Pharmacological Effects of a Novel Bradykinin-Related Peptide (RR-18) from the Skin Secretion of the Hejiang Frog (Ordorrana hejiangensis) on Smooth Muscle
Abstract
1. Introduction
2. Experimental Section
2.1. Skin Secretion Acquisition
2.2. “Shotgun” Cloning of cDNA Encoding RR-18 Biosynthetic Precursor from Skin Secretion
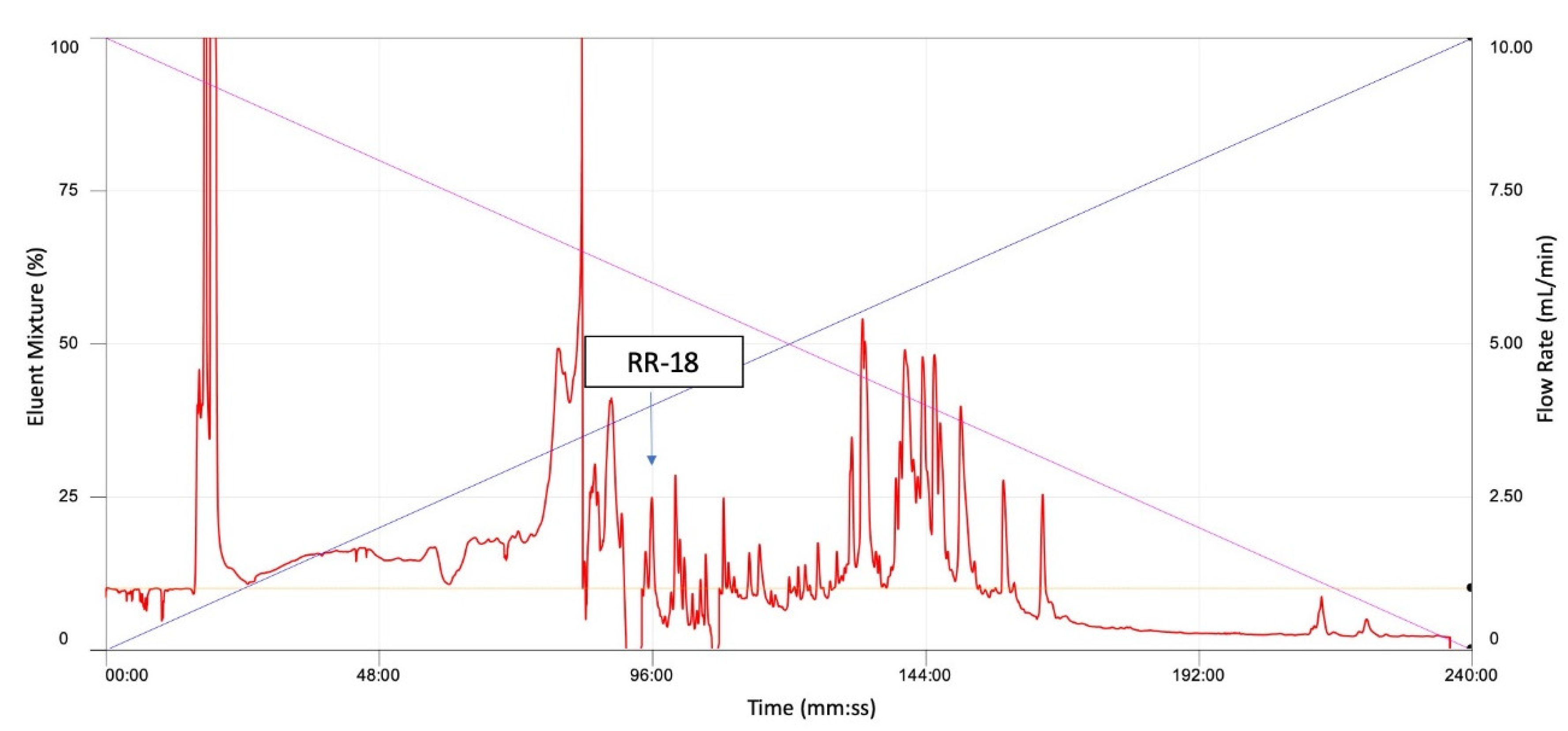
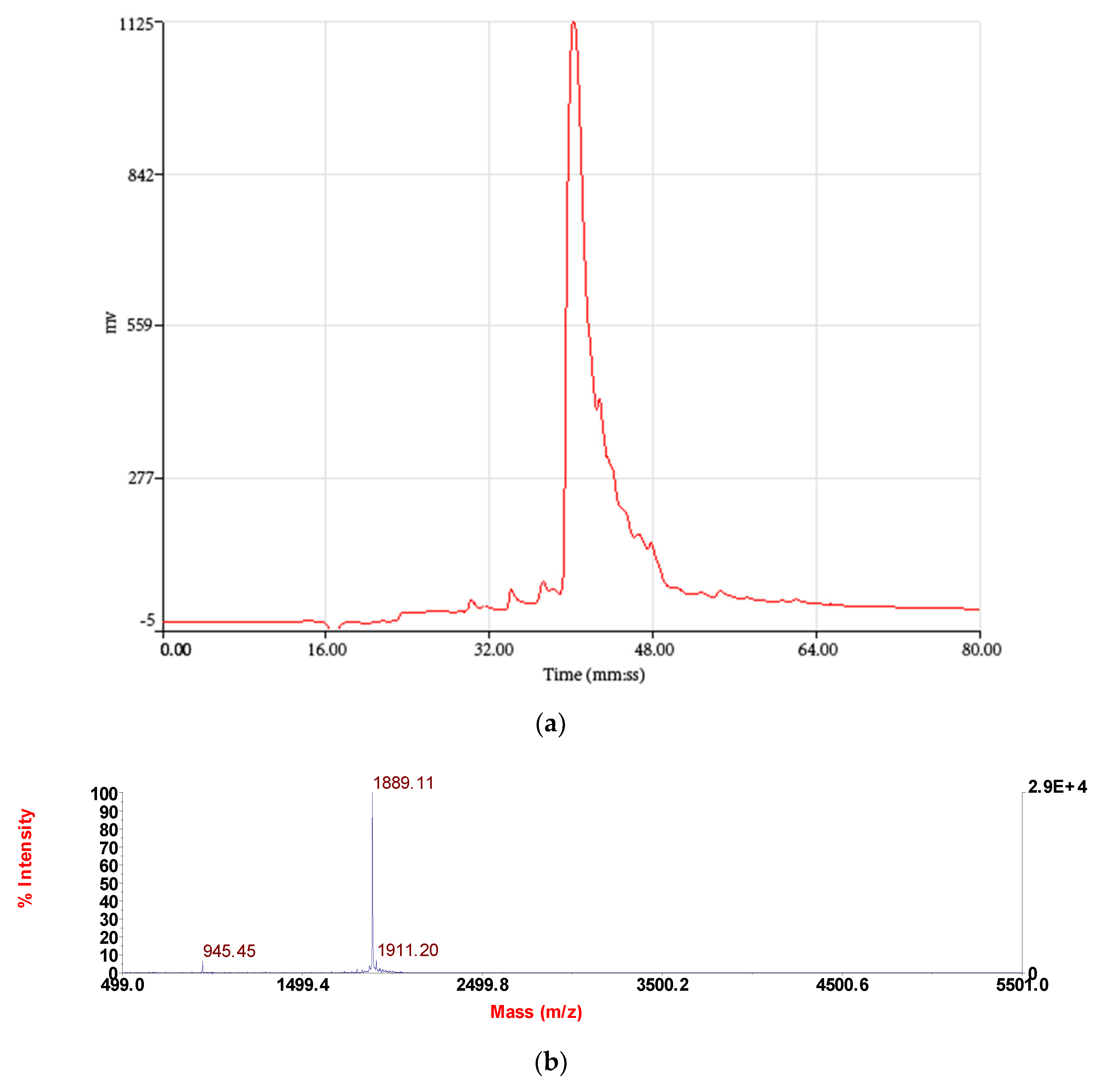
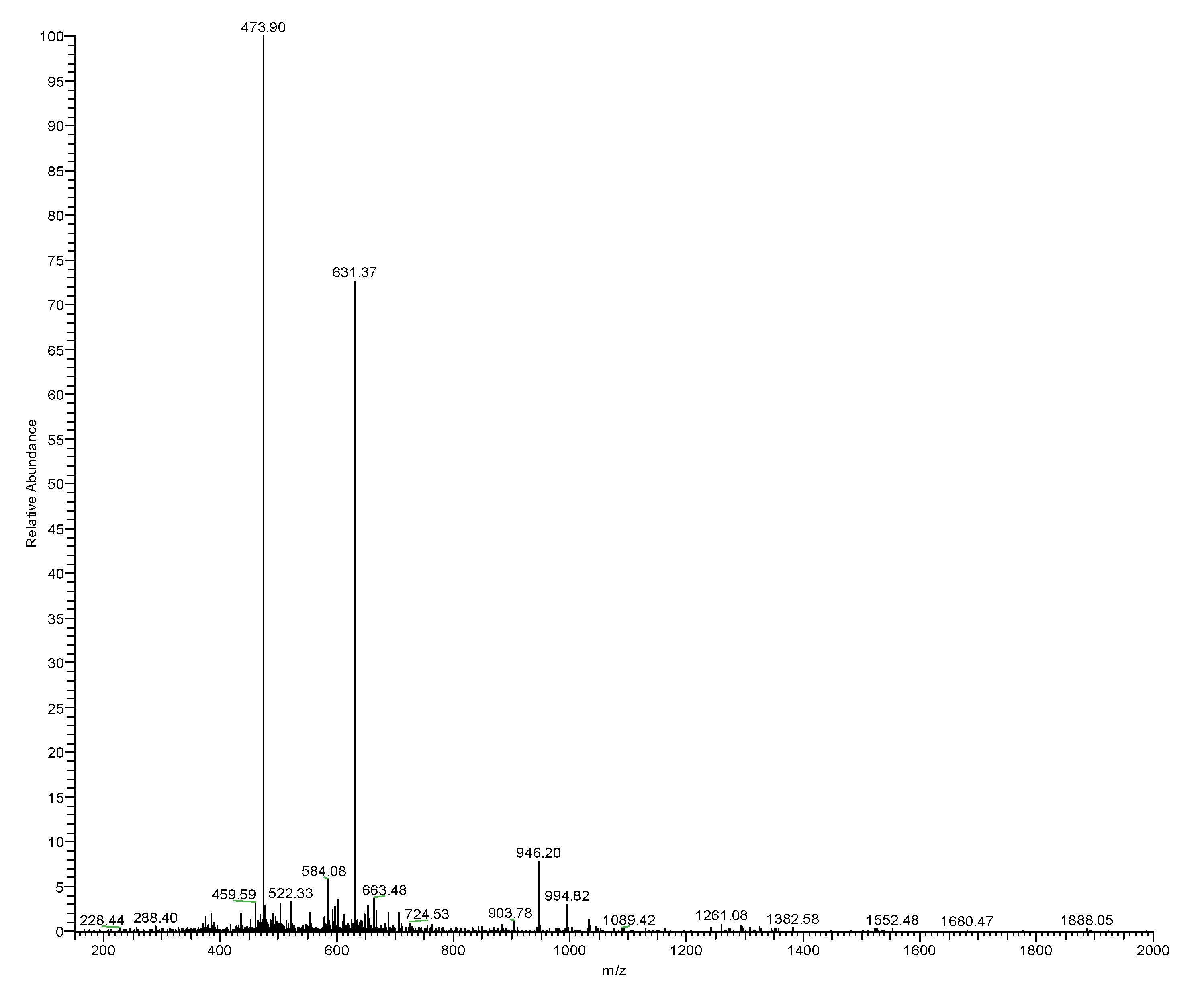
2.3. Isolation and Structural Characterisation of RR-18 from Skin Secretion
2.4. Solid-Phase Peptide Synthesis of Peptides
2.5. Myotropic Activity Evaluation on Smooth Muscles
2.6. Statistical Analysis
3. Results
3.1. Molecular Cloning of RR-18 Precursor-Encoding cDNAs
3.2. Isolation and Identification and Structural Characterisation of RR-18
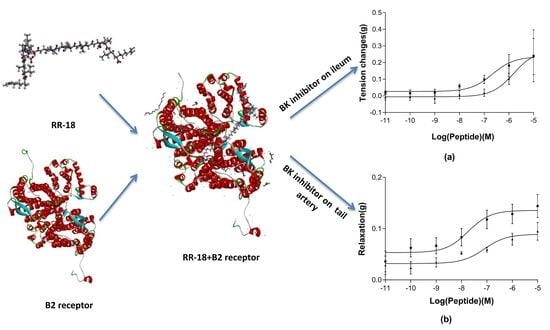
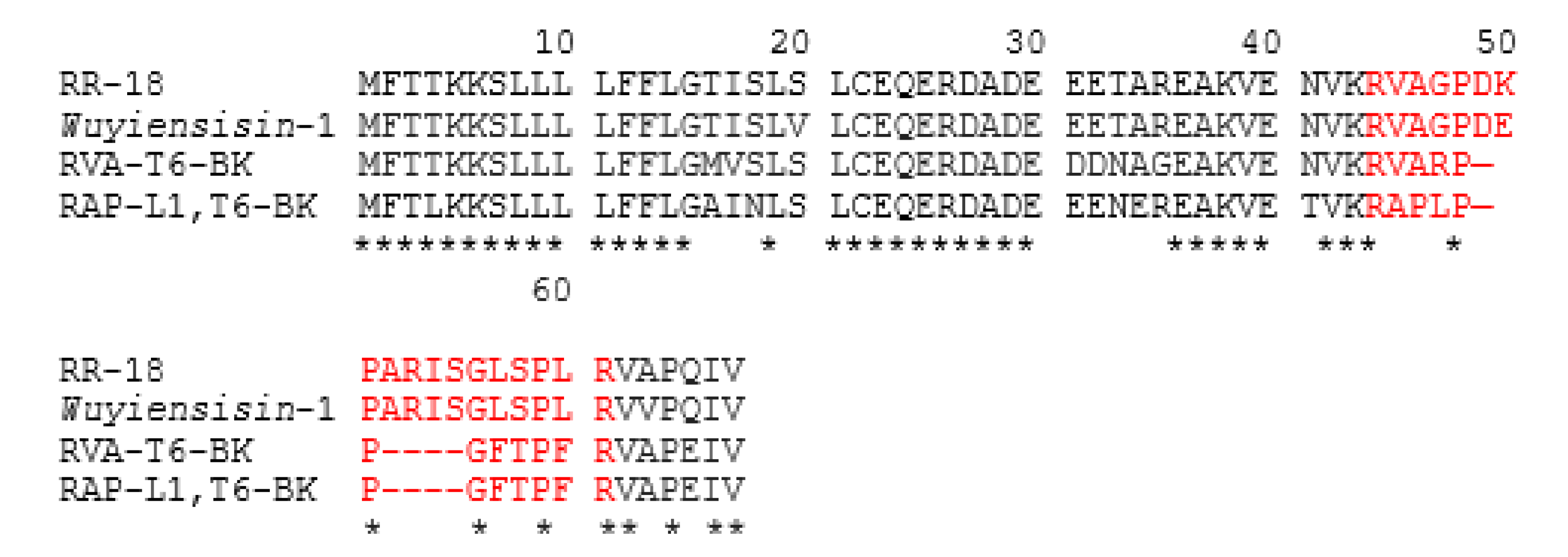
3.3. Bioinformatic Analysis of Novel RR-18
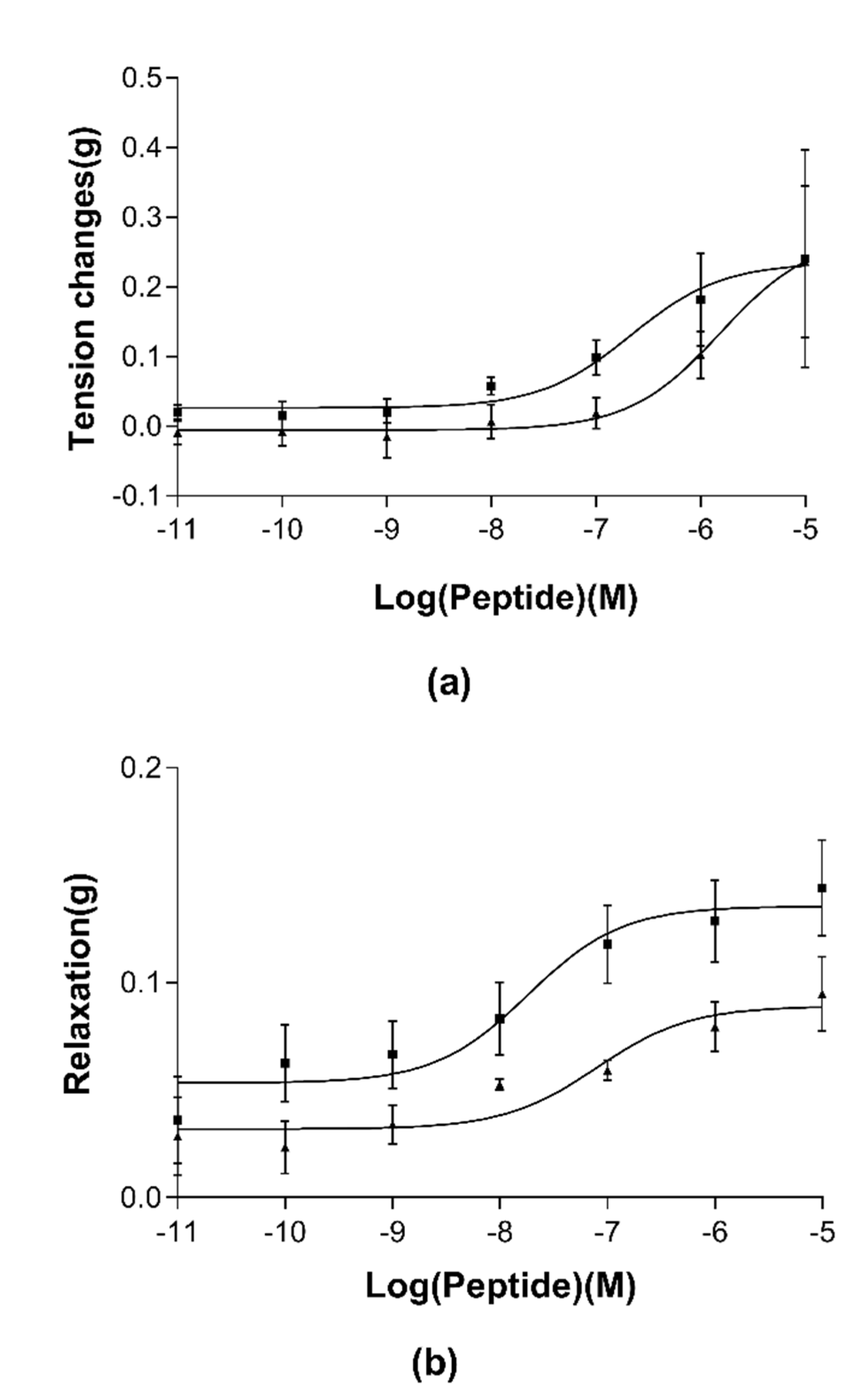
3.4. Pharmacological Effects of RR-18 on Smooth Muscle
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Erspamer, V.; Bertaccini, G. Occurrence of bradykinin in the skin of Rana temperaria. Comp. Biochem. Physiol. 1965, 14, 43–52. [Google Scholar]
- Wu, D.; Gao, Y.; Tan, Y.; Liu, Y.; Wang, L.; Zhou, M.; Xi, X.; Ma, C.; Bininda-Emonds, O.R.; Chen, T. Discovery of Distinctin-Like-Peptide-PH (DLP-PH) From the Skin Secretion of Phyllomedusa hypochondrialis, a Prototype of a Novel Family of Antimicrobial Peptide. Front. Microbiol. 2018, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Bininda-Emonds, O.R.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, M.; Bai, B.; Ma, C.B.; Wei, L.; Wang, L.; Chen, T.B.; Shaw, C. Peptide IC-20, encoded by skin kininogen-1 of the European yellow-bellied toad, Bombina variegata, antagonizes bradykinin-induced arterial smooth muscle relaxation. J. Pharm. Bioallied Sci. 2011, 3, 221–225. [Google Scholar]
- Xi, X.P.; Li, B.; Chen, T.B.; Kwok, H.F. A Review on Bradykinin-Related Peptides Isolated from Amphibian Skin Secretion. Toxins 2015, 7, 951–970. [Google Scholar] [CrossRef]
- Sin, Y.T.; Zhou, M.; Chen, W.; Wang, L.; Chen, T.B.; Walker, B.; Shaw, C. Skin bradykinin-related peptides (BRPs) and their biosynthetic precursors (kininogens): Comparisons between various taxa of Chinese and North American ranid frogs. Peptides 2008, 29, 393–403. [Google Scholar] [CrossRef]
- Rouhiainen, A.; Kulesskaya, N.; Mennesson, M.; Misiewicz, Z.; Sipila, T.; Sokolowska, E.; Trontti, K.; Urpa, L.; McEntegart, W.; Saarnio, S.; et al. The bradykinin system in stress and anxiety in humans and mice. Sci. Rep. 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shi, D.N.; Chen, X.L.; Wang, L.; Ying, Y.; Ma, C.B.; Xi, X.P.; Zhou, M.; Chen, T.B.; Shaw, C. A Novel Bradykinin-Related Peptide, RVA-Thr(6)-BK, from the Skin Secretion of the Hejiang Frog; Ordorrana hejiangensis: Effects of Mammalian Isolated Smooth Muscle. Toxins 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Marceau, F.; Bachelard, H.; Bouthillier, J.; Fortin, J.P.; Morissette, G.; Bawolak, M.T.; Charest-Morin, X.; Gera, L. Bradykinin receptors: Agonists, antagonists, expression, signaling, and adaptation to sustained stimulation. Int. Immunopharmacol. 2020, 82, 7. [Google Scholar] [CrossRef]
- Couture, R.; Blaes, N.; Girolami, J.-P. Kinin receptors in vascular biology and pathology. Curr. Vasc. Pharmacol. 2014, 12, 223–248. [Google Scholar] [CrossRef]
- Figueroa, C.D.; Ehrenfeld, P.; Bhoola, K.D. Kinin receptors as targets for cancer therapy. Expert Opin. Ther. Targets 2012, 16, 299–312. [Google Scholar] [CrossRef]
- Calixto, J.B.; Medeiros, R.; Fernandes, E.S.; Ferreira, J.; Cabrini, D.A.; Campos, M.M. Kinin B1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br. J. Pharmacol. 2004, 143, 803–818. [Google Scholar] [CrossRef]
- Sang, H.; Liu, L.; Wang, L.; Qiu, Z.; Li, M.; Yu, L.; Zhang, H.; Shi, R.; Yu, S.; Guo, R. Opposite roles of bradykinin B1 and B2 receptors during cerebral ischaemia–reperfusion injury in experimental diabetic rats. Eur. J. Neurosci. 2016, 43, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Ye, Y.W.; Zhang, X.F.; Song, J.M. Bradykinin stimulates IL-6 production and cell invasion in colorectal cancer cells. Oncol. Rep. 2014, 32, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Regoli, D.; Gobeil, F. Kinins and peptide receptors. Biol. Chem. 2016, 397, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Ma, C.B.; Zhou, M.; Zhang, Y.N.; Xi, X.P.; Zhong, R.M.; Chen, T.B.; Shaw, C.; Wang, L. Pharmacological Effects of Two Novel Bombesin-Like Peptides from the Skin Secretions of Chinese Piebald Odorous Frog (Odorrana schmackeri) and European Edible Frog (Pelophylax kl. esculentus) on Smooth Muscle. Molecules 2017, 22, 1798. [Google Scholar] [CrossRef]
- Kumar, V.T.V.; Holthausen, D.; Jacob, J.; George, S. Host Defense Peptides from Asian Frogs as Potential Clinical Therapies. Antibiotics 2015, 4, 136–159. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Foster, D.C.; Woeller, C.F.; Pollock, S.J.; Bonham, A.D.; Haidaris, C.G.; Phipps, R.P. A Role for Bradykinin Signaling in Chronic Vulvar Pain. J. Pain 2016, 17, 1183–1197. [Google Scholar] [CrossRef]
- Wang, L.; Evaristo, G.; Zhou, M.; Pinkse, M.; Wang, M.; Xu, Y.; Jiang, X.F.; Chen, T.B.; Rao, P.F.; Verhaert, P.; et al. Nigrocin-2 peptides from Chinese Odorrana frogs—Integration of UPLC/MS/MS with molecular cloning in amphibian skin peptidome analysis. FEBS J. 2010, 277, 1519–1531. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Chen, T.B.; Walker, B.; Zhou, M.; Sui, D.Y.; Conlon, J.M.; Shaw, C. Identification and molecular cloning of a novel amphibian Bowman Birk-type trypsin inhibitor from the skin of the Hejiang Odorous Frog; Odorrana hejiangensis. Peptides 2012, 33, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, D.; Xi, X.; Wu, Y.; Ma, C.; Zhou, M.; Wang, L.; Yang, M.; Chen, T.; Shaw, C. Identification and Characterisation of the Antimicrobial Peptide, Phylloseptin-PT, from the Skin Secretion of Phyllomedusa tarsius, and Comparison of Activity with Designed, Cationicity-Enhanced Analogues and Diastereomers. Molecules 2016, 21, 1667. [Google Scholar] [CrossRef] [PubMed]
- Benyó, Z.; Lacza, Z.; Hortobágyi, T.; Görlach, C.; Wahl, M. Functional importance of neuronal nitric oxide synthase in the endothelium of rat basilar arteries. Brain Res. 2000, 877, 79–84. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, H.; Ma, C.B.; Zhou, M.; Wu, Y.X.; Wang, L.; Guo, S.D.; Chen, T.B.; Shaw, C. Ex Vivo Smooth Muscle Pharmacological Effects of a Novel Bradykinin-Related Peptide, and Its Analogue, from Chinese Large Odorous Frog, Odorrana livida Skin Secretions. Toxins 2016, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Wang, L.; Lin, C.; Lin, Y.; Zhou, M.; Chen, L.; Connolly, B.; Zhang, Y.Q.; Chen, T.B.; Shaw, C. Vasorelaxin: A Novel Arterial Smooth Muscle-Relaxing Eicosapeptide from the Skin Secretion of the Chinese Piebald Odorous Frog (Odorrana schmackeri). PLoS ONE 2013, 8, e55739. [Google Scholar] [CrossRef]
- Conlon, J.M. Bradykinin and its receptors in non-mammalian vertebrates. Regul. Pept. 1999, 79, 71–81. [Google Scholar] [CrossRef]
- Martin, R.P.; Filippelli-Silva, R. Non-radioactive binding assay for bradykinin and angiotensin receptors. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 149, pp. 77–85. [Google Scholar]
- Shi, D.N.; Luo, Y.; Du, Q.; Wang, L.; Zhou, M.; Ma, J.; Li, R.J.; Chen, T.B.; Shaw, C. A Novel Bradykinin-Related Dodecapeptide (RVALPPGFTPLR) from the Skin Secretion of the Fujian Large-Headed Frog (Limnonectes fujianensis) Exhibiting Unusual Structural and Functional Features. Toxins 2014, 6, 2886–2898. [Google Scholar] [CrossRef]
- Bonechi, C.; Ristori, S.; Martini, G.; Martini, S.; Rossi, C. Study of bradykinin conformation in the presence of model membrane by Nuclear Magnetic Resonance and molecular modelling. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 708–716. [Google Scholar] [CrossRef]
- Lyu, P.; Ge, L.L.; Wang, L.; Guo, X.X.; Zhang, H.L.; Li, Y.H.; Zhou, Y.; Zhou, M.; Chen, T.B.; Shaw, C. Ornithokinin (avian bradykinin) from the skin of the Chinese bamboo odorous frog, Odorrana versabilis. J. Pept. Sci. 2014, 20, 618–624. [Google Scholar] [CrossRef]
- Ohnishi, M.; Yukawa, R.; Akagi, M.; Ohsugi, Y.; Inoue, A. Bradykinin and interleukin-1β synergistically increase the expression of cyclooxygenase-2 through the RNA-binding protein HuR in rat dorsal root ganglion cells. Neurosci. Lett. 2019, 694, 215–219. [Google Scholar] [CrossRef]
- Gonçalves, E.C.D.; Vieira, G.; Gonçalves, T.R.; Simões, R.R.; Brusco, I.; Oliveira, S.M.; Calixto, J.B.; Cola, M.; Santos, A.R.; Dutra, R.C. Bradykinin Receptors Play a Critical Role in the Chronic Post-ischaemia Pain Model. Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.L.; Fan, L.; Zhao, L.; Liu, Y.L.; Lin, C.; Xu, X.P.; Li, Z.; Bao, Z.Y.; Liu, Y.; Wang, X.M.; et al. Activation of bradykinin B2 receptor induced the inflammatory responses of cytosolic phospholipase A(2) after the early traumatic brain injury. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 2957–2971. [Google Scholar] [CrossRef] [PubMed]

| #1 | b(1+) | b(2+) | b(3+) | Seq. | y(1+) | y(2+) | y(3+) | #2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 157.10840 | 79.05784 | 53.04098 | R | 18 | |||
| 2 | 256.17682 | 128.59205 | 86.06379 | V | 1734.00218 | 867.50473 | 578.67224 | 17 |
| 3 | 327.21394 | 164.11061 | 109.74283 | A | 1634.93376 | 817.97052 | 545.64944 | 16 |
| 4 | 384.23541 | 192.62134 | 128.74999 | G | 1563.89664 | 782.45196 | 521.97040 | 15 |
| 5 | 481.28818 | 241.14773 | 161.10091 | P | 1506.87517 | 753.94122 | 502.96324 | 14 |
| 6 | 596.31513 | 298.66120 | 199.44323 | D | 1409.82240 | 705.41484 | 470.61232 | 13 |
| 7 | 724.41010 | 362.70869 | 242.14155 | K | 1294.79545 | 647.90136 | 432.27000 | 12 |
| 8 | 821.46287 | 411.23507 | 274.49247 | P | 1166.70048 | 583.85388 | 389.57168 | 11 |
| 9 | 892.49999 | 446.75363 | 298.17151 | A | 1069.64771 | 535.32749 | 357.22075 | 10 |
| 10 | 1048.60111 | 524.80419 | 350.20522 | R | 998.61059 | 499.80893 | 333.54171 | 9 |
| 11 | 1161.68518 | 581.34623 | 387.89991 | I | 842.50947 | 421.75837 | 281.50801 | 8 |
| 12 | 1248.71721 | 624.86224 | 416.91059 | S | 729.42540 | 365.21634 | 243.81332 | 7 |
| 13 | 1305.73868 | 653.37298 | 435.91774 | G | 642.39337 | 321.70032 | 214.80264 | 6 |
| 14 | 1418.82275 | 709.91501 | 473.61243 | L | 585.37190 | 293.18959 | 195.79548 | 5 |
| 15 | 1505.85478 | 753.43103 | 502.62311 | S | 472.28783 | 236.64755 | 158.10079 | 4 |
| 16 | 1602.90755 | 801.95741 | 534.97403 | P | 385.25580 | 193.13154 | 129.09012 | 3 |
| 17 | 1715.99162 | 858.49945 | 572.66872 | L | 288.20303 | 144.60515 | 96.73919 | 2 |
| 18 | R | 175.11896 | 88.06312 | 59.04450 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Xu, J.; Zhong, R.; Ma, C.; Zhou, M.; Cao, Z.; Xi, X.; Shaw, C.; Chen, T.; Wang, L.; et al. Pharmacological Effects of a Novel Bradykinin-Related Peptide (RR-18) from the Skin Secretion of the Hejiang Frog (Ordorrana hejiangensis) on Smooth Muscle. Biomedicines 2020, 8, 225. https://doi.org/10.3390/biomedicines8070225
Zhou X, Xu J, Zhong R, Ma C, Zhou M, Cao Z, Xi X, Shaw C, Chen T, Wang L, et al. Pharmacological Effects of a Novel Bradykinin-Related Peptide (RR-18) from the Skin Secretion of the Hejiang Frog (Ordorrana hejiangensis) on Smooth Muscle. Biomedicines. 2020; 8(7):225. https://doi.org/10.3390/biomedicines8070225
Chicago/Turabian StyleZhou, Xiaowei, Jie Xu, Ruimin Zhong, Chengbang Ma, Mei Zhou, Zhijian Cao, Xinping Xi, Chris Shaw, Tianbao Chen, Lei Wang, and et al. 2020. "Pharmacological Effects of a Novel Bradykinin-Related Peptide (RR-18) from the Skin Secretion of the Hejiang Frog (Ordorrana hejiangensis) on Smooth Muscle" Biomedicines 8, no. 7: 225. https://doi.org/10.3390/biomedicines8070225
APA StyleZhou, X., Xu, J., Zhong, R., Ma, C., Zhou, M., Cao, Z., Xi, X., Shaw, C., Chen, T., Wang, L., & Kwok, H. F. (2020). Pharmacological Effects of a Novel Bradykinin-Related Peptide (RR-18) from the Skin Secretion of the Hejiang Frog (Ordorrana hejiangensis) on Smooth Muscle. Biomedicines, 8(7), 225. https://doi.org/10.3390/biomedicines8070225






_Kwok.png)