Pharmacological Treatment for Neuroinflammation in Stress-Related Disorder
Abstract
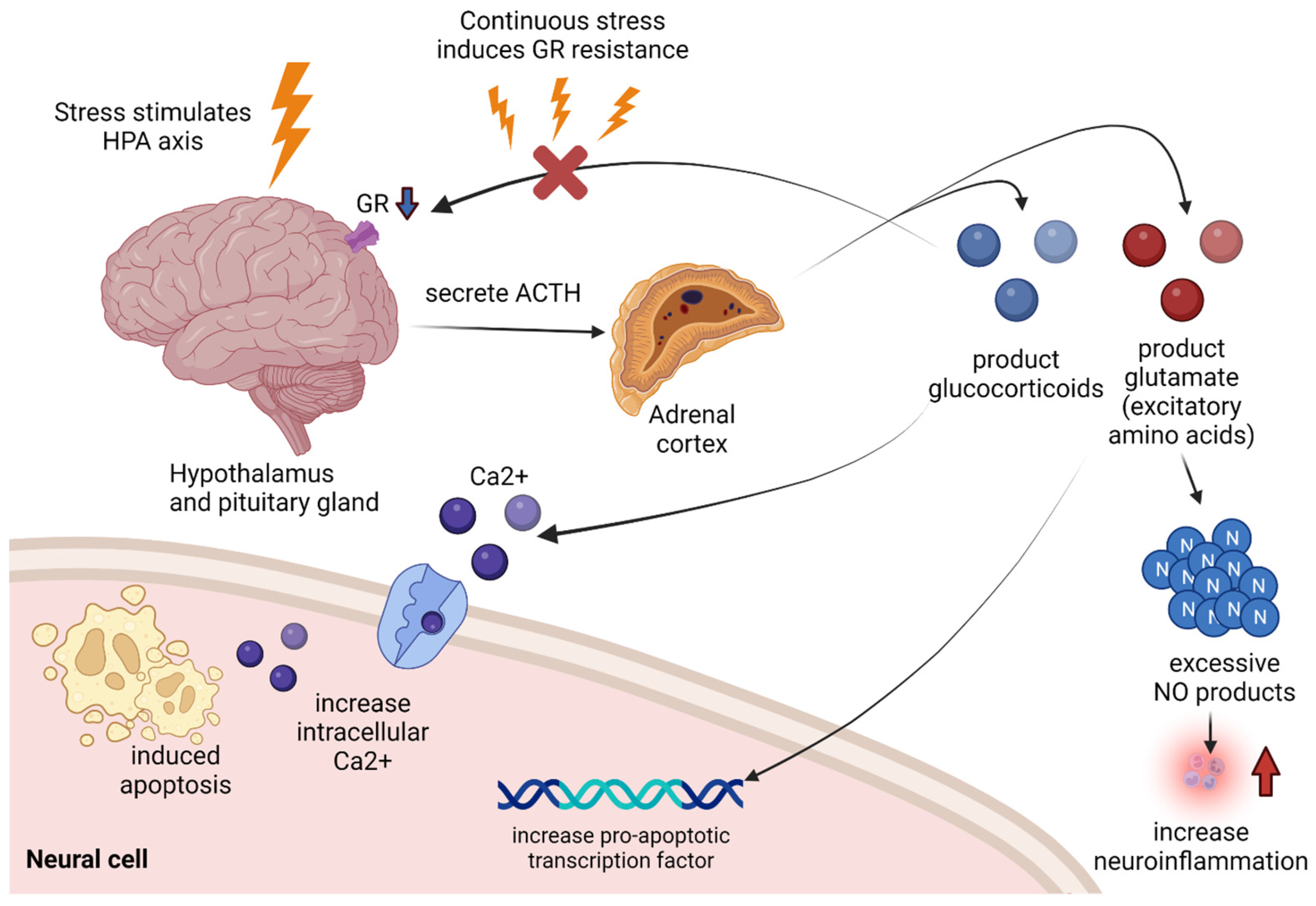
:1. Introduction
2. Stress-Related Neuroinflammatory Mechanism
2.1. HPA Axis and Glucocorticoids
2.2. Glutamate (Excitatory Amino Acids)
2.3. Renin–Angiotensin System
2.4. Neuroinflammation by Stress-Related Oxidative and Nitric Oxide Products
3. NMDA Glutamate Receptor Inhibition for Treatment of Neuroinflammation
4. Peroxisome Proliferator-Activated Receptor (PPAR) agonist for Treatment Neuroinflammation
5. Angiotensin-Converting Enzyme (ACE) Inhibition and Angiotensin Receptor Blocking (ARB) for Treatment of Neuroinflammation
6. Other Pharmacological Treatments for Neuroinflammation
7. New Candidates for Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muthukumar, K.; Nachiappan, V. Cadmium-induced oxidative stress in Saccharomyces cerevisiae. Indian J. Biochem. Biophys. 2010, 47, 383–387. [Google Scholar]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S. Stress-induced activation of the sympathetic nervous system. Baillieres Clin. Endocrinol. Metab. 1987, 1, 253–278. [Google Scholar] [CrossRef]
- Miki, K.; Yoshimoto, M. Sympathetic nerve activity during sleep, exercise, and mental stress. Auton. Neurosci. 2013, 174, 15–20. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [Green Version]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, S.Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef]
- Zhong, J.; Shi, G. Editorial: Regulation of Inflammation in Chronic Disease. Front. Immunol. 2019, 10, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Michels da Silva, D.; Langer, H.; Graf, T. Inflammatory and Molecular Pathways in Heart Failure-Ischemia, HFpEF and Transthyretin Cardiac Amyloidosis. Int. J. Mol. Sci. 2019, 20, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The Role of Chemokines in the Pathophysiology of Major Depressive Disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef] [Green Version]
- Needham, E.J.; Helmy, A.; Zanier, E.R.; Jones, J.L.; Coles, A.J.; Menon, D.K. The immunological response to traumatic brain injury. J. Neuroimmunol. 2019, 332, 112–125. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.E.; Jensen, P.; Ozenne, B.; Armand, S.; Svarer, C.; Stenbaek, D.S.; Moeller, K.; Dyssegaard, A.; Thomsen, G.; Steinmetz, J.; et al. Molecular imaging of neuroinflammation in patients after mild traumatic brain injury: A longitudinal (123) I-CLINDE single photon emission computed tomography study. Eur. J. Neurol. 2019, 26, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, H.E. Neural immunity: Friend or foe? J. Neurovirol. 2002, 8, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.Y.; Lee, D.H.; Lee, J.Y.; Lee, E.C.; Park, S.W.; Lee, M.R.; Oh, J.S. Relationship between Brain Metabolic Disorders and Cognitive Impairment: LDL Receptor Defect. Int. J. Mol. Sci. 2022, 23, 8384. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.R.; Rowitch, D.H. Evolving concepts of gliogenesis: A look way back and ahead to the next 25 years. Neuron 2013, 80, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, C.; Hartupee, J.; Altuntas, C.Z.; Gulen, M.F.; Jane-Wit, D.; Xiao, J.; Lu, Y.; Giltiay, N.; Liu, J.; et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 2007, 8, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Trzyna, W.C.; McClintock, D.S.; Schumacker, P.T. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J. Immunol. 2000, 165, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Bezzi, P.; Domercq, M.; Brambilla, L.; Galli, R.; Schols, D.; De Clercq, E.; Vescovi, A.; Bagetta, G.; Kollias, G.; Meldolesi, J.; et al. CXCR4-activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001, 4, 702–710. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y.; Hong, D.Y.; Lee, E.C.; Park, S.W.; Lee, M.R.; Oh, J.S. Neuroinflammation in Post-Traumatic Stress Disorder. Biomedicines 2022, 10, 953. [Google Scholar] [CrossRef]
- Raadsheer, F.C.; van Heerikhuize, J.J.; Lucassen, P.J.; Hoogendijk, W.J.; Tilders, F.J.; Swaab, D.F. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am. J. Psychiatry 1995, 152, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- De Cristobal, J.; Madrigal, J.L.; Lizasoain, I.; Lorenzo, P.; Leza, J.C.; Moro, M.A. Aspirin inhibits stress-induced increase in plasma glutamate, brain oxidative damage and ATP fall in rats. Neuroreport 2002, 13, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef]
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta 1999, 1411, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Shinohe, A.; Hashimoto, K.; Nakamura, K.; Tsujii, M.; Iwata, Y.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.; et al. Increased serum levels of glutamate in adult patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1472–1477. [Google Scholar] [CrossRef] [Green Version]
- Leza, J.C.; Salas, E.; Sawicki, G.; Russell, J.C.; Radomski, M.W. The effects of stress on homeostasis in JCR-LA-cp rats: The role of nitric oxide. J. Pharmacol. Exp. Ther. 1998, 286, 1397–1403. [Google Scholar]
- Madrigal, J.L.; Garcia-Bueno, B.; Caso, J.R.; Perez-Nievas, B.G.; Leza, J.C. Stress-induced oxidative changes in brain. CNS Neurol. Disord. Drug Targets 2006, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Yang, G.; Xi, Z.X.; Wan, Y.; Wang, H.; Bi, G. Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol. Signals 1993, 2, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wan, Y.; Zhu, Y. Angiotensin II—An important stress hormone. Biol. Signals 1996, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, J.P.; Sriramula, S.; Mariappan, N.; Agarwal, D.; Francis, J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension 2012, 59, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Zhang, Z.H.; Wei, S.G.; Serrats, J.; Weiss, R.M.; Felder, R.B. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension 2010, 55, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Saavedra, J.M. Brain and pituitary angiotensin. Endocr. Rev. 1992, 13, 329–380. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G. Factors controlling steroid biosynthesis in the zona glomerulosa of the adrenal. J. Steroid Biochem. Mol. Biol. 1993, 45, 147–151. [Google Scholar] [CrossRef]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Zorumski, C.F.; Izumi, Y. Nitric oxide and hippocampal synaptic plasticity. Biochem. Pharmacol. 1993, 46, 777–785. [Google Scholar] [CrossRef]
- Huang, E.P. Synaptic plasticity: A role for nitric oxide in LTP. Curr. Biol. 1997, 7, R141–R143. [Google Scholar] [CrossRef] [Green Version]
- Schini, V.B.; Busse, R.; Vanhoutte, P.M. Inducible nitric oxide synthase in vascular smooth muscle. Arzneimittelforschung 1994, 44, 432–435. [Google Scholar] [PubMed]
- Ferrari, A.U.; Radaelli, A.; Mori, T.; Mircoli, L.; Perlini, S.; Meregalli, P.; Fedele, L.; Mancia, G. Nitric oxide-dependent vasodilation and the regulation of arterial blood pressure. J. Cardiovasc. Pharmacol. 2001, 38 (Suppl. S2), S19–S22. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Moro, M.A.; Lizasoain, I.; Lorenzo, P.; Castrillo, A.; Bosca, L.; Leza, J.C. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J. Neurochem. 2001, 76, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Hinz, B.; Brune, K. Cyclooxygenase-2—10 years later. J. Pharmacol. Exp. Ther. 2002, 300, 367–375. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, G.P.; Ford-Hutchinson, A.W. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993, 330, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Yasojima, K.; Schwab, C.; McGeer, E.G.; McGeer, P.L. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999, 830, 226–236. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Nogawa, S.; Zhang, F.; Ross, M.E.; Iadecola, C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997, 17, 2746–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minghetti, L.; Pocchiari, M. Cyclooxygenase-2, prostaglandin E2, and microglial activation in prion diseases. Int. Rev. Neurobiol. 2007, 82, 265–275. [Google Scholar] [PubMed]
- Muller-Decker, K.; Furstenberger, G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol. Carcinog. 2007, 46, 705–710. [Google Scholar] [CrossRef]
- Vesce, S.; Rossi, D.; Brambilla, L.; Volterra, A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int. Rev. Neurobiol. 2007, 82, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Andreasson, K.I.; Kaufmann, W.E.; Barnes, C.A.; Worley, P.F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: Regulation by synaptic activity and glucocorticoids. Neuron 1993, 11, 371–386. [Google Scholar] [CrossRef]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Lipton, S.A. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006, 97, 1611–1626. [Google Scholar] [CrossRef]
- Kemp, J.A.; McKernan, R.M. NMDA receptor pathways as drug targets. Nat. Neurosci. 2002, 5, 1039–1042. [Google Scholar] [CrossRef]
- Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat. Rev. Drug Discov. 2006, 5, 160–170. [Google Scholar] [CrossRef]
- Koch, H.J.; Szecsey, A.; Haen, E. NMDA-antagonism (memantine): An alternative pharmacological therapeutic principle in Alzheimer’s and vascular dementia. Curr. Pharm. Des. 2004, 10, 253–259. [Google Scholar] [CrossRef]
- Lipton, S.A. Failures and successes of NMDA receptor antagonists: Molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx 2004, 1, 101–110. [Google Scholar] [CrossRef]
- Sonkusare, S.K.; Kaul, C.L.; Ramarao, P. Dementia of Alzheimer’s disease and other neurodegenerative disorders—Memantine, a new hope. Pharmacol. Res. 2005, 51, 1–17. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Hurtado, O.; Moro, M.A.; Lizasoain, I.; Lorenzo, P.; Castrillo, A.; Bosca, L.; Leza, J.C. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology 2002, 26, 155–163. [Google Scholar] [CrossRef]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Jenum, P.A. Rapid identification of Escherichia coli from routine urine specimens based on macroscopic criteria. Acta Pathol. Microbiol. Immunol. Scand. B 1985, 93, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, D.L. Therapeutic potential of peroxisome proliferator-activated receptor agonists for neurological disease. Diabetes Technol. Ther. 2003, 5, 67–73. [Google Scholar] [CrossRef]
- Luna-Medina, R.; Cortes-Canteli, M.; Alonso, M.; Santos, A.; Martinez, A.; Perez-Castillo, A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J. Biol. Chem. 2005, 280, 21453–21462. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Bueno, B.; Madrigal, J.L.; Lizasoain, I.; Moro, M.A.; Lorenzo, P.; Leza, J.C. Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol. Psychiatry 2005, 57, 885–894. [Google Scholar] [CrossRef]
- Garcia-Bueno, B.; Madrigal, J.L.; Lizasoain, I.; Moro, M.A.; Lorenzo, P.; Leza, J.C. The anti-inflammatory prostaglandin 15d-PGJ2 decreases oxidative/nitrosative mediators in brain after acute stress in rats. Psychopharmacology 2005, 180, 513–522. [Google Scholar] [CrossRef]
- Park, E.Y.; Cho, I.J.; Kim, S.G. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid X receptor heterodimer. Cancer Res. 2004, 64, 3701–3713. [Google Scholar] [CrossRef] [Green Version]
- Gaddam, R.R.; Chambers, S.; Bhatia, M. ACE and ACE2 in inflammation: A tale of two enzymes. Inflamm. Allergy Drug Targets 2014, 13, 224–234. [Google Scholar] [CrossRef]
- Coates, D. The angiotensin converting enzyme (ACE). Int. J. Biochem. Cell Biol. 2003, 35, 769–773. [Google Scholar] [CrossRef]
- Thompson, D.; Pepys, M.B.; Wood, S.P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 1999, 7, 169–177. [Google Scholar] [CrossRef]
- Khoury, N.M.; Marvar, P.J.; Gillespie, C.F.; Wingo, A.; Schwartz, A.; Bradley, B.; Kramer, M.; Ressler, K.J. The renin-angiotensin pathway in posttraumatic stress disorder: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J. Clin. Psychiatry 2012, 73, 849–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.A.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silveira, K.D.; Coelho, F.M.; Vieira, A.T.; Sachs, D.; Barroso, L.C.; Costa, V.V.; Bretas, T.L.; Bader, M.; de Sousa, L.P.; da Silva, T.A.; et al. Anti-inflammatory effects of the activation of the angiotensin-(1-7) receptor, MAS, in experimental models of arthritis. J. Immunol. 2010, 185, 5569–5576. [Google Scholar] [CrossRef] [Green Version]
- Feltenberger, J.D.; Andrade, J.M.; Paraiso, A.; Barros, L.O.; Filho, A.B.; Sinisterra, R.D.; Sousa, F.B.; Guimaraes, A.L.; de Paula, A.M.; Campagnole-Santos, M.J.; et al. Oral formulation of angiotensin-(1-7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension 2013, 62, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Khajah, M.A.; Fateel, M.M.; Ananthalakshmi, K.V.; Luqmani, Y.A. Anti-Inflammatory Action of Angiotensin 1-7 in Experimental Colitis. PLoS ONE 2016, 11, e0150861. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Li, T.; Zhou, G.S.; Chen, Y.; Yu, C.H.; Pang, M.X.; Li, W.; Li, Y.; Zhang, W.Y.; Li, X. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxid. Redox Signal. 2015, 22, 241–258. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, J.; Hao, P.; Chen, W.; Meng, X.; Li, H.; Zhang, Y.; Zhang, C.; Yang, J. Imbalance between angiotensin II and angiotensin-(1-7) in human coronary atherosclerosis. J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1470320316659618. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zeng, Z.; Cao, Y.; Liu, Y.; Ping, F.; Liang, M.; Xue, Y.; Xi, C.; Zhou, M.; Jiang, W. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-kappaB signaling pathways. Sci. Rep. 2016, 6, 27911. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, G.S.; Rodrigues-Machado, M.G.; Motta-Santos, D.; Silva, A.R.; Caliari, M.V.; Prata, L.O.; Abreu, S.C.; Rocco, P.R.; Barcelos, L.S.; Santos, R.A.; et al. Angiotensin-(1-7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation. Br. J. Pharmacol. 2015, 172, 2330–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues-Machado, M.G.; Magalhaes, G.S.; Cardoso, J.A.; Kangussu, L.M.; Murari, A.; Caliari, M.V.; Oliveira, M.L.; Cara, D.C.; Noviello, M.L.; Marques, F.D.; et al. AVE 0991, a non-peptide mimic of angiotensin-(1-7) effects, attenuates pulmonary remodelling in a model of chronic asthma. Br. J. Pharmacol. 2013, 170, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, G.S.; Barroso, L.C.; Reis, A.C.; Rodrigues-Machado, M.G.; Gregorio, J.F.; Motta-Santos, D.; Oliveira, A.C.; Perez, D.A.; Barcelos, L.S.; Teixeira, M.M.; et al. Angiotensin-(1-7) Promotes Resolution of Eosinophilic Inflammation in an Experimental Model of Asthma. Front. Immunol. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ena, P.; Madeddu, P.; Glorioso, N.; Cerimele, D.; Rappelli, A. High prevalence of cardiovascular diseases and enhanced activity of the renin-angiotensin system in psoriatic patients. Acta Cardiol. 1985, 40, 199–205. [Google Scholar]
- Kawajiri, M.; Mogi, M.; Higaki, N.; Matsuoka, T.; Ohyagi, Y.; Tsukuda, K.; Kohara, K.; Horiuchi, M.; Miki, T.; Kira, J.I. Angiotensin-converting enzyme (ACE) and ACE2 levels in the cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. 2009, 15, 262–265. [Google Scholar] [CrossRef]
- Sagawa, K.; Nagatani, K.; Komagata, Y.; Yamamoto, K. Angiotensin receptor blockers suppress antigen-specific T cell responses and ameliorate collagen-induced arthritis in mice. Arthritis Rheum. 2005, 52, 1920–1928. [Google Scholar] [CrossRef]
- Timmermans, S.; Bogie, J.F.; Vanmierlo, T.; Lutjohann, D.; Stinissen, P.; Hellings, N.; Hendriks, J.J. High fat diet exacerbates neuroinflammation in an animal model of multiple sclerosis by activation of the Renin Angiotensin system. J. Neuroimmune Pharmacol. 2014, 9, 209–217. [Google Scholar] [CrossRef]
- Torika, N.; Asraf, K.; Danon, A.; Apte, R.N.; Fleisher-Berkovich, S. Telmisartan Modulates Glial Activation: In Vitro and In Vivo Studies. PLoS ONE 2016, 11, e0155823. [Google Scholar] [CrossRef] [Green Version]
- Benter, I.F.; Yousif, M.H.; Al-Saleh, F.M.; Raghupathy, R.; Chappell, M.C.; Diz, D.I. Angiotensin-(1-7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-L-arginine methyl ester. J. Cardiovasc. Pharmacol. 2011, 57, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, S.; Jaffray, E.; Farrow, S.N.; Rossi, A.G.; Haslett, C.; Hay, R.T. Inhibition of NF-kappa B by a cell permeable form of I kappa B alpha induces apoptosis in eosinophils. Biochem. Biophys. Res. Commun. 2005, 326, 632–637. [Google Scholar] [CrossRef]
- Sousa, L.P.; Carmo, A.F.; Rezende, B.M.; Lopes, F.; Silva, D.M.; Alessandri, A.L.; Bonjardim, C.A.; Rossi, A.G.; Teixeira, M.M.; Pinho, V. Cyclic AMP enhances resolution of allergic pleurisy by promoting inflammatory cell apoptosis via inhibition of PI3K/Akt and NF-kappaB. Biochem. Pharmacol. 2009, 78, 396–405. [Google Scholar] [CrossRef]
- Sousa, L.P.; Lopes, F.; Silva, D.M.; Tavares, L.P.; Vieira, A.T.; Rezende, B.M.; Carmo, A.F.; Russo, R.C.; Garcia, C.C.; Bonjardim, C.A.; et al. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-kappaB-independent manner. J. Leukoc. Biol. 2010, 87, 895–904. [Google Scholar] [CrossRef]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New. Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Sethi, R.; Gomez-Coronado, N.; Walker, A.J.; Robertson, O.D.; Agustini, B.; Berk, M.; Dodd, S. Neurobiology and Therapeutic Potential of Cyclooxygenase-2 (COX-2) Inhibitors for Inflammation in Neuropsychiatric Disorders. Front. Psychiatry 2019, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Antman, E.M.; Bennett, J.S.; Daugherty, A.; Furberg, C.; Roberts, H.; Taubert, K.A.; American Heart, A. Use of nonsteroidal antiinflammatory drugs: An update for clinicians: A scientific statement from the American Heart Association. Circulation 2007, 115, 1634–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philp, R.B.; Arora, P.; McIver, D.J. Effects of gaseous anesthetics and ultrashort and short-acting barbiturates on human blood platelet free cytosolic calcium: Relevance to their effects on platelet aggregation. Can. J. Physiol. Pharmacol. 1992, 70, 1161–1166. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Kirkby, K.C.; Lichtblau, N. Inflammation and Immune Regulation as Potential Drug Targets in Antidepressant Treatment. Curr. Neuropharmacol. 2016, 14, 674–687. [Google Scholar] [CrossRef] [Green Version]
- Strawbridge, R.; Arnone, D.; Danese, A.; Papadopoulos, A.; Herane Vives, A.; Cleare, A.J. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1532–1543. [Google Scholar] [CrossRef]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Obuchowicz, E.; Bielecka, A.M.; Paul-Samojedny, M.; Pudelko, A.; Kowalski, J. Imipramine and fluoxetine inhibit LPS-induced activation and affect morphology of microglial cells in the rat glial culture. Pharmacol. Rep. 2014, 66, 34–43. [Google Scholar] [CrossRef]
- Himmerich, H.; Milenovic, S.; Fulda, S.; Plumakers, B.; Sheldrick, A.J.; Michel, T.M.; Kircher, T.; Rink, L. Regulatory T cells increased while IL-1beta decreased during antidepressant therapy. J. Psychiatr. Res. 2010, 44, 1052–1057. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, H.; Li, M.; Zhuang, J.; Peng, Y.; Zhou, H.; Xu, C.; Yu, Q.; Fu, X.; Cao, S.; et al. Autophagy protein NRBF2 attenuates endoplasmic reticulum stress-associated neuroinflammation and oxidative stress via promoting autophagosome maturation by interacting with Rab7 after SAH. J. Neuroinflamm. 2021, 18, 210. [Google Scholar] [CrossRef]
- Lu, J.; He, L.; Behrends, C.; Araki, M.; Araki, K.; Jun Wang, Q.; Catanzaro, J.M.; Friedman, S.L.; Zong, W.X.; Fiel, M.I.; et al. NRBF2 regulates autophagy and prevents liver injury by modulating Atg14L-linked phosphatidylinositol-3 kinase III activity. Nat. Commun. 2014, 5, 3920. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.Y.; Liu, L.; Wang, E.J.; Xiao, H.T.; Cai, C.Z.; Wang, J.; Su, H.; Wang, Y.; Tan, J.; Zhang, Z.; et al. PI3KC3 complex subunit NRBF2 is required for apoptotic cell clearance to restrict intestinal inflammation. Autophagy 2021, 17, 1096–1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cai, C.Z.; Song, J.X.; Tan, J.Q.; Durairajan, S.S.K.; Iyaswamy, A.; Wu, M.Y.; Chen, L.L.; Yue, Z.; Li, M.; et al. NRBF2 is involved in the autophagic degradation process of APP-CTFs in Alzheimer disease models. Autophagy 2017, 13, 2028–2040. [Google Scholar] [CrossRef]
- Sinha, P.; Verma, B.; Ganesh, S. Trehalose Ameliorates Seizure Susceptibility in Lafora Disease Mouse Models by Suppressing Neuroinflammation and Endoplasmic Reticulum Stress. Mol. Neurobiol. 2021, 58, 1088–1101. [Google Scholar] [CrossRef]
- Acosta, S.A.; Tajiri, N.; Hoover, J.; Kaneko, Y.; Borlongan, C.V. Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke. Stroke 2015, 46, 2616–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.S.; Jeong, S.Y.; Yang, J.; Kim, S.D.; Zhang, B.; Yoo, H.S.; Song, S.U.; Jeon, M.S.; Song, Y.S. Neuroprotective effect of mesenchymal stem cell through complement component 3 downregulation after transient focal cerebral ischemia in mice. Neurosci. Lett. 2016, 633, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Ryu, J.K.; McLarnon, J.G. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J. Cell. Mol. Med. 2009, 13, 2911–2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [Green Version]
- Musa, N.H.; Mani, V.; Lim, S.M.; Vidyadaran, S.; Abdul Majeed, A.B.; Ramasamy, K. Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 2017, 84, 488–495. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]

| Types of Treatments | Mechanism | Typical Drugs |
|---|---|---|
| ACE inhibitor and ARB | Inhibits converting enzymes that activate Ang II, or converts Ang II into Ang(1–7), which exhibits an opposite effect | Benazepril, captopril, enalapril, lisinopril, perindopril, ramipril, trandolapril |
| Antidepressant | Stress suppression, or up-regulation of Treg cells that limit cytokines | Amitriptyline (Elavil), fluoxetine, imipramine, paroxetine |
| COX-2 inhibitor | Direct inhibition of COX-2, which is important for inflammatory reactions, improves neuroinflammatory pathways, and improves depression | celecoxib, etoricoxib, rofecoxib |
| NMDA receptor antagonist | Reduces oxidative and NO damage by inhibiting excessive glutamate in cells and improves neuroinflammation | dizocilpine |
| PPAR agonist | Prevents various post-stress neuroinflammatory pathways by inhibiting NF-κB, TNF-α, COX-2 and iNOS when injecting agents. | ciglitazone, rosiglitazone, troglitazone, pioglitazone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Lee, J.-Y.; Hong, D.-Y.; Lee, E.-C.; Park, S.-W.; Lee, Y.-K.; Oh, J.-S. Pharmacological Treatment for Neuroinflammation in Stress-Related Disorder. Biomedicines 2022, 10, 2518. https://doi.org/10.3390/biomedicines10102518
Lee D-H, Lee J-Y, Hong D-Y, Lee E-C, Park S-W, Lee Y-K, Oh J-S. Pharmacological Treatment for Neuroinflammation in Stress-Related Disorder. Biomedicines. 2022; 10(10):2518. https://doi.org/10.3390/biomedicines10102518
Chicago/Turabian StyleLee, Dong-Hun, Ji-Young Lee, Dong-Yong Hong, Eun-Chae Lee, Sang-Won Park, Yun-Kyung Lee, and Jae-Sang Oh. 2022. "Pharmacological Treatment for Neuroinflammation in Stress-Related Disorder" Biomedicines 10, no. 10: 2518. https://doi.org/10.3390/biomedicines10102518






