The Epidemiology of Transition into Adulthood of Rare Diseases Patients: Results from a Population-Based Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. RD Framework
2.2. The RD Care Network
2.3. The RD Registry
2.4. Setting and Subjects
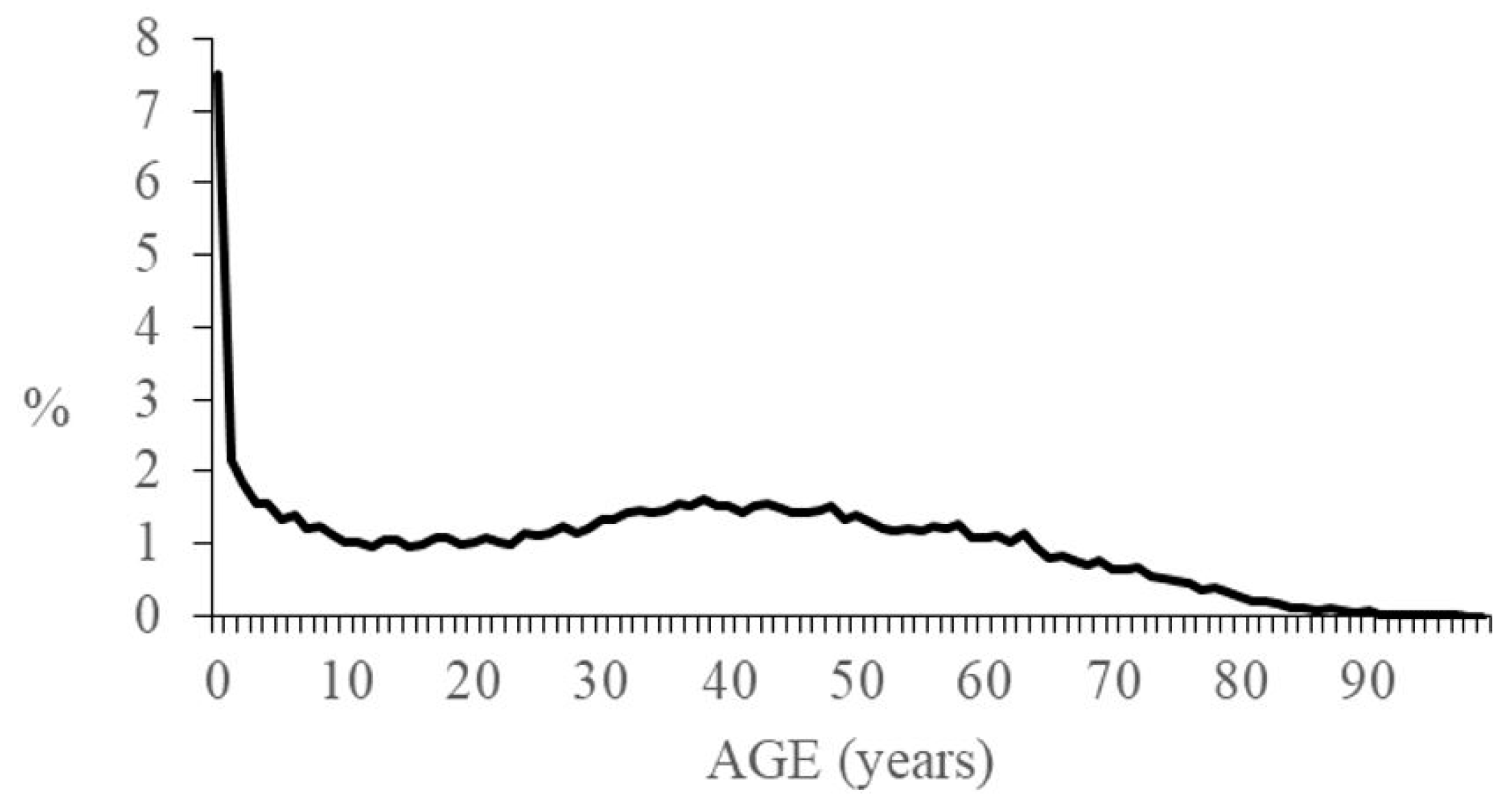
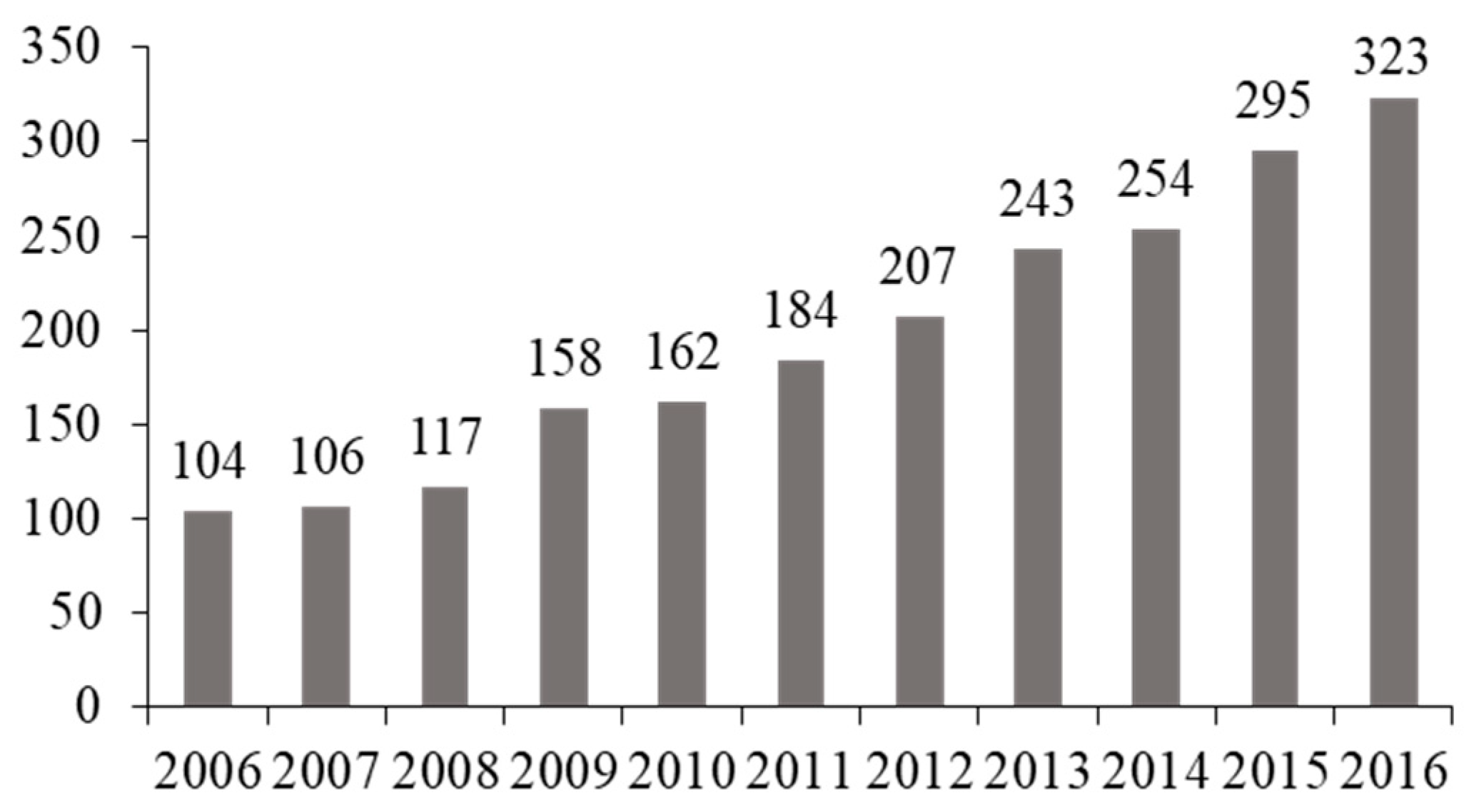
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Organisation for Rare Diseases. Rare Diseases: Understanding This Public Health Priority. Eurordis: Paris, France, 2005. Available online: http://www.eurordis.org/IMG/pdf/princeps_document-EN.pdf (accessed on 20 May 2018).
- Fredericks, E.M.; Magee, J.C.; Opipari-Arrigan, L.; Shieck, V.; Well, A.; Lopez, M.J. Adherence and health-related quality of life in adolescent liver transplant recipients. Pediatr. Transplant. 2008, 12, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.C.; Marciel, K.K.; Slater, S.K.; Drotar, D.; Quittner, A.L. The influence of parental supervision on medical adherence in adolescents with cystic fibrosis: developmental shifts from pre to late adolescence. Child. Health Care 2008, 37, 78–92. [Google Scholar] [CrossRef]
- Reed-Knight, B.; Lewis, J.D.; Blount, R.L. Association of disease, adolescent, and family factors with medication adherence in pediatric inflammatory bowel disease. J. Pediatr. Psychol. 2011, 36, 308–3171. [Google Scholar] [CrossRef] [PubMed]
- Bryden, K.S.; Peveler, R.C.; Stein, A.; Neil, A.; Mayou, R.A.; Dunger, D.B. Clinical and psychological course of diabetes from adolescence to young adulthood: A longitudinal cohort study. Diabetes Care 2001, 24, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, E.M.; Dore-Stites, D.; Well, A.; Magee, J.C.; Freed, G.L.; Shieck, V.; James, L.M. Assessment of transition readiness skills and adherence in pediatric liver transplant recipients. Pediatr. Transplant. 2010, 14, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.; Kay, J.; Roosevelt, G.E.; Brandon, M.; Yetman, A.T. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int. J. Cardiol. 2008, 125, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.E.; Bartosh, S.M.; Davis, C.L.; Dobbels, F.; Al-Uzri, A.; Lotstein, D.; Reiss, J.; Dharnidharka, V.R. Adolescent transition to adult care in solid organ transplantation: A consensus conference report. Am. J. Transplant. 2008, 8, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, R.A.; Emre, S.; Shneider, B.; Barton, C.; Dugan, C.A.; Shemesh, E. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatr. Transplant. 2007, 11, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.W.; Garell, D.; Hodgman, C.H.; Jorissen, T.W.; Okinow, N.A.; Orr, D.P.; Slap, G.B. Transition from child-centered to adult health-care systems for adolescents with chronic conditions. A position paper of the Society for Adolescent Medicine. J. Adolesc. Health 1993, 14, 570–576. [Google Scholar] [CrossRef]
- Van den Akker, A.L.; Deković, M.; Prinzie, P. Transitioning to adolescence: how changes in child personality and overreactive parenting predict adolescent adjustment problems. Dev. Psychopathol. 2010, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Yurgelun-Todd, D. Emotional and cognitive changes during adolescence. Curr. Opin. Neurobiol. 2007, 17, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.S.; Behrmann, M.; Dahl, R.E. Facing changes and changing faces in adolescence: A new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev. Cogn. Neurosci. 2012, 2, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.K.; Becker, D.J.; Helgeson, V.S. Transfer from pediatric to adult health care: Effects on diabetes outcomes. Pediatr. Diabetes 2014, 15, 10–17. [Google Scholar] [CrossRef]
- Wijlaars, L.P.M.M.; Hardelid, P.; Guttmann, A.; Gilbert, R. Emergency admissions and long-term conditions during transition from paediatric to adult care: A cross-sectional study using Hospital Episode Statistics data. BMJ Open 2018, 8, e021015. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.R.; Kuhlthau, K.; Van Cleave, J.; Knapp, A.A.; Newacheck, P.; Perrin, J.M. Health care transition for youth with special health care needs. J. Adolesc. Health 2012, 51, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Crone, M.R.; van Spronsen, F.J.; Oudshoorn, K.; Bekhof, J.; van Rijn, G.; Verkerk, P.H. Behavioural factors related to metabolic control in patients with phenylketonuria. J. Inherit. Metab. Dis. 2005, 28, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.M.; Mendez, E.; Throop, C.; Ogata, E.S. Adult survivors of pediatric illness: The impact on pediatric hospitals. Pediatrics 2002, 110, 583–589. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians Transitioning Clinical Report Authoring Group. Clinical Report—Supporting the Health Care Transition from Adolescence to Adulthood in the Medical Home. Pediatrics 2011. [Google Scholar] [CrossRef]
- EURORDIS. Recommendations for the Development of National Plans for Rare Disease. Guidance Document. Available online: https://download2.eurordis.org/europlan/2_EUROPLAN_Guidance_Documents_for_the_National_Conference/2_EUROPLAN_Recommendations_for_Rare_Disease_National_Plans_Final.pdf (accessed on 15 June 2018).
- Ministero della Salute. Piano Nazionale Malattie Rare 2013–2016. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2153_allegato.pdf (accessed on 15 June 2018).
- Ministry of Health and Social Policy. Rare Diseases Strategy of the Spanish National Health System Strategy. Available online: http://www.msc.es/organizacion/sns/planCalidadSNS/docs/RareDiseases.pdf (accessed on 15 June 2018).
- Minister of Health and Social Protection. Minister of Research. Plan National Maladies Rares 2011–2014. Available online: http://solidarites-sante.gouv.fr/IMG/pdf/Plan_national_maladies_rares.pdf (accessed on 15 June 2018).
- The UK Strategy for Rare Diseases. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/260562/UK_Strategy_for_Rare_Diseases.pdf (accessed on 15 June 2018).
- Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the Application of Patients’ Rights in Cross-Border Healthcare. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:088:0045:0065:en:PDF (accessed on 15 June 2018).
- 2014/286/EU. Commission Delegated Decision of 10 March 2014 Setting out Criteria and Conditions that European Reference Networks and Healthcare Providers Wishing to Join a European Reference Network Must Fulfil. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014D0286&from=EN (accessed on 15 June 2018).
- 2014/287/EU. Commission Implementing Decision of 10 March 2014 Setting out Criteria for Establishing and Evaluating European Reference Networks and Their Members and for Facilitating the Exchange of Information and Expertise on Establishing and Evaluating Such Networks. 2014. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014D0287&from=EN (accessed on 15 June 2018).
- Prior, M.; McManus, M.; White, P.; Davidson, L. Measuring the “triple aim” in transition care: a systematic review. Pediatrics 2014, 134, e1648–e1661. [Google Scholar] [CrossRef] [PubMed]
- Mazzucato, M.; Visonà Dalla Pozza, L.; Manea, S.; Minichiello, C.; Facchin, P. A population-based registry as a source of health indicators for rare diseases: the ten-year experience of the Veneto Region’s rare diseases registry. Orphanet J. Rare Dis. 2014, 9, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.E.; Mahede, T.; Davis, G.; Miller, L.J.; Girschik, J.; Brameld, K.; Sun, W.; Rath, A.; Aymé, S.; Zubrick, S.R.; et al. The collective impact of rare diseases in Western Australia: An estimate using a population-based cohort. Genet. Med. 2017, 19, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Decreto 18 Maggio 2001, n. 279. Regolamento di Istituzione della Rete Nazionale delle Malattie Rare e di Esenzione Dalla Partecipazione al Costo delle Relative Prestazioni Sanitarie, ai sensi dell’Articolo 5, Comma 1, Lettera b), del Decreto Legislativo 29 Aprile 1998, n. 124. (Gazzetta Ufficiale n. 160 of 12/07/2001 Suppl. Ordinario n.180/L). Available online: http://www.salute.gov.it/imgs/C_17_normativa_194_allegato.pdf (accessed on 15 June 2018).
- Ministero della Salute. Decreto del Presidente del Consiglio dei Ministri 12 Gennaio 2017. Definizione e Aggiornamento dei Livelli Essenziali di Assistenza, di cui al’Articolo 1, Comma 7, del Decreto Legislativo 30 Dicembre 1992, n. 502. (17A02015). G.U. Serie Generale, n. 65 del 18 Marzo 2017. Available online: http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=58669 (accessed on 16 June 2018).
- Orphanet. Inventory of Rare Diseases with Annotations. Available online: http://www.orphadata.org/cgi-bin/index.php/ (accessed on 16 June 2018).
- European Commission. European Reference Networks. Available online: https://ec.europa.eu/health/ern_en (accessed on 16 June 2018).
- McPherson, M.; Arango, P.; Fox, H.; Lauver, C.; McManus, M.; Newacheck, P.W.; Perrin, J.M.; Shonkoff, J.P.; Strickland, B. A new definition of children with special healthcare needs. Pediatrics 1998, 102, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Perrin, J.M.; Bloom, S.R.; Gortmaker, S.L. The increase of childhood chronic conditions in the United States. JAMA 2007, 297, 2755–2759. [Google Scholar] [CrossRef] [PubMed]
- Lotstein, D.S.; McPherson, M.; Strickland, B.; Newacheck, P.W. Transition planning for youth with special health care needs: results from the National Survey of Children with Special Health Care Needs. Pediatrics 2005, 115, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- European Parliament: Decision No 1295/1999/EC of the European Parliament and of the Council of 29 April 1999 Adopting a Programme of Community Action on Rare Diseases within the Framework for Action in the Field of Public Health (1999 to 2003). Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1395750802170&uri=CELEX:31999D1295 (accessed on 16 June 2018).
- Bowes, G.; Sinnema, G.; Suris, J.-C.; Buhlmann, U. Transition health services for youth with disabilities: A global perspective. J. Adolesc. Health 1995, 17, 23–31. [Google Scholar] [CrossRef]
- Viner, R.M. National survey of use of hospital beds by adolescents aged 12 to 19 in the United Kingdom. BMJ 2001, 322, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Tennant, P.W.; Pearce, M.S.; Bythell, M.; Rankin, J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet 2010, 375, 649–656. [Google Scholar] [CrossRef]
- Dastgiri, S.; Gilmour, W.H.; Stone, D.H. Survival of children born with congenital anomalies. Arch. Dis. Child. 2003, 88, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Darlison, M.; Birgens, H.; Cario, H.; Faustino, P.; Giordano, P.C.; Gulbis, B.; Hopmeier, P.; Lena-Russo, D.; Romao, L.; et al. Epidemiology of haemoglobin disorders in Europe: An overview. Scand. J. Clin. Lab. Investig. 2007, 67, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Dalla Pozza, L.V.; Mazzucato, M.; Sainati, L.; Pierobon, M.; Facchin, P. Hospitalization of children with sickle cell disease in a region with increasing immigration rates. Haematologica 2008, 93, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.L. Newborn screening conditions: What we know, what we do not know, and how we will know it. Genet. Med. 2010, 12, S213–S214. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, S.; Ronis, T.; White, P.H. Pediatric rheumatology: Addressing the transition to adult-orientated health care. Open Access Rheumatol. 2018, 10, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Van der Bent, A.; Duggan, E.M.; Fishman, L.N.; Dickie, B.H. Reality check: What happens when patients with anorectal malformations grow up? A pilot study of medical care transition from the adult patient perspective. J. Pediatr. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chabrol, B.; Jacquin, P.; Francois, L.; Broué, P.; Dobbelaere, D.; Douillard, C.; Dubois, S.; Feillet, F.; Perrier, A.; Fouilhoux, A.; et al. Transition from pediatric to adult care in adolescents with hereditary metabolic diseases: Specific guidelines from the French network for rare inherited metabolic diseases (G2M). Arch. Pediatr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, T.S.; van Berkel, P.; Hammerschmidt, G.; Lhotáková, M.; Saludes, R.P. Requirements for a minimum standard of care for phenylketonuria: The patients’ perspective. Orphanet J. Rare Dis. 2013, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Grano, C.; Aminoff, D.; Schwarzer, N.; Van De Vorle, M.; Cretolle, C.; Haanen, M.; Brisighelli, G.; Marzheuser, S.; Connor, M. ARM-net Consortium. Transition of care in patients with anorectal malformations: Consensus by the ARM-net consortium. J. Pediatr. Surg. 2017, 52, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Hokken-Koelega, A.; van der Lely, A.J.; Hauffa, B.; Häusler, G.; Johannsson, G.; Maghnie, M.; Argente, J.; DeSchepper, J.; Gleeson, H.; Gregory, J.W.; et al. Bridging the gap: Metabolic and endocrine care of patients during transition. Endocr. Connect. 2016, 5, R44–R54. [Google Scholar] [CrossRef] [PubMed]
- Mütze, U.; Roth, A.; Weigel, F.; Beblo, S.; Baerwald, C.G.; Bührdel, P.; Kiess, W. Transition of young adults with phenylketonuria from pediatric to adult care. J. Inherit. Metab. Dis. 2011, 34, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, L.; Schwartz, M. Health outcomes associated with transition from pediatric to adult cystic fibrosis care. Pediatrics 2013, 132, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; O’Hare, K.; Antonelli, R.C.; Sawicki, G.S. Transition care: future directions in education, health policy, and outcomes research. Acad. Pediatr. 2014, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Gibson, R. Health care transition: destinations unknown. Pediatrics 2002, 110, 1307–1314. [Google Scholar] [PubMed]
- Mazur, A.; Dembinski, L.; Schrier, L.; Hadjipanayis, A.; Michaud, P.A. European Academy of Paediatric consensus statement on successful transition from paediatric to adult care for adolescents with chronic conditions. Acta Paediatr. 2017, 106, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
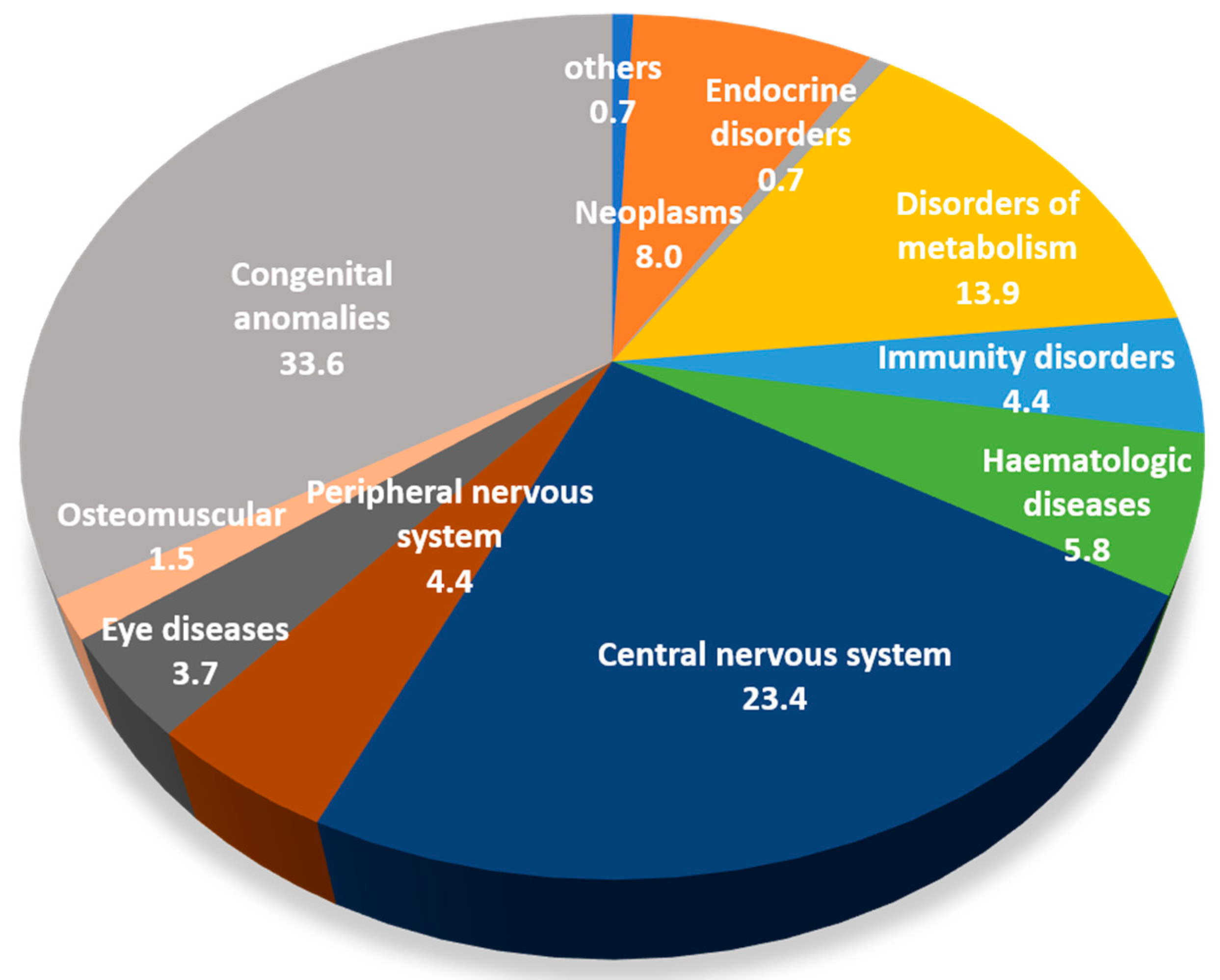
| GROUPS OF DISEASES (ICD9-CM) | |
|---|---|
| Infectious and parasitic diseases | 0.0 |
| Neoplasms | 7.9 |
| Endocrine disorders | 3.6 |
| Disorders of metabolism | 7.7 |
| Immunity disorders | 4.1 |
| Diseases of the blood and blood-forming organs | 15.9 |
| Central nervous system disorders | 2.6 |
| Peripheral nervous system disorders | 4.5 |
| Disorders of the eye and adnexa | 12.1 |
| Diseases of the circulatory system | 3.9 |
| Diseases of the digestive system | 0.7 |
| Diseases of the genitourinary system | 0.1 |
| Diseases of the skin and subcutaneous tissue | 0.7 |
| Diseases of the musculoskeletal system and connective tissue | 4.0 |
| Congenital anomalies | 32.0 |
| Certain conditions originating in the perinatal period | 0.3 |
| Patients | Patients | |
|---|---|---|
| GROUPS OF DISEASES (ICD9-CM) | (0–17 Years) | (=>18 Years) |
| Infectious and parasitic diseases | 0.0 | 0.4 |
| Neoplasms | 6.6 | 2.8 |
| Endocrine disorders | 2.4 | 2.0 |
| Disorders of metabolism | 7.8 | 9.1 |
| Immunity disorders | 3.4 | 4.8 |
| Diseases of the blood and blood-forming organs | 17.2 | 11.7 |
| Central nervous system disorders | 3.0 | 10.3 |
| Peripheral nervous system disorders | 3.1 | 6.9 |
| Disorders of the eye and adnexa | 6.2 | 19.5 |
| Diseases of the circulatory system | 3.7 | 4.1 |
| Diseases of the digestive system | 0.4 | 2.7 |
| Diseases of the genitourinary system | 0.1 | 1.2 |
| Diseases of the skin and subcutaneous tissue | 0.7 | 5.5 |
| Diseases of the musculoskeletal system and connective tissue | 2.2 | 10.1 |
| Congenital anomalies | 42.9 | 8.7 |
| Certain conditions originating in the perinatal period | 0.2 | 0.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzucato, M.; Visonà Dalla Pozza, L.; Minichiello, C.; Manea, S.; Barbieri, S.; Toto, E.; Vianello, A.; Facchin, P. The Epidemiology of Transition into Adulthood of Rare Diseases Patients: Results from a Population-Based Registry. Int. J. Environ. Res. Public Health 2018, 15, 2212. https://doi.org/10.3390/ijerph15102212
Mazzucato M, Visonà Dalla Pozza L, Minichiello C, Manea S, Barbieri S, Toto E, Vianello A, Facchin P. The Epidemiology of Transition into Adulthood of Rare Diseases Patients: Results from a Population-Based Registry. International Journal of Environmental Research and Public Health. 2018; 15(10):2212. https://doi.org/10.3390/ijerph15102212
Chicago/Turabian StyleMazzucato, Monica, Laura Visonà Dalla Pozza, Cinzia Minichiello, Silvia Manea, Sara Barbieri, Ema Toto, Andrea Vianello, and Paola Facchin. 2018. "The Epidemiology of Transition into Adulthood of Rare Diseases Patients: Results from a Population-Based Registry" International Journal of Environmental Research and Public Health 15, no. 10: 2212. https://doi.org/10.3390/ijerph15102212
APA StyleMazzucato, M., Visonà Dalla Pozza, L., Minichiello, C., Manea, S., Barbieri, S., Toto, E., Vianello, A., & Facchin, P. (2018). The Epidemiology of Transition into Adulthood of Rare Diseases Patients: Results from a Population-Based Registry. International Journal of Environmental Research and Public Health, 15(10), 2212. https://doi.org/10.3390/ijerph15102212





