcidin through an Estrogen Responsive Element Half-Site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef] [Green Version]
- Thompson, B.; Almarjawi, A.; Sculley, D.; De Jonge, X.A.J. The Effect of the Menstrual Cycle and Oral Contraceptives on Acute Responses and Chronic Adaptations to Resistance Training: A Systematic Review of the Literature. Sports Med. 2020, 50, 171–185. [Google Scholar] [CrossRef]
- Barba-Moreno, L.; Alfaro-Magallanes, V.; Calderón, F.; Peinado, A. Systemic iron homeostasis in female athletes: Hepcidin, exercise and sex influence. Arch. Med. Deport. 2020, 37, 348–353. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Coad, J.; Pedley, K. Iron deficiency and iron deficiency anemia in women. Scand. J. Clin. Lab. Investig. 2014, 74, 82–89. [Google Scholar] [CrossRef]
- McClung, J.P. Iron status and the female athlete. J. Trace Elements Med. Biol. 2012, 26, 124–126. [Google Scholar] [CrossRef]
- Milić, R.; Martinovic, J.; Dopsaj, M.; Dopsaj, V. Haematological and iron-related parameters in male and female athletes according to different metabolic energy demands. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 111, 449–458. [Google Scholar] [CrossRef]
- Schumacher, Y.O.; Schmid, A.; Grathwohl, D.; Bültermann, D.; Berg, A. Hematological indices and iron status in athletes of various sports and performances. Med. Sci. Sports Exerc. 2002, 34, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Suedekum, N.A.; Dimeff, R.J. Iron and the Athlete. Curr. Sports Med. Rep. 2005, 4, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Mclnnis, M.D.; Newhouse, I.J.; Von Duvillard, S.P.; Thayer, R. The effect of exercise intensity on hematuria in healthy male runners. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 79, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Quintas, L. Iron deficiency without anemia: Indications for treatment. Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 215–222. [Google Scholar]
- Kamel, H.K. Sarcopenia and Aging. Nutr. Rev. 2003, 61, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Tian, F.; Shen, H.; Jiang, J.; Zhang, Y.; Chai, Y.; Xie, Y. Identifying risk factors for bone mass transition states for postmenopausal osteoporosis. Eur. J. Integr. Med. 2017, 14, 7–12. [Google Scholar] [CrossRef]
- Kendall, B.; Eston, R. Exercise-Induced Muscle Damage and the Potential Protective Role of Estrogen. Sports Med. 2002, 32, 103–123. [Google Scholar] [CrossRef]
- Greising, S.M.; Baltgalvis, K.A.; Lowe, D.A.; Warren, G.L. Hormone Therapy and Skeletal Muscle Strength: A Meta-Analysis. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2009, 64, 1071–1081. [Google Scholar] [CrossRef]
- Tiidus, P.M.; Lowe, D.A.; Brown, M. Estrogen replacement and skeletal muscle: Mechanisms and population health. J. Appl. Physiol. 2013, 115, 569–578. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.M.; Rebar, R.W.; Sherman, S.S.; Sluss, P.M.; De Villiers, T.J. Executive summary of the Stages of Reproductive Aging Workshop + 10. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, R.W.; Tjalsma, H.; Yeap, B.B.; Trinder, D.; Peeling, P. Oral contraception does not alter typical post-exercise interleukin-6 and hepcidin levels in females. J. Sci. Med. Sport 2015, 18, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Sipavičienė, S.; Daniusevičiūtė, L.; Klizienė, I.; Kamandulis, S.; Skurvydas, A. Effects of Estrogen Fluctuation during the Menstrual Cycle on the Response to Stretch-Shortening Exercise in Females. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, X.J.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, M.A.; Jenkins, D.G.; De Jonge, X.A.J.; Emmerton, L.M.; Skinner, T.L. Three-step method for menstrual and oral contraceptive cycle verification. J. Sci. Med. Sport 2017, 20, 965–969. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.B.; Soules, M.R. The Usefulness of a Urinary LH Kit for Ovulation Prediction during Menstrual Cycles of Normal Women. Obstet. Gynecol. 1996, 87, 13–17. [Google Scholar] [CrossRef]
- McGovern, P.G.; Myers, E.R.; Silva, S.; Coutifaris, C.; Carson, S.A.; Legro, R.S.; Schlaff, W.D.; Carr, B.R.; Steinkampf, M.P.; Giudice, L.C.; et al. Absence of secretory endometrium after false-positive home urine luteinizing hormone testing. Fertil. Steril. 2004, 82, 1273–1277. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ishijima, T.; Hayashida, H.; Suzuki, K.; Higuchi, M. Menstrual cycle phase and carbohydrate ingestion alter immune response following endurance exercise and high intensity time trial performance test under hot conditions. J. Int. Soc. Sports Nutr. 2014, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Vaiksaar, S.; Jürimäe, J.; Mäestu, J.; Purge, P.; Kalytka, S.; Shakhlina, L.; Jürimäe, T. No Effect of Menstrual Cycle Phase and Oral Contraceptive Use on Endurance Performance in Rowers. J. Strength Cond. Res. 2011, 25, 1571–1578. [Google Scholar] [CrossRef]
- Tsampoukos, A.; Peckham, E.A.; James, R.; Nevill, M.E. Effect of menstrual cycle phase on sprinting performance. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 109, 659–667. [Google Scholar] [CrossRef]
- Chai, W.; Morimoto, Y.; Cooney, R.V.; Franke, A.A.; Shvetsov, Y.B.; Le Marchand, L.; Haiman, C.A.; Kolonel, L.N.; Goodman, M.T.; Maskarinec, G. Dietary Red and Processed Meat Intake and Markers of Adiposity and Inflammation: The Multiethnic Cohort Study. J. Am. Coll. Nutr. 2017, 36, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; D’Souza, R.F.; Aasen, K.M.M.; Mitchell, S.M.; Durainayagam, B.R.; Sinclair, A.J.; Peake, J.M.; Egner, I.M.; Raastad, T.; Cameron-Smith, D.; et al. Arachidonic acid supplementation transiently augments the acute inflammatory response to resistance exercise in trained men. J. Appl. Physiol. 2018, 125, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Margolis, L.M.; Murphy, N.E.; McClung, H.L.; Martini, S.; Gundersen, Y.; Castellani, J.W.; Karl, J.P.; Teien, H.K.; Madslien, E.H.; et al. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol. Rep. 2016, 4, e12820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwedhelm, C.; Pischon, T.; Rohrmann, S.; Himmerich, H.; Linseisen, J.; Nimptsch, K. Plasma Inflammation Markers of the Tumor Necrosis Factor Pathway but Not C-Reactive Protein Are Associated with Processed Meat and Unprocessed Red Meat Consumption in Bavarian Adults. J. Nutr. 2016, 147, 78–85. [Google Scholar] [CrossRef]
- Berge, H.M.; Isern, C.B.; Berge, E. Blood pressure and hypertension in athletes: A systematic review. Br. J. Sports Med. 2015, 49, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Jeukendrup, A.E. Validity and reliability of three commercially available breath-by-breath respiratory systems. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 86, 435–441. [Google Scholar] [CrossRef]
- Foss, Ø.; Hallén, J. Validity and Stability of a Computerized Metabolic System with Mixing Chamber. Int. J. Sports Med. 2004, 26, 569–575. [Google Scholar] [CrossRef]
- Nolan, P.B.; Beaven, M.L.; Dalleck, L. Comparison of Intensities and Rest Periods for VO2max Verification Testing Procedures. Int. J. Sports Med. 2014, 35, 1024–1029. [Google Scholar] [CrossRef]
- Poole, D.C.; Jones, A.M. Measurement of the maximum oxygen uptake Vo2max: Vo2peak is no longer acceptable. J. Appl. Physiol. 2017, 122, 997–1002. [Google Scholar] [CrossRef]
- Astorino, T.A.; Edmunds, R.; Clark, A.; King, L.; Gallant, R.M.; Namm, S.; Fischer, A.; Wood, K.A. Increased cardiac output and maximal oxygen uptake in response to 10 sessions of high intensity interval training. J. Sports Med. Phys. Fit. 2016, 58, 164–171. [Google Scholar]
- Cortes, N.; Onate, J.; Morrison, S. Differential effects of fatigue on movement variability. Gait Posture 2014, 39, 888–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billat, V.; Renoux, J.C.; Pinoteau, J.; Petit, B.; Koralsztein, J.P. Reproducibility of running time to exhaustion at VO2max in subelite runners. Med. Sci. Sports Exerc. 1994, 26, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Troutt, J.S.; Rudling, M.; Persson, L.; Ståhle, L.; Angelin, B.; Butterfield, A.M.; Schade, A.E.; Cao, G.; Konrad, R.J. Circulating Human Hepcidin-25 Concentrations Display a Diurnal Rhythm, Increase with Prolonged Fasting, and Are Reduced by Growth Hormone Administration. Clin. Chem. 2012, 58, 1225–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, D.W.; Tjalsma, H.; Trinder, D.; Peeling, P. Effect of Exercise Modality and Intensity on Postexercise Interleukin-6 and Hepcidin Levels. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Nurmekivi, A.; Pihl, E.; Jürimäe, T.; Karu, T.; Lemberg, H. Blood Lactate Recovery and Perceived Readiness to Start a New Run in Middle-Distance Runners during Interval Training. Percept. Mot. Ski. 2001, 93, 397–404. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Marchante, D.; Baz-Valle, E.; Alonso-Molero, I.; Jiménez, S.L.; Muñóz-López, M. Analysis of Wearable and Smartphone-Based Technologies for the Measurement of Barbell Velocity in Different Resistance Training Exercises. Front. Physiol. 2017, 8, 649. [Google Scholar] [CrossRef] [Green Version]
- González-Badillo, J.J.; Sánchez-Medina, L. Movement Velocity as a Measure of Loading Intensity in Resistance Training. Int. J. Sports Med. 2010, 31, 347–352. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Marchante, D.; Muñoz-López, M.; Jiménez, S.L. Validity and reliability of a novel iPhone app for the measurement of barbell velocity and 1RM on the bench-press exercise. J. Sports Sci. 2018, 36, 64–70. [Google Scholar] [CrossRef]
- Macdonald, G.Z.; Button, D.C.; Drinkwater, E.J.; Behm, D.G. Foam Rolling as a Recovery Tool after an Intense Bout of Physical Activity. Med. Sci. Sports Exerc. 2014, 46, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the Visual Analog Scale for Measurement of Acute Pain. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.M.; Onambélé, G.L.; Winwood, K.; Morse, C.I. Muscle Damage following Maximal Eccentric Knee Extensions in Males and Females. PLoS ONE 2016, 11, e0150848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhne, J.L.; Ormsbee, M.J.; McKune, A.J. The effects of a multi-ingredient supplement on markers of muscle damage and inflammation following downhill running in females. J. Int. Soc. Sports Nutr. 2016, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- French, D.N.; Thomas, K.; Garland, S.W.; Barnes, C.A.; Portas, M.D.; Hood, P.E.; Wilkes, G. The Effects of Contrast Bathing and Compression Therapy on Muscular Performance. Med. Sci. Sports Exerc. 2008, 40, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Fuentes, F.; Gallardo-Fuentes, J.; Ramírez-Campillo, R.; Balsalobre-Fernández, C.; Martínez, C.; Caniuqueo, A.; Cañas, R.; Banzer, W.; LoTurco, I.; Nakamura, F.Y.; et al. Intersession and Intrasession Reliability and Validity of the My Jump App for Measuring Different Jump Actions in Trained Male and Female Athletes. J. Strength Cond. Res. 2016, 30, 2049–2056. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent Validation of the OMNI Perceived Exertion Scale for Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Diepeveen, L.E.; Laarakkers, C.M.; Martos, G.; Pawlak, M.E.; Uğuz, F.F.; Verberne, K.E.; Van Swelm, R.P.L.; Klaver, S.; De Haan, A.F.; Pitts, K.R.; et al. Provisional standardization of hepcidin assays: Creating a traceability chain with a primary reference material, candidate reference method and a commutable secondary reference material. Clin. Chem. Lab. Med. 2018, 57, 864–872. [Google Scholar] [CrossRef]
- Laarakkers, C.M.M.; Wiegerinck, E.T.; Klaver, S.; Kolodziejczyk, M.; Gille, H.; Hohlbaum, A.M.; Tjalsma, H.; Swinkels, D.W. Improved Mass Spectrometry Assay For Plasma Hepcidin: Detection and Characterization of a Novel Hepcidin Isoform. PLoS ONE 2013, 8, e75518. [Google Scholar] [CrossRef]
- An, P.; Wu, Q.; Wang, H.; Guan, Y.; Mu, M.; Liao, Y.; Zhou, D.; Song, P.; Wang, C.; Meng, L.; et al. TMPRSS6, but not TF, TFR2 or BMP2 variants are associated with increased risk of iron-deficiency anemia. Hum. Mol. Genet. 2012, 21, 2124–2131. [Google Scholar] [CrossRef] [Green Version]
- Benyamin, B.; Ferreira, M.A.R.; Willemsen, G.; Gordon, S.D.; Middelberg, R.P.S.; McEvoy, B.P.; Hottenga, J.-J.; Henders, A.K.; Campbell, M.J.; Wallace, L.; et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat. Genet. 2009, 41, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Galesloot, T.E.; Geurts-Moespot, A.J.; Heijer, M.D.; Sweep, F.C.G.J.; E Fleming, R.; Kiemeney, L.A.L.M.; Vermeulen, S.H.; Swinkels, D.W. Associations of common variants inHFEandTMPRSS6with iron parameters are independent of serum hepcidin in a general population: A replication study. J. Med Genet. 2013, 50, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, B.; McRae, A.F.; Zhu, G.; Gordon, S.; Henders, A.K.; Palotie, A.; Peltonen, L.; Martin, N.G.; Montgomery, G.W.; Whitfield, J.B.; et al. Variants in TF and HFE Explain ∼40% of Genetic Variation in Serum-Transferrin Levels. Am. J. Hum. Genet. 2009, 84, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Rojo, R.; Baeza-Richer, C.; López-Parra, A.M.; Pérez-Granados, A.M.; Brichs, A.; Bertoncini, S.; Buil, A.; Arroyo-Pardo, E.; Soria, J.M.; Vaquero, M.P. Four variants in transferrin and HFE genes as potential markers of iron deficiency anaemia risk: An association study in menstruating women. Nutr. Metab. 2011, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Chicharro, J.L.; Hoyos, J.; Gallego, F.G.; Villa, J.G.; Bandrés, F.; Celaya, P.; Jiménez, F.; Alonso, J.M.; Córdova, A.; Lucia, A. Mutations in the hereditary haemochromatosis gene HFE in professional endurance athletes. Br. J. Sports Med. 2004, 38, 418–421. [Google Scholar] [CrossRef]
- Ropero, P.; Briceño, O.; Mateo, M.; Polo, M.; Mora, A.; González, F.A.; Villegas, A. Frequency of the C282Y and H63D mutations of the hemochromatosis gene (HFE) in a cohort of 1000 neonates in Madrid (Spain). Ann. Hematol. 2006, 85, 323–326. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hoffman, E.P.; Zambraski, E.; Gordish-Dressman, H.; Kearns, A.; Hubal, M.; Harmon, B.; Devaney, J.M. ACTN3 and MLCK genotype associations with exertional muscle damage. J. Appl. Physiol. 2005, 99, 564–569. [Google Scholar] [CrossRef]
- Coelho, D.B.; Pimenta, E.M.; Rosse, I.C.; Veneroso, C.; Pussieldi, G.D.A.; Becker, L.K.; Oliveira, E.C.; Carvalho, M.R.; Silami-Garcia, E. Alpha-Actinin-3 R577X Polymorphism Influences Muscle Damage and Hormonal Responses After a Soccer Game. J. Strength Cond. Res. 2019, 33, 2655–2664. [Google Scholar] [CrossRef]
- Del Coso, J.; Valero, M.; Lara, B.; Salinero, J.J.; Gallo-Salazar, C.; Areces, F. Myosin Light Chain Kinase (MLCK) Gene Influences Exercise Induced Muscle Damage during a Competitive Marathon. PLoS ONE 2016, 11, e0160053. [Google Scholar] [CrossRef]
- Heled, Y.; Bloom, M.S.; Wu, T.J.; Stephens, Q.; Deuster, P.A. CM-MM and ACE genotypes and physiological prediction of the creatine kinase response to exercise. J. Appl. Physiol. 2007, 103, 504–510. [Google Scholar] [CrossRef]
- Rivera, M.A.; Dionne, F.T.; Wolfarth, B.; Chagnon, M.; Simoneau, J.A.; Pérusse, L.; Boulay, M.R.; Gagnon, J.; Song, T.M.; Keul, J.; et al. Muscle-specific creatine kinase gene polymorphisms in elite endurance athletes and sedentary controls. Med. Sci. Sports Exerc. 1997, 29, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; MacArthur, D.G.; Gulbin, J.P.; Hahn, A.G.; Beggs, A.H.; Easteal, S.; North, K.N. ACTN3 Genotype Is Associated with Human Elite Athletic Performance. Am. J. Hum. Genet. 2003, 73, 627–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Zaken, S.; Meckel, Y.; Nemet, D.; Kassem, E.; Eliakim, A. Increased Prevalence of the IL-6 -174C Genetic Polymorphism in Long Distance Swimmers. J. Hum. Kinet. 2017, 58, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eynon, N.; Ruiz, J.R.; Meckel, Y.; Santiago, C.; Fiuza-Luces, C.; Gomez-Gallego, F.; Oliveira, J.; Lucía, A. Is the −174 C/G polymorphism of theIL6gene associated with elite power performance? A replication study with two different Caucasian cohorts. Exp. Physiol. 2010, 96, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Funghetto, S.S.; Prestes, J.; Silva, A.D.O.; Farias, D.L.; Teixeira, T.G.; Vieira, D.C.L.; Souza, V.C.; De Sousa, N.M.F.; Navalta, J.W.; Melo, G.F.; et al. Interleukin-6-174G/C gene polymorphism affects muscle damage response to acute eccentric resistance exercise in elderly obese women. Exp. Gerontol. 2013, 48, 1255–1259. [Google Scholar] [CrossRef]
- Pereira, D.; Garcia, D.; Narciso, F.; Santos, M.; Dias, J.; Queiroz, B.; Souza, E.; Nóbrega, O.; Pereira, L. Effects of 174 G/C polymorphism in the promoter region of the interleukin-6 gene on plasma IL-6 levels and muscle strength in elderly women. Braz. J. Med Biol. Res. 2011, 44, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.S.; Mateo, E.C.; de Queiroz, B.Z.; Assumpcao, A.M.; Miranda, A.S.; Felicio, D.C.; Rocha, N.P.; da Cruz dos Anjos, D.M.; Pereira, D.A.; Teixeira, A.L.; et al. TNF-α, IL6, and IL10 polymorphisms and the effect of physical exercise on inflammatory parameters and physical performance in elderly women. AGE 2013, 35, 2455–2463. [Google Scholar] [CrossRef] [Green Version]
- Yamin, C.; Duarte, J.A.; Oliveira, J.; Amir, O.; Sagiv, M.; Eynon, N.; Sagiv, M.; Amir, R. IL6 (-174) and TNFA (-308) promoter polymorphisms are associated with systemic creatine kinase response to eccentric exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 104, 579–586. [Google Scholar] [CrossRef]
- Costello, J.T.; Bieuzen, F.; Bleakley, C.M. Where are all the female participants in Sports and Exercise Medicine research? Eur. J. Sport Sci. 2014, 14, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Bruinvels, G.; Burden, R.J.; McGregor, A.J.; Ackerman, K.E.; Dooley, M.; Richards, T.; Pedlar, C. Sport, exercise and the menstrual cycle: Where is the research? Br. J. Sports Med. 2016, 51, 487–488. [Google Scholar] [CrossRef]
- Mujika, I.; Taipale, R.S. Sport Science on Women, Women in Sport Science. Int. J. Sports Physiol. Perform. 2019, 14, 1013–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
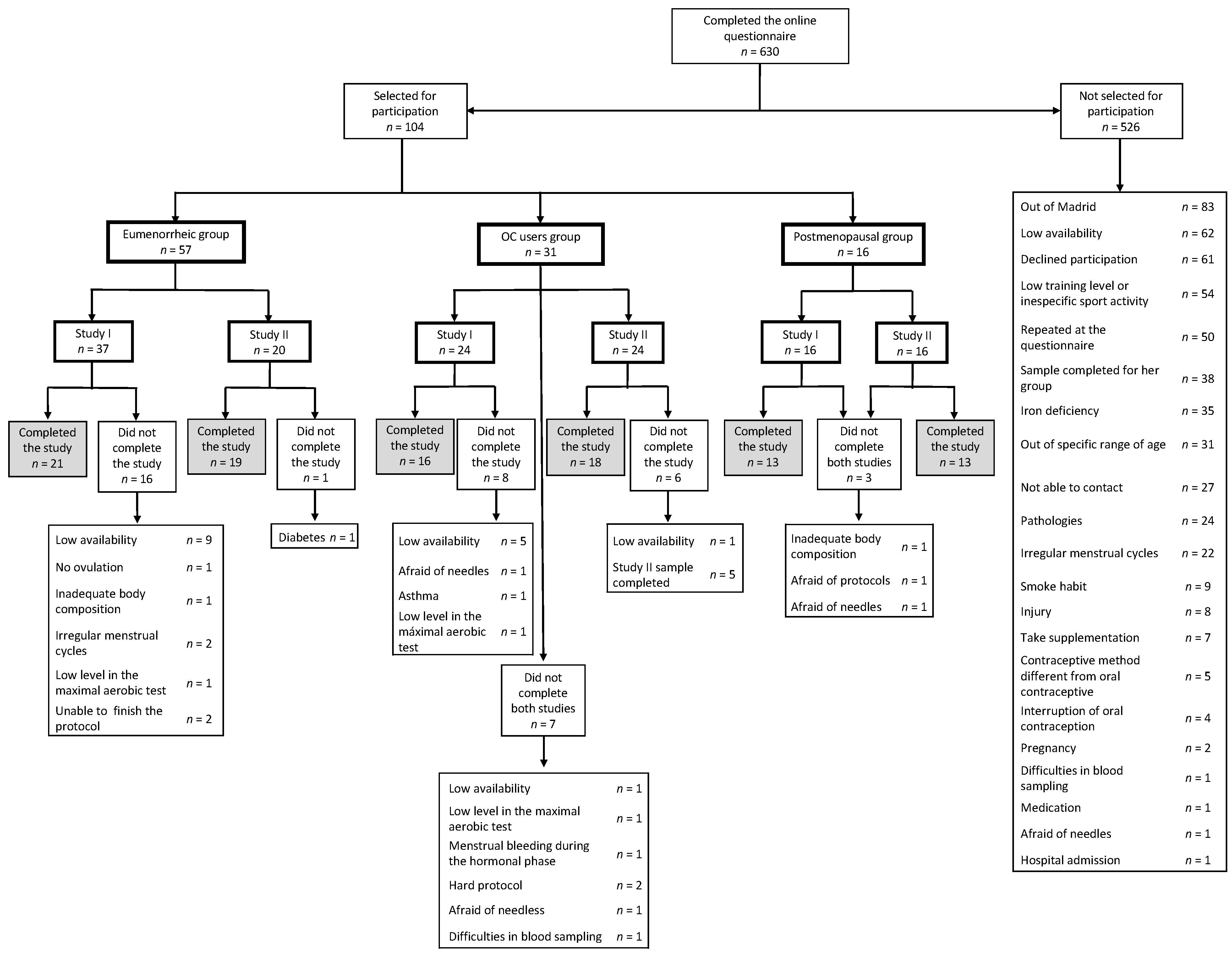

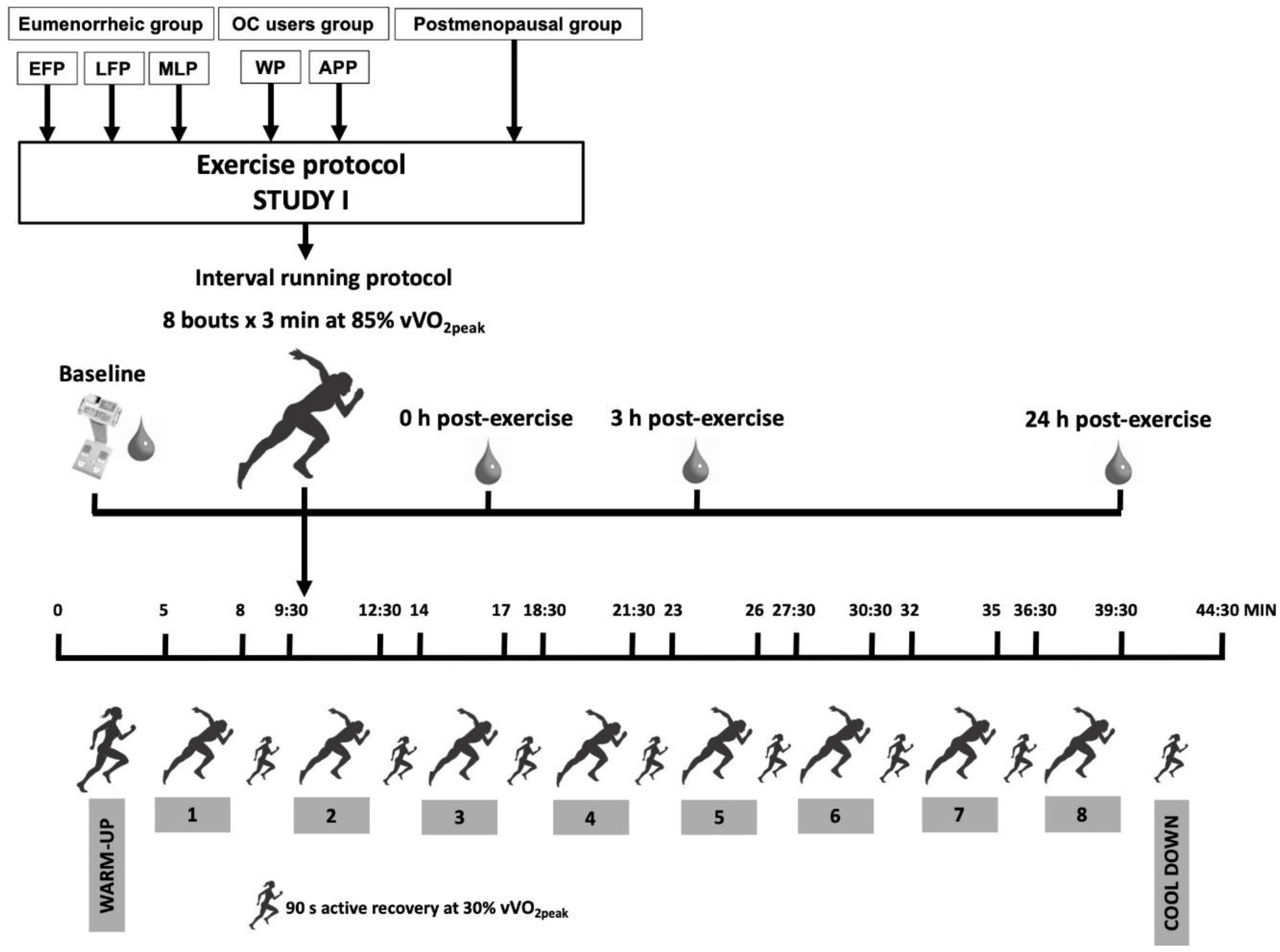
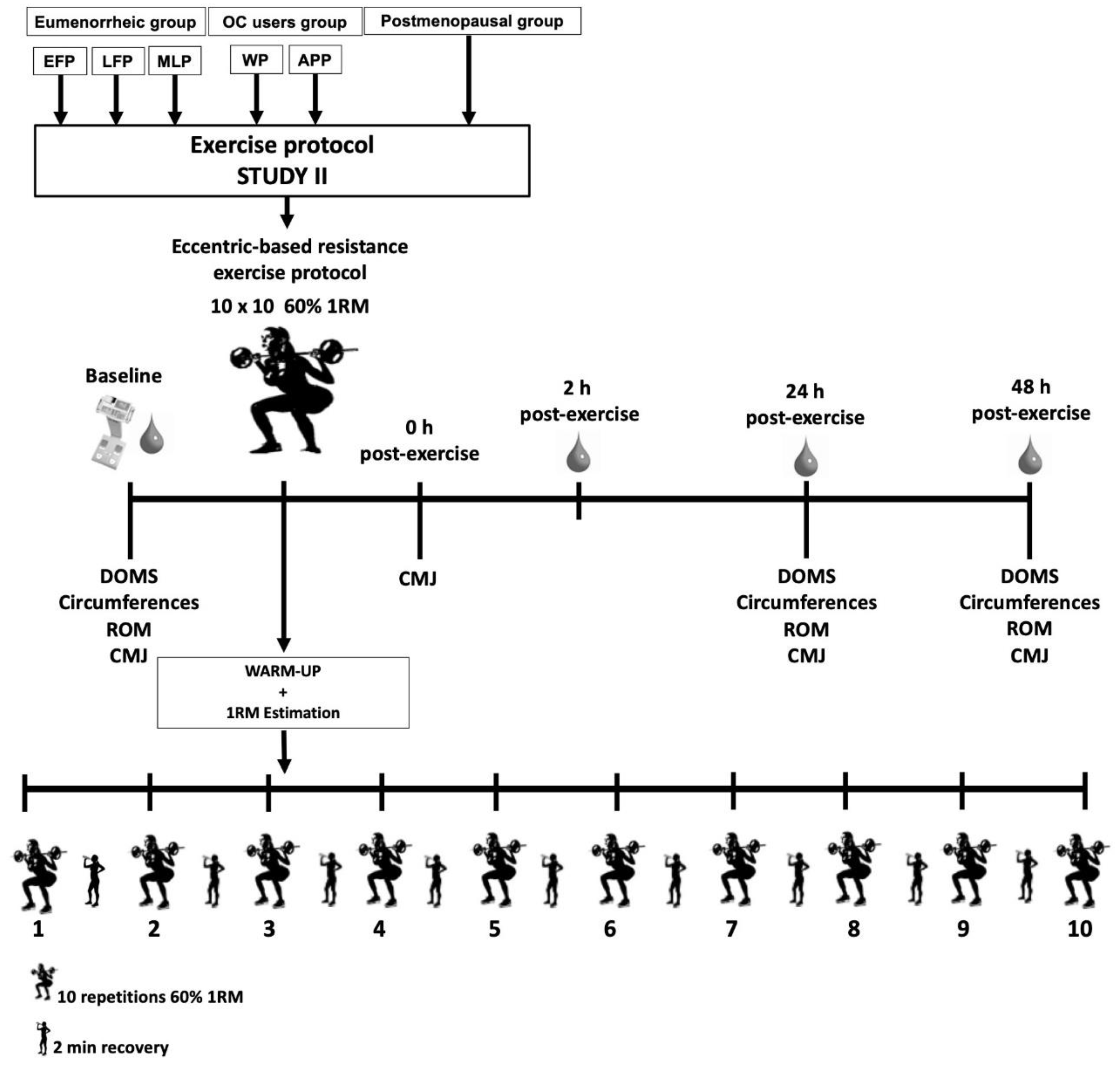
| Study I | Study II | |||||
|---|---|---|---|---|---|---|
| Variables | Eumenorrheic | OC Users | Postmenopausal | Eumenorrheic | OC Users | Postmenopausal |
| n | 37 | 31 | 16 | 20 | 31 | 16 |
| Age (years) | 30.0 ± 6.3 | 25.1 ± 4.3 | 51.4 ± 3.7 | 28.8 ± 6.2 | 25.1 ± 4.3 | 51.4 ± 3.7 |
| Body weight (kg) | 59.8 ± 15.7 | 56.2 ± 10.9 | 56.7 ± 8.3 | 57.5 ± 13.8 | 56.2 ± 10.9 | 56.7 ± 8.3 |
| Height (cm) | 163.7 ± 6.3 | 163.1 ± 5.5 | 161.7 ± 4.9 | 163.9 ± 6.4 | 163.1 ± 5.5 | 161.7 ± 4.9 |
| Training experience * (years) | 7.7 ± 5.1 | 7.3 ± 5.5 | 7.9 ± 3.4 | 6.4 ± 4.1 | 3.1 ± 1.9 | 3.1 ± 1.9 |
| Training volume * (h/week) | 5.5 ± 0.9 | 3.4 ± 1.5 | 4.1 ± 1.2 | 7.5 ± 2.1 | 2.5 ± 1.4 | 1.6 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peinado, A.B.; Alfaro-Magallanes, V.M.; Romero-Parra, N.; Barba-Moreno, L.; Rael, B.; Maestre-Cascales, C.; Rojo-Tirado, M.A.; Castro, E.A.; Benito, P.J.; Ortega-Santos, C.P.; et al. Methodological Approach of the Iron and Muscular Damage: Female Metabolism and Menstrual Cycle during Exercise Project (IronFEMME Study). Int. J. Environ. Res. Public Health 2021, 18, 735. https://doi.org/10.3390/ijerph18020735
Peinado AB, Alfaro-Magallanes VM, Romero-Parra N, Barba-Moreno L, Rael B, Maestre-Cascales C, Rojo-Tirado MA, Castro EA, Benito PJ, Ortega-Santos CP, et al. Methodological Approach of the Iron and Muscular Damage: Female Metabolism and Menstrual Cycle during Exercise Project (IronFEMME Study). International Journal of Environmental Research and Public Health. 2021; 18(2):735. https://doi.org/10.3390/ijerph18020735
Chicago/Turabian StylePeinado, Ana B., Victor M. Alfaro-Magallanes, Nuria Romero-Parra, Laura Barba-Moreno, Beatriz Rael, Cristina Maestre-Cascales, Miguel A. Rojo-Tirado, Eliane A. Castro, Pedro J. Benito, Carmen P. Ortega-Santos, and et al. 2021. "Methodological Approach of the Iron and Muscular Damage: Female Metabolism and Menstrual Cycle during Exercise Project (IronFEMME Study)" International Journal of Environmental Research and Public Health 18, no. 2: 735. https://doi.org/10.3390/ijerph18020735







