Association between Sudden Sensorineural Hearing Loss and Preexisting Thyroid Diseases: A Nationwide Case-Control Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Design
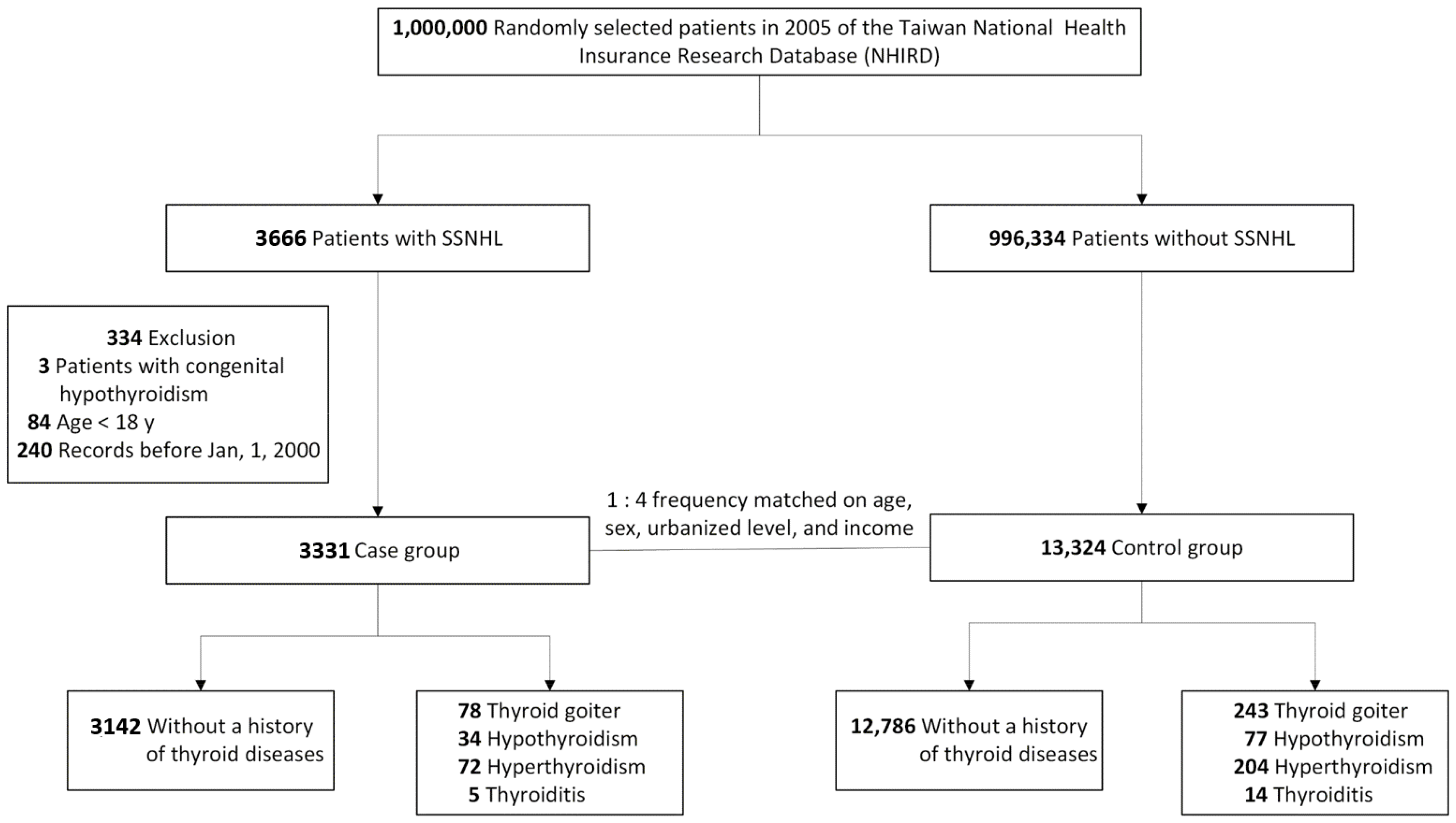
2.2. Patient Selection and Case-Control Matching
2.3. Thyroid Disorders and Other Adjustments
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.-S.; Lin, H.-C.; Chao, P.-Z. Sudden Sensorineural Hearing Loss: Evidence from Taiwan. Audiol. Neurotol. 2006, 11, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.K.; Lin, J.R.J.; Atashband, S.; Irvine, R.A.; Westerberg, B.D. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 2010, 120, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.D.; Chen, P.Y.; Lin, H.C.; Hung, S.H. Sudden sensorineural hearing loss associated with iron-deficiency anemia: A population-based study. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Hsu, H.T.; Lin, Y.S.; Weng, S.F. Increased risk of getting sudden sensorineural hearing loss in patients with chronic kidney disease: A population-based cohort study. Laryngoscope 2013, 123, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, S.-W.; Weng, S.-F.; Lin, Y.-S. Risk of Sudden Sensorineural Hearing Loss in Patients with Systemic Lupus Erythematosus: A Population-Based Cohort Study. Audiol. Neurotol. 2013, 18, 95–100. [Google Scholar] [CrossRef]
- Lin, C.; Lin, S.W.; Weng, S.F.; Lin, Y.S. Increased risk of sudden sensorineural hearing loss in patients with human immunodeficiency virus aged 18 to 35 years: A population-based cohort study. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 251–255. [Google Scholar] [CrossRef]
- Lin, S.W.; Lin, Y.S.; Weng, S.F.; Chou, C.W. Risk of developing sudden sensorineural hearing loss in diabetic patients: A population-based cohort study. Otol. Neurotol. 2012, 33, 1482–1488. [Google Scholar] [CrossRef]
- Yeh, M.-C.; Weng, S.-F.; Shen, Y.-C.; Chou, C.-W.; Yang, C.-Y.; Wang, J.-J.; Tien, K.-J. Increased Risk of Sudden Sensorineural Hearing Loss in Patients with Osteoporosis: A Population-based, Propensity Score-matched, Longitudinal Follow-up Study. J. Clin. Endocrinol. Metab. 2015, 100, 2413–2419. [Google Scholar] [CrossRef]
- Yen, Y.-C.; Lin, C.; Weng, S.-F.; Lin, Y.-S. Higher Risk of Developing Sudden Sensorineural Hearing Loss in Patients with Chronic Otitis Media. JAMA Otolaryngol. Neck Surg. 2015, 141, 429. [Google Scholar] [CrossRef]
- Yen, Y.-C.; Lin, Y.-S.; Weng, S.-F.; Lai, F.-J. Risk of Sudden Sensorineural Hearing Loss in Patients with Psoriasis: A Retrospective Cohort Study. Am. J. Clin. Dermatol. 2015, 16, 213–220. [Google Scholar] [CrossRef]
- Himelfarb, M.Z.; Lakretz, T.; Gold, S.; Shanon, E. Auditory brain stem responses in thyroid dysfunction. J. Laryngol. Otol. 1981, 95, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, W.L. Hypothyroidism and the ear: Electrophysiological, morphological, and chemical considerations. Laryngoscope 1979, 89, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Oiticica, J.; Bittar, R.S.M. Metabolic disorders prevalence in sudden deafness. Clinics 2010, 65, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Chen, Y.-J.; Ho, H.J.; Hsu, Y.-C.; Kuo, K.N.; Wu, M.-S.; Lin, J.-T. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA 2012, 308, 1906–1913. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Chen, W.-C.; Tsan, Y.-T.; Chen, M.-J.; Shih, W.-T.; Tsai, Y.-H.; Chen, P.-C. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J. Hepatol. 2015, 63, 1111–1117. [Google Scholar] [CrossRef]
- Sohmer, H.; Freeman, S. The Importance of Thyroid Hormone for Auditory Development in the Fetus and Neonate. Audiol. Neurotol. 1996, 1, 137–147. [Google Scholar] [CrossRef]
- Lautermann, J.; Cate, W.J.T. Postnatal expression of the alpha-thyroid hormone receptor in the rat cochlea. Hear. Res. 1997, 107, 23–28. [Google Scholar] [CrossRef]
- Cordas, E.A.; Ng, L.; Hernandez, A.; Kaneshige, M.; Cheng, S.-Y.; Forrest, U. Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinol. 2012, 153, 1548–1560. [Google Scholar] [CrossRef]
- Bhatia, P.L.; Gupta, O.P.; Agrawal, M.K.; Mishr, S.K. Audiological and vestibular function tests in hypothyroidism. Laryngoscope 1977, 87, 2082. [Google Scholar] [CrossRef]
- Debruyne, F.; Vanderschueren-Lodeweyckx, M.; Bastijns, P. Hearing in Congenital Hypothyroidism. Int. J. Audiol. 1983, 22, 404–409. [Google Scholar] [CrossRef]
- Wasniewska, M.; De Luca, F.; Siclari, S.; Salzano, G.; Messina, M.F.; Lombardo, F.; Valenzise, M.; Ruggeri, C.; Arrigo, T. Hearing loss in congenital hypothalamic hypothyroidism: A wide therapeutic window. Hear. Res. 2002, 172, 87–91. [Google Scholar] [CrossRef]
- Di Lorenzo, L.; Foggia, L.; Panza, N.; Calabrese, M.R.; Motta, G.; Tranchino, G.; Lombardi, G.; Orio, J. Auditory Brainstem Responses in Thyroid Diseases before and after Therapy. Horm. Res. 1995, 43, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.; Jarvis, S. Auditory brainstem response findings in hypothyroid and hyperthyroid disease. Clin. Neurophysiol. 2008, 119, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Psaltakos, V.; Balatsouras, D.G.; Sengas, I.; Ferekidis, E.; Riga, M.; Korres, S.G. Cochlear dysfunction in patients with acute hypothyroidism. Eur. Arch. Oto-Rhino-Laryngology 2013, 270, 2839–2848. [Google Scholar] [CrossRef]
- Nakashima, T.; Tanabe, T.; Yanagita, N.; Wakai, K.; Ohno, Y. Risk factors for sudden deafness: A case-control study. Auris Nasus Larynx 1997, 24, 265–270. [Google Scholar] [CrossRef]
- Narozny, W.; Kuczkowski, J.; Mikaszewski, B. Thyroid dysfunction--underestimated but important prognostic factor in sudden sensorineural hearing loss. Otolaryngol. Neck Surg. 2006, 135, 995–996. [Google Scholar] [CrossRef]
- Chiarella, G.; Monzani, F.; Petrolo, C.; Fattori, B.; Pasqualetti, G.; Cassandro, E.; Costante, G.; Russo, D. Hashimoto thyroiditis and vestibular dysfunction. Endocr. Pr. 2017, 23, 863–868. [Google Scholar] [CrossRef]
- Hostiuc, M.; Curcă, G.; Dermengiu, D.; Sinescu, C.; Hostiuc, S. Can subclinical hypothyroidism explain some sudden deaths due to pulmonary embolism without evident risk factors? Med. Hypotheses 2011, 76, 855–857. [Google Scholar] [CrossRef]
- Segna, D.; Méan, M.; Limacher, A.; Baumgartner, C.; Blum, M.R.; Beer, J.-H.; Kucher, N.; Righini, M.; Matter, C.M.; Frauchiger, B.; et al. Association between thyroid dysfunction and venous thromboembolism in the elderly: A prospective cohort study. J. Thromb. Haemost. 2016, 14, 685–694. [Google Scholar] [CrossRef]
- Berker, D.; Karabulut, H.; Isik, S.; Tutuncu, Y.; Ozuguz, U.; Erden, G.; Aydin, Y.; Dagli, M.; Guler, S. Evaluation of hearing loss in patients with Graves’ disease. Endocrine 2011, 41, 116–121. [Google Scholar] [CrossRef]
- Stuijver, D.J.; van Zaane, B.; Romualdi, E.; Brandjes, D.P.; Gerdes, V.E.; Squizzato, A. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: A systematic review and meta-analysis. Thromb. Haemost. 2012, 108, 1077–1088. [Google Scholar] [PubMed]
- Squizzato, A.; Romualdi, E.; Buller, H.R.; Gerdes, V.E. Clinical review: Thyroid dysfunction and effects on coagulation and fibrinolysis: A systematic review. J. Clin. Endocrinol. Metab. 2007, 92, 2415–2420. [Google Scholar] [CrossRef]
- Passamonti, S.M.; Di Berardino, F.; Bucciarelli, P.; Berto, V.; Artoni, A.; Gianniello, F.; Ambrosetti, U.; Cesarani, A.; Pappalardo, E.; Martinelli, I. Risk factors for idiopathic sudden sensorineural hearing loss and their association with clinical outcome. Thromb. Res. 2015, 135, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, Z.; Ulu, A.; Incesulu, A.; Ozkaptan, Y.; Akar, N. The Importance of Thrombotic Risk Factors in the Development of Idiopathic Sudden Hearing Loss. Clin. Appl. Thromb. 2008, 14, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, M.; Zhang, Q.; Liu, L.; Song, K.; Tan, J.; Jia, Q.; Zhang, G.; Wang, R.; He, Y.; et al. Gender and Age Impacts on the Association Between Thyroid Function and Metabolic Syndrome in Chinese. Medicine 2015, 94, e2193. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedus, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef]
- Ahad, F.; Ganie, S.A. Iodine, Iodine metabolism and Iodine deficiency disorders revisited. Indian J. Endocrinol. Metab. 2010, 14, 13–17. [Google Scholar]
- Lima, L.P.; Barros, I.A.; Lisboa, P.C.; Araújo, R.L.; Silva, A.C.; Rosenthal, D.; Ferreira, A.C.; Carvalho, D.P. Estrogen effects on thyroid iodide uptake and thyroperoxidase activity in normal and ovariectomized rats. Steroids 2006, 71, 653–659. [Google Scholar] [CrossRef]
- Umesawa, M.; Kobashi, G.; Kitoh, R.; Nishio, S.-Y.; Ogawa, K.; Hato, N.; Sone, M.; Fukuda, S.; Hara, A.; Ikezono, T.; et al. Relationships among drinking and smoking habits, history of diseases, body mass index and idiopathic sudden sensorineural hearing loss in Japanese patients. Acta Oto-Laryngologica 2017, 137, S17–S23. [Google Scholar] [CrossRef]
| Variables | SSNHL (N = 3331) | Non-SSNHL (N = 13,324) | p-Value a | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | 1.000 | ||||
| Men | 1786 | 53.6 | 7144 | 53.6 | |
| Women | 1545 | 46.4 | 6180 | 46.4 | |
| Age (years) | 1.000 | ||||
| 18–49 | 1294 | 38.9 | 5176 | 38.9 | |
| ≥50 | 2037 | 61.2 | 8148 | 61.2 | |
| Monthly income (NTD) 0 | 856 | 25.7 | 3424 | 25.7 | 1.000 |
| 1–15840 | 577 | 17.3 | 2308 | 17.3 | |
| 15841–25000 | 988 | 29.7 | 3952 | 29.7 | |
| ≥25001 | 910 | 27.3 | 3640 | 27.3 | |
| Urbanization level | 1.000 | ||||
| 1(City) | 920 | 27.6 | 3680 | 27.6 | |
| 2 | 1595 | 47.9 | 6380 | 47.9 | |
| 3 | 573 | 17.2 | 2292 | 17.2 | |
| 4 (Village) | 243 | 7.3 | 972 | 7.3 | |
| Thyroid diseases | 0.001 | ||||
| Thyroid goiter | 78 | 2.3 | 243 | 1.8 | |
| Hypothyroidism | 34 | 1.0 | 77 | 0.6 | |
| Hyperthyroidism | 72 | 2.2 | 204 | 1.5 | |
| Thyroiditis | 5 | 0.2 | 14 | 0.1 | |
| None | 3142 | 94.3 | 12786 | 96.0 | |
| Age < 50 y/o | 0.074 | ||||
| Thyroid goiter | 19 | 1.5 | 59 | 1.1 | |
| Hypothyroidism | 7 | 0.5 | 19 | 0.4 | |
| Hyperthyroidism | 26 | 2.0 | 67 | 1.3 | |
| Thyroiditis | 3 | 0.2 | 4 | 0.1 | |
| None | 1239 | 95.8 | 5027 | 97.1 | |
| Age ≥ 50 y/o | 0.008 | ||||
| Thyroid goiter | 59 | 2.9 | 184 | 2.3 | |
| Hypothyroidism | 27 | 1.3 | 58 | 0.7 | |
| Hyperthyroidism | 46 | 2.3 | 137 | 1.7 | |
| Thyroiditis | 2 | 0.1 | 10 | 0.1 | |
| None | 1903 | 93.4 | 7759 | 95.2 | |
| Female | 0.003 | ||||
| Thyroid goiter | 58 | 3.8 | 192 | 3.1 | |
| Hypothyroidism | 25 | 1.6 | 58 | 0.9 | |
| Hyperthyroidism | 60 | 3.9 | 162 | 2.6 | |
| Thyroiditis | 1 | 0.1 | 14 | 0.2 | |
| None | 1401 | 90.7 | 5754 | 93.1 | |
| Male | 0.001 | ||||
| Thyroid goiter | 20 | 1.1 | 51 | 0.7 | |
| Hypothyroidism | 9 | 0.5 | 19 | 0.3 | |
| Hyperthyroidism | 12 | 0.7 | 42 | 0.6 | |
| Thyroiditis | 4 | 0.2 | 0 | 0 | |
| None | 1741 | 97.5 | 7032 | 98.4 | |
| Medication for thyroid dysfunction | 135 | 4.1 | 427 | 3.2 | 0.015 |
| Thyroidectomy | 12 | 0.4 | 51 | 0.4 | 0.850 |
| Covariates | |||||
| History of DM | 650 | 19.5 | 1713 | 12.9 | <0.001 |
| History of COM | 62 | 1.9 | 98 | 0.7 | <0.001 |
| History of HTN | 1175 | 35.3 | 3879 | 29.1 | <0.001 |
| History of hyerlipidemia | 786 | 23.6 | 2340 | 17.6 | <0.001 |
| Variables | Adjusted OR a (95% CI) | p-Value |
|---|---|---|
| Overall | ||
| Without thyroid disorders | 1 [Reference] | NA |
| Thyroid goiter | 1.24 (0.96−1.61) | 0.106 |
| Hypothyroidism | 1.54 (1.02−2.32) | 0.042 |
| Hyperthyroidism | 1.41 (1.07−1.85) | 0.015 |
| Thyroiditis | 1.35 (0.48−3.78) | 0.569 |
| Age < 50 y/o | ||
| Without thyroid disorders | 1 [Reference] | NA |
| Thyroid goiter | 1.22 (0.72−2.07) | 0.462 |
| Hypothyroidism | 1.40 (0.57−3.39) | 0.462 |
| Hyperthyroidism | 1.41 (0.88−2.26) | 0.151 |
| Thyroiditis | 3.06 (0.68−13.74) | 0.145 |
| Age ≥ 50 y/o | ||
| Without thyroid disorders | 1 [Reference] | NA |
| Thyroid goiter | 1.25 (0.92−1.68) | 0.153 |
| Hypothyroidism | 1.61 (1.01−2.57) | 0.045 |
| Hyperthyroidism | 1.36 (0.97−1.92) | 0.075 |
| Thyroiditis | 0.73 (0.16−3.38) | 0.690 |
| Women | ||
| Without thyroid disorders | 1 [Reference] | NA |
| Thyroid goiter | 1.17 (0.86−1.58) | 0.320 |
| Hypothyroidism | 1.46 (0.90−2.37) | 0.125 |
| Hyperthyroidism | 1.48 (1.09−2.01) | 0.012 |
| Thyroiditis | 0.28 (0.04−2.12) | 0.217 |
| Men | ||
| Without thyroid disorders | 1 [Reference] | NA |
| Thyroid goiter | 1.54 (0.91−2.61) | 0.106 |
| Hypothyroidism | 1.73 (0.77−3.85) | 0.183 |
| Hyperthyroidism | 1.14 (0.60−2.18) | 0.688 |
| Thyroiditis | NA | NA |
| DM | 1.47 (1.31−1.64) | <0.001 |
| COM | 2.50 (1.81−3.45) | <0.001 |
| HTN | 1.21 (1.09−1.33) | <0.001 |
| Hyerlipidemia | 1.21 (1.09−1.35) | <0.001 |
| Variable | Adjusted OR a (95% CI) | p-Value |
|---|---|---|
| Participants with hypothyroidism | ||
| Without TD medications | 1 [Reference] | NA |
| With TD medications b | 0.61 (0.21–1.76) | 0.358 |
| Participants with hyperthyroidism | ||
| Without TD medications and thyroidectomy | 1 [Reference] | NA |
| With TD medications c or thyroidectomy d | 0.77 (0.43–1.38) | 0.379 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-T.; Chang, I.-J.; Hsu, C.-M.; Yang, Y.-H.; Liu, C.-Y.; Tsai, M.-S.; Chang, G.-H.; Lee, Y.-C.; Huang, E.I.; Lin, M.-H.; et al. Association between Sudden Sensorineural Hearing Loss and Preexisting Thyroid Diseases: A Nationwide Case-Control Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 834. https://doi.org/10.3390/ijerph17030834
Tsai Y-T, Chang I-J, Hsu C-M, Yang Y-H, Liu C-Y, Tsai M-S, Chang G-H, Lee Y-C, Huang EI, Lin M-H, et al. Association between Sudden Sensorineural Hearing Loss and Preexisting Thyroid Diseases: A Nationwide Case-Control Study in Taiwan. International Journal of Environmental Research and Public Health. 2020; 17(3):834. https://doi.org/10.3390/ijerph17030834
Chicago/Turabian StyleTsai, Yao-Te, I-Jen Chang, Cheng-Ming Hsu, Yao-Hsu Yang, Chia-Yen Liu, Ming-Shao Tsai, Geng-He Chang, Yi-Chan Lee, Ethan I. Huang, Meng-Hung Lin, and et al. 2020. "Association between Sudden Sensorineural Hearing Loss and Preexisting Thyroid Diseases: A Nationwide Case-Control Study in Taiwan" International Journal of Environmental Research and Public Health 17, no. 3: 834. https://doi.org/10.3390/ijerph17030834
APA StyleTsai, Y.-T., Chang, I.-J., Hsu, C.-M., Yang, Y.-H., Liu, C.-Y., Tsai, M.-S., Chang, G.-H., Lee, Y.-C., Huang, E. I., Lin, M.-H., & Luan, C.-W. (2020). Association between Sudden Sensorineural Hearing Loss and Preexisting Thyroid Diseases: A Nationwide Case-Control Study in Taiwan. International Journal of Environmental Research and Public Health, 17(3), 834. https://doi.org/10.3390/ijerph17030834







