Regulation of Sphingolipid Metabolism by MicroRNAs: A Potential Approach to Alleviate Atherosclerosis
Abstract
1. Introduction
2. Regulation of Lipid and Lipoprotein Metabolism by miRNAs
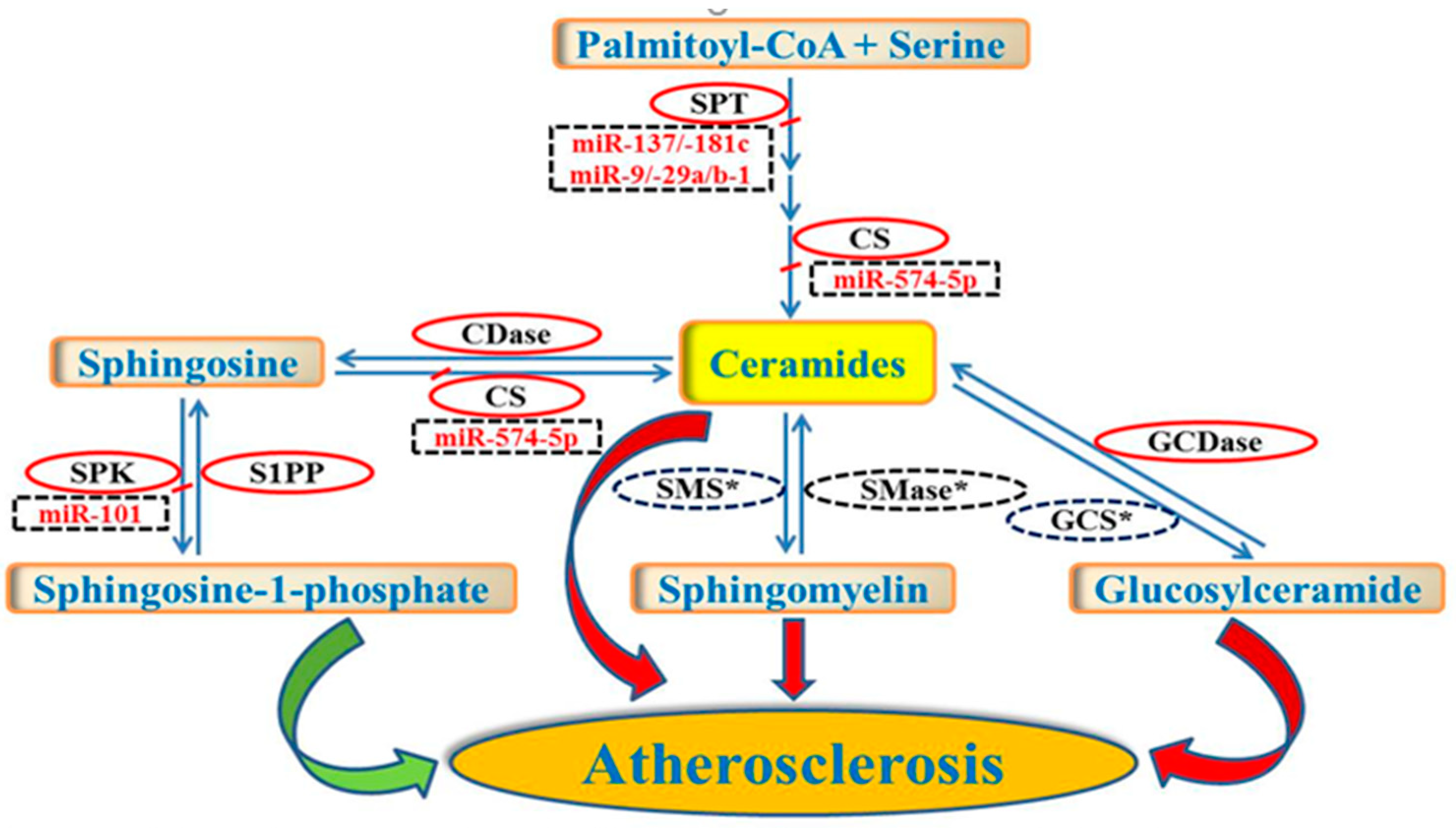
3. Regulation of Sphingolipid Metabolism by miRNAs
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| ACACA | acetyl-CoA carboxylase alpha |
| ACC2 | acetyl-coA carboxylase 2 |
| ACLY | ATP citrate lyase |
| ApoA1 | apolipoprotein A1 |
| CerS | ceramide synthase |
| CPT1A | carnitine palmitoyltransferase 1A |
| CROT | carnitine O-octanoyltransferase |
| CVD | cardiovascular disease |
| ELOVL5 | ELOVL fatty acid elongase 5 |
| FASN | fatty acid synthase |
| HADHB | hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit beta |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| LPGAT1 | lysophosphatidylglycerol acyltransferase 1 |
| LXRα | liver X receptor alpha |
| MBOAT1 | membrane bound O-acyltransferase domain containing 1 |
| miRNAs | microRNAs |
| MTTP | microsomal triglyceride transfer protein |
| SCD1 | steroyl-coA desaturase 1 |
| SM | sphingomyelin |
| SMS | sphingomyelin synthase |
| S1P | sphingosine-1-phosphate |
| SPK | sphingosine kinase |
| SPT | serine-palmitoyl transferase |
| SPTLC1 | serine-palmitoyl transferase long chain base subunit 1 |
| SPTLC2 | serine-palmitoyl transferase long chain base subunit 2 |
| SR-B1 | scavenger receptor class B type 1 |
| nSMase2 | type 2-neutral sphingomyelinase |
| SREBF1 | sterol regulatory element binding factor 1 |
| STARD3 | stAR related lipid transfer domain containing 3 |
| 3′-UTR | 3′ untranslated region |
| VLDL | very low-density lipoprotein |
References
- Glass, C.K.; Witztum, J.L. Atherosclerosis. The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Borodzicz, S.; Czarzasta, K.; Kuch, M.; Cudnoch-Jedrzejewska, A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol. Metab. 2017, 28, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Ben-Dor, S.; Futerman, A.H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 2006, 281, 25001–25005. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Tarugi, P.; Hegele, R.A.; Davidson, N.O.; Rader, D.J.; Klein, R.L.; Hussain, M.M. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J. Biol. Chem. 2015, 290, 25863–25875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Paultre, F.; Pearson, T.A.; Reed, R.G.; Francis, C.K.; Lin, M.; Berglund, L.; Tall, A.R. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Y.; Liu, J.; Li, Y.; Huan, C.; Bui, H.H.; Kuo, M.S.; Park, T.S.; Cao, G.; Jiang, X.C. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huan, C.; Chakraborty, M.; Zhang, H.; Lu, D.; Kuo, M.S.; Cao, G.; Jiang, X.C. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ. Res. 2009, 105, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kasumov, T.; Li, L.; Li, M.; Gulshan, K.; Kirwan, J.P.; Liu, X.; Previs, S.; Willard, B.; Smith, J.D.; McCullough, A. Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis. PLoS. ONE 2015, 10, e0126910. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, T.; Rouahi, M.; Swiader, A.; Grazide, M.H.; Geoffre, N.; Alayrac, P.; Recazens, E.; Coste, A.; Salvayre, R.; Negre-Salvayre, A.; et al. nSMase2 (Type 2-Neutral Sphingomyelinase) Deficiency or Inhibition by GW4869 Reduces Inflammation and Atherosclerosis in Apoe (−/−) Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Kruski, A.W.; Scanu, A.M. Distribution of glycosphingolipids in the serum lipoproteins of normal human subjects and patients with hypo- and hyperlipidemias. J. Lipid Res. 1976, 17, 125–131. [Google Scholar] [PubMed]
- Chatterjee, S.; Bedja, D.; Mishra, S.; Amuzie, C.; Avolio, A.; Kass, D.A.; Berkowitz, D.; Renehan, M. Inhibition of glycosphingolipid synthesis ameliorates atherosclerosis and arterial stiffness in apolipoprotein E-/- mice and rabbits fed a high-fat and -cholesterol diet. Circulation 2014, 129, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Lisowska, A.; Zabielski, P.; Musial, W.; Baranowski, M. Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostag. Other Lipid Mediat. 2013, 106, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Egom, E.E.; Mamas, M.A.; Chacko, S.; Stringer, S.E.; Charlton-Menys, V.; El-Omar, M.; Chirico, D.; Clarke, B.; Neyses, L.; Cruickshank, J.K.; et al. Serum sphingolipids level as a novel potential marker for early detection of human myocardial ischaemic injury. Front. Physiol. 2013, 4, 130. [Google Scholar] [CrossRef] [PubMed]
- Sattler, K.; Lehmann, I.; Graler, M.; Brocker-Preuss, M.; Erbel, R.; Heusch, G.; Levkau, B. HDL-bound sphingosine 1-phosphate (S1P) predicts the severity of coronary artery atherosclerosis. Cell. Physiol. Biochem. 2014, 34, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Yatomi, Y. Sphingosine 1-Phosphate and Atherosclerosis. J. Atheroscler. Thromb. 2018, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Effects of low-carbohydrate and low-fat diets. Ann. Intern. Med. 2015, 162, 392. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Su, Q. MicroRNA regulation of mitochondrial and ER stress signaling pathways: Implications for lipoprotein metabolism in metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E729–E737. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Bienertova-Vasku, J.; Kara, T.; Novak, M. MicroRNAs involved in the lipid metabolism and their possible implications for atherosclerosis development and treatment. Mediat. Inflamm. 2014, 2014, 275867. [Google Scholar] [CrossRef] [PubMed]
- Wronska, A.; Kurkowska-Jastrzebska, I.; Santulli, G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol. (Oxf.) 2015, 213, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Wilczynska, A.; Bushell, M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015, 22, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.Y. MicroRNAs in Human Diseases: From Cancer to Cardiovascular Disease. Immune Netw. 2011, 11, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Scholer, N.; Langer, C.; Dohner, H.; Buske, C.; Kuchenbauer, F. Serum microRNAs as a novel class of biomarkers: A comprehensive review of the literature. Exp. Hematol. 2010, 38, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 2014, 6, 239ps3. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernandez-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M.; Bakillah, A. Epigenetic Regulation of ATP-Binding Cassette Protein A1 (ABCA1) Gene Expression: A New Era to Alleviate Atherosclerotic Cardiovascular Disease. Diseases 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Suarez, Y.; Davalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernandez-Hernando, C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Sud, N.; Taher, J.; Su, Q. MicroRNAs and Noncoding RNAs in Hepatic Lipid and Lipoprotein Metabolism: Potential Therapeutic Targets of Metabolic Disorders. Drug Dev. Res. 2015, 76, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Olejnickova, V.; Tkacova, N.; Santulli, G. Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. Adv. Exp. Med. Biol. 2015, 887, 79–100. [Google Scholar] [PubMed]
- Soh, J.; Iqbal, J.; Queiroz, J.; Fernandez-Hernando, C.; Hussain, M.M. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat. Med. 2013, 19, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Gilchrist, D.S.; Nijjar, J.; Araldi, E.; Ramirez, C.M.; Lavery, C.A.; Fernandez-Hernando, C.; McInnes, I.B.; Kurowska-Stolarska, M. MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PLoS ONE 2013, 8, e72324. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Yang, L.; Lu, P.H.; Fu, X.L.; Zhang, Y.; Zhu, Y.Q.; Tian, Y. MicroRNA-101 down-regulates sphingosine kinase 1 in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2015, 463, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Geekiyanage, H.; Chan, C. MicroRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer′s disease. J. Neurosci. 2011, 31, 14820–14830. [Google Scholar] [CrossRef] [PubMed]
- Meyers-Needham, M.; Ponnusamy, S.; Gencer, S.; Jiang, W.; Thomas, R.J.; Senkal, C.E.; Ogretmen, B. Concerted functions of HDAC1 and microRNA-574-5p repress alternatively spliced ceramide synthase 1 expression in human cancer cells. EMBO Mol. Med. 2012, 4, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Hojjati, M.R.; Li, Z.; Zhou, H.; Tang, S.; Huan, C.; Ooi, E.; Lu, S.; Jiang, X.C. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 2005, 280, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Panek, R.L.; Mueller, S.B.; Hanselman, J.C.; Rosebury, W.S.; Robertson, A.W.; Kindt, E.K.; Homan, R.; Karathanasis, S.K.; Rekhter, M.D. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 2004, 110, 3465–3471. [Google Scholar] [CrossRef] [PubMed]
- Glaros, E.N.; Kim, W.S.; Wu, B.J.; Suarna, C.; Quinn, C.M.; Rye, K.A.; Stocker, R.; Jessup, W.; Garner, B. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol. 2007, 73, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Glaros, E.N.; Kim, W.S.; Quinn, C.M.; Jessup, W.; Rye, K.A.; Garner, B. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J. Lipid Res. 2008, 49, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Takabe, K.; Kapitonov, D.; Milstien, S.; Spiegel, S. Targeting SphK1 as a new strategy against cancer. Curr. Drug Targets 2008, 9, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Vadas, M.; Xia, P.; McCaughan, G.; Gamble, J. The role of sphingosine kinase 1 in cancer: Oncogene or non-oncogene addiction? Biochim. Biophys. Acta 2008, 1781, 442–447. [Google Scholar] [CrossRef] [PubMed]
| miRNAs | Predicted Seed Sequence * | Target Genes | Target Tissues |
|---|---|---|---|
| miR-33 | GUUACGU | ABCA1, CROT, CPT1A, HADHB, ACLY, SREBF1, ACACA | Liver |
| miR-144 | UAUGACA | ABCA1 | Liver |
| miR-758 | CAGUGUU | ABCA1 | Liver |
| miR-26 | AUGAACU | ABCA1 | Liver |
| miR-106b | CGUGAAA | ABCA1 | Liver |
| miR-27 | AUUCGAG | SR-B1 | Liver |
| miR-185 | AGAGAGG | SR-B1 | Liver |
| miR-96 | CACGGUU | SR-B1, ABCA1 | Liver |
| miR-223 | UUGACUG | SR-B1 | Liver |
| miR-30c | CAAAUG | MTTP, LPGAT1, ELOVL5, STARD3, MBOAT1 | Liver |
| miR-128-1 | GCCGGGG | LDLR | Liver |
| miR-148 | ACGUGAC | LDLR, ABCA1 | Liver |
| miR-122 | UGUGAGG | FASN, SCD1, ACLY, ACC2 | Liver |
| miR-155 | CGUAAU | LXRα | Liver |
| miR-574 | GUGUGAG | CerS | Multiple human cancer cells |
| miR-9 | UGGUUUC | SPTLC1, SPTLC2 | Primary astrocytes |
| miR-29a | UUUAGUC | SPTLC1, SPTLC2 | Primary astrocytes |
| miR-29b-1 | UUUGGUC | SPTLC1, SPTLC2 | Primary astrocytes |
| miR-101 | CUAUUGA | SPK | Colorectal cancer cells |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahangir, Z.; Bakillah, A.; Iqbal, J. Regulation of Sphingolipid Metabolism by MicroRNAs: A Potential Approach to Alleviate Atherosclerosis. Diseases 2018, 6, 82. https://doi.org/10.3390/diseases6030082
Jahangir Z, Bakillah A, Iqbal J. Regulation of Sphingolipid Metabolism by MicroRNAs: A Potential Approach to Alleviate Atherosclerosis. Diseases. 2018; 6(3):82. https://doi.org/10.3390/diseases6030082
Chicago/Turabian StyleJahangir, Zainab, Ahmed Bakillah, and Jahangir Iqbal. 2018. "Regulation of Sphingolipid Metabolism by MicroRNAs: A Potential Approach to Alleviate Atherosclerosis" Diseases 6, no. 3: 82. https://doi.org/10.3390/diseases6030082
APA StyleJahangir, Z., Bakillah, A., & Iqbal, J. (2018). Regulation of Sphingolipid Metabolism by MicroRNAs: A Potential Approach to Alleviate Atherosclerosis. Diseases, 6(3), 82. https://doi.org/10.3390/diseases6030082






