Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy
Abstract
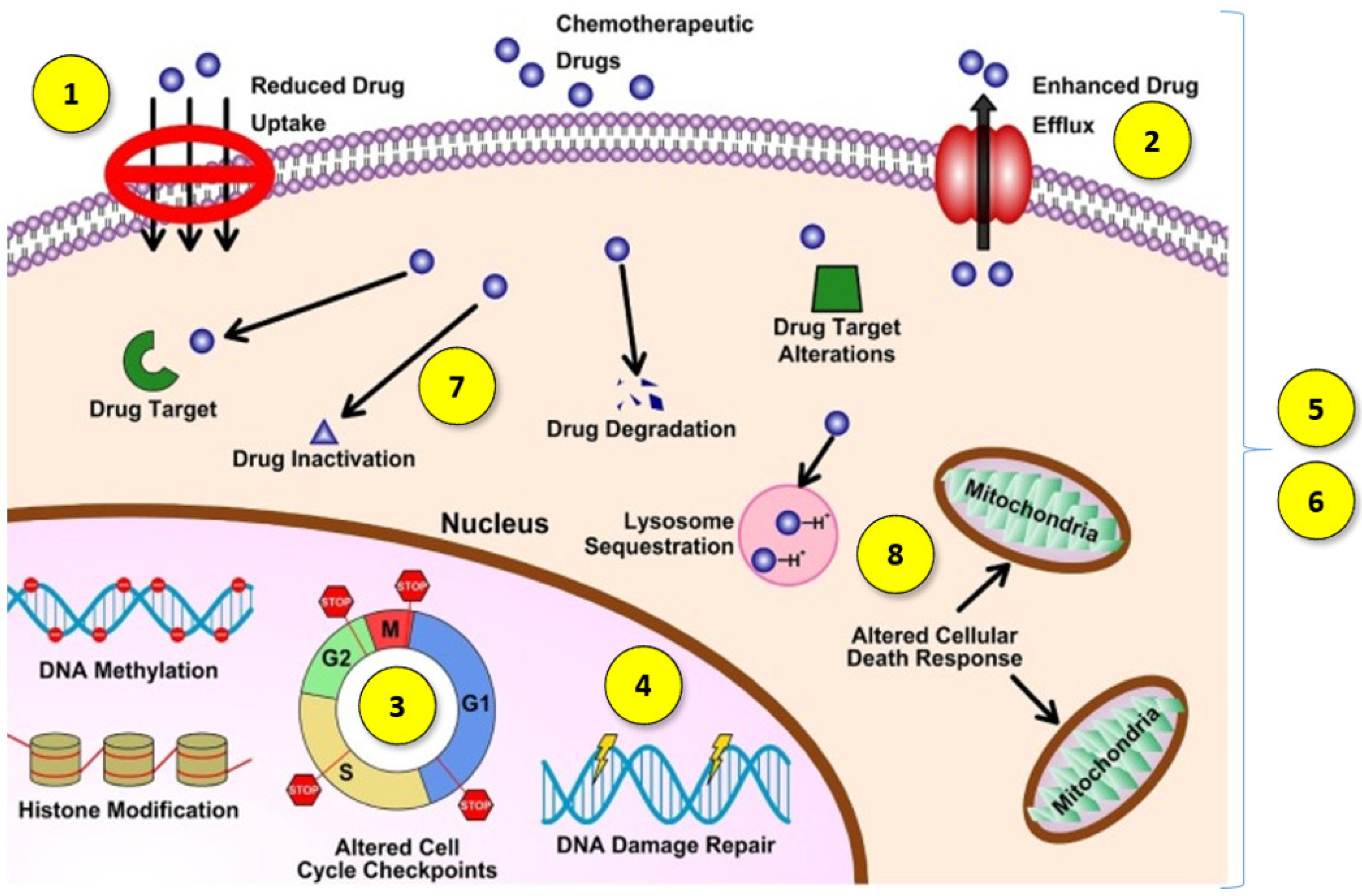
1. Introduction
- (1)
- Decreased drug absorption by cancer cells [5];
- (2)
- The increased expression of certain ATP-binding cassette efflux transporters, including P-glycoprotein (P-gp/ABCB1), multidrug resistance protein 1 (MRP1/ABCC1) and BCRP (ABCG2), which lower the cytosolic concentration of the active agents through increased drug transportation outside the cell [4];
- (3)
- The impaired function of pro-apoptotic factors, resulting in cancer cells avoiding programmed death [6];
- (4)
- A better ability to repair damaged DNA [6];
- (5)
- Qualitative or quantitative changes in specific cell targets [5];
- (6)
- Changes that allow cancer cells to tolerate adverse or stressful conditions caused by treatment with antineoplastic agents by transforming them into less effective or inactive metabolites [7];
- (7)
- An increasing in the efficiency of the metabolism and biotransformation of cytostatic drugs, leading to their conversion into metabolites without cytostatic effect [8];
- (8)
- The intracellular and intercellular sequestration of drugs in well-defined organelles away from the cellular target, including the lysosomal compartmentalization of hydrophobic, weakly basic anticancer drugs [9].
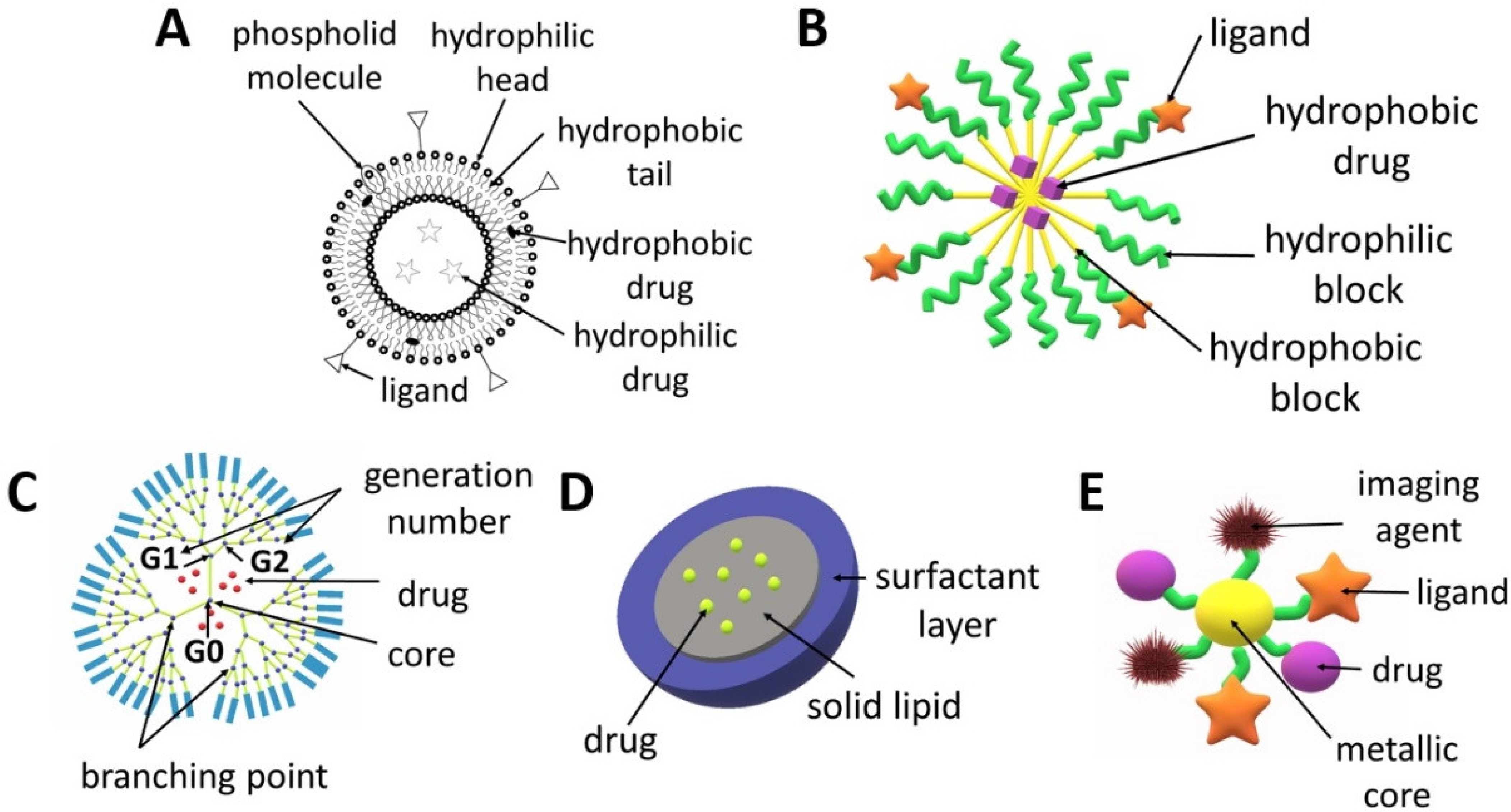
2. Nanoparticles for Drug Delivery Application
3. Docetaxel
3.1. Docetaxel as an Anticancer Drug
3.2. Docetaxel Drug Delivery Systems
3.2.1. Carriers for Co-Delivery of Dtx with Another Drug
3.2.2. Clinical Trials of Docetaxel Nanoparticles
3.3. Summary
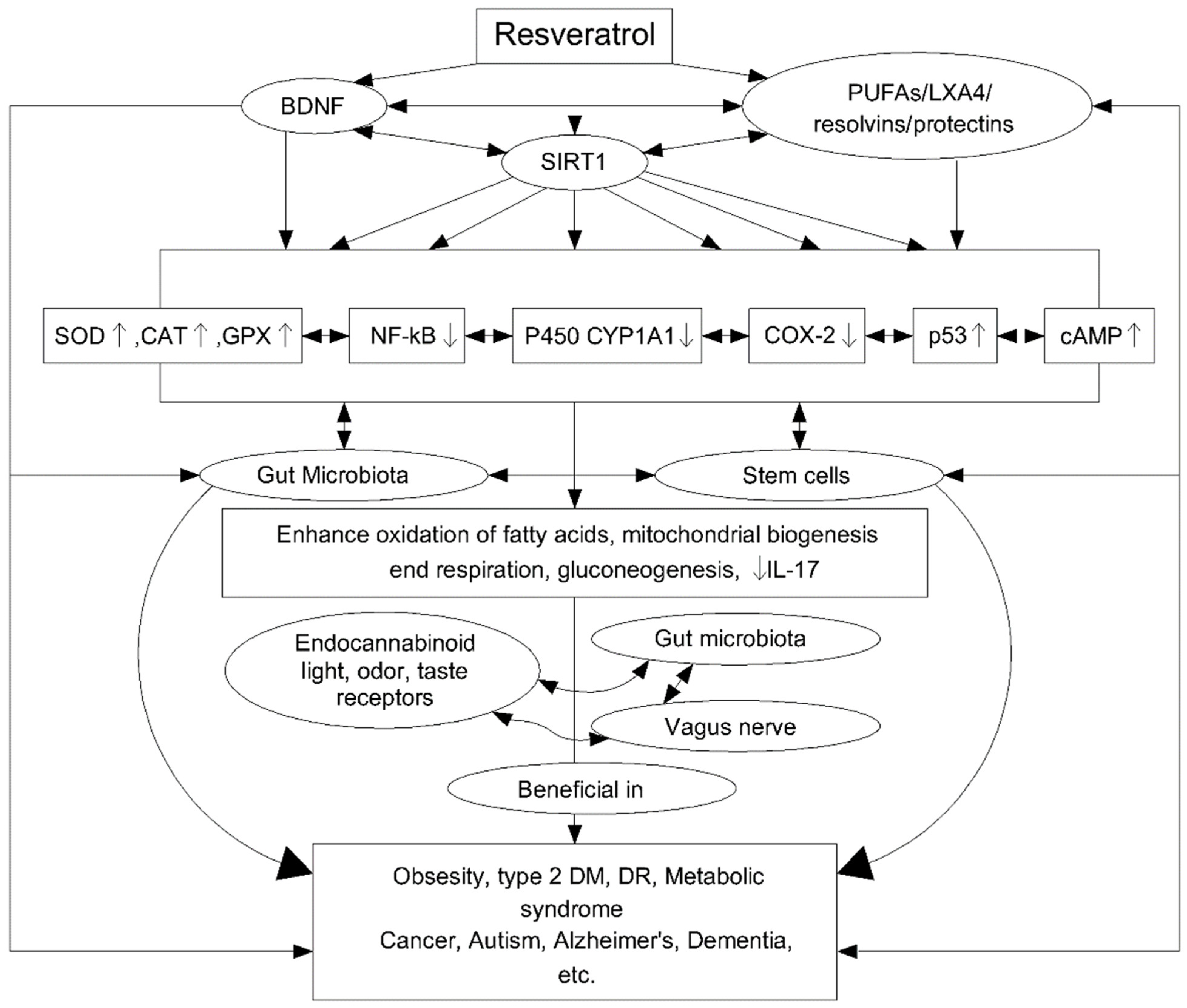
4. Resveratrol
4.1. Resveratrol as an Active Agent
4.2. RES-Loaded Nanoparticles
4.3. Resveratrol Clinical Trials
4.4. Summary
5. Co-Delivery of Docetaxel and Resveratrol
5.1. Synergistic Effect of Docetaxel with Resveratrol
5.2. Nanoparticles for Co-Delivery of Docetaxel and Resveratrol
5.3. Summary
6. Challenges and Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4T1 cells | murine mammary carcinoma cells |
| γ-PGA | poly(γ-glutamic acid) |
| A549 cells | human epithelial carcinoma cell line |
| ACUPA | ((S)-2-(3-((S)-5-amino-1-carboxypentyl) ureido) pentanedioic acid |
| AMPK | AMP-activated protein kinase |
| AP-1 | activator protein-1 |
| ATRA | all-trans-retinoic acid |
| Au | gold |
| B16F10 cells | melanoma lung metastasis cell line |
| B6 cells | melanoma lung metastasis cell line |
| A375 cells | melanoma lung metastasis cell line |
| BSA | bovine serum albumin |
| BT-474 cells | human breast cancer cell line |
| CA | caffeic acid |
| Caco-2 cells | colon carcinoma cell line |
| Cap | calcium phosphate |
| CB | carbon black |
| CHOL | cholesterol |
| cho-CpG | cholesterol-modified Toll-like receptor 9 (TLR9) agonist oligonucleotide |
| COX | cyclooxygenase enzymes |
| COX-2 | cyclooxygenase-2 enzymes |
| CS | chitosan |
| CRPC cells | castration-resistant prostate cancer cell line |
| CT26 cells | murine colorectal carcinoma cell line |
| Cur | curcumin |
| DA | deoxycholic acid |
| DDS | drug delivery system |
| DGC | N-deoxycholic acid glycol chitosan |
| Dha | dihydroartemisin |
| DLT | dose limiting toxicity |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(poly(ethylene glycol)-2000] |
| Dtx | docetaxel |
| Dtx-CCL-PMs | docetaxel-entrapped core-cross-linked polymeric micelles |
| DU-145 cells | docetaxel-resistant prostate carcinoma cell line |
| EGFR | epidermal growth factor receptor |
| eSM | egg sphingomyelin |
| FA | folic acid |
| FDA | Food and Drug Administration |
| FRα | folic acid α receptor |
| fWGA | fluorescein-labelled wheat germ agglutinin |
| G-CSF | granulocyte colony-stimulating factors |
| Gba | galbanic acid |
| GNRs | gold nanorods |
| GX1 | gastric cancer angiogenesis marker peptide |
| H520 cell line | human lung squamous carcinoma cell lines |
| HA | hyaluronic acid |
| HCC827 cells | non-small cell lung cancer cell line |
| hCMEC/D3 cells | brain microvascular endothelial cell line |
| HepG2 cells | human hepatocellular carcinoma cell line |
| HIF-1α | hypoxia-induced factor |
| HSA | human serum albumin |
| HSPC | hydrogenated phosphatidylcholine |
| HSR | hypersensitivity reaction |
| HT-29 cells | human colorectal adenocarcinoma cell line |
| HUVEC cells | human umbilical vein endothelial cell line |
| IL-17 | interleukin-17 |
| LNCaP | prostate cancer cells |
| LPNPs | lipid-polymer nanoparticles |
| MAPK | p38-Mitogen activated protein kinase |
| MC-38 cells | murine colon adenocarcinoma cell line |
| MCF-7 | human breast carcinoma cell line |
| MCF-10A cells | non-malignant breast epithelial cell line |
| MDA-MB-231 cells | human breast adenocarcinoma cell line |
| MDA-MB-453 cells | human breast adenocarcinoma cell line |
| MDR | multidrug resistance |
| mEHT | modulated electro-hyperthermia |
| MMPs | metalloproteinases |
| mPEG-PDLA | methoxyl poly(ethylene glycol)-poly(D,L-lactide) |
| MTD | maximum tolerated dose |
| Myrj52 | polyoxyethylene (40) stearate |
| NCIH2135 cells | non-small cell lung cancer cell line |
| NF-κB | nuclear transcription factor-κB |
| NIR | near-infrared radiation |
| NPs | nanoparticles |
| NSCLC | non-small cell lung cancer |
| P-gp | P-glycoprotein |
| PAMAM | poly(amidoamine) |
| PAMAMOS | PAMAM-organosilicon |
| PBAE | poly (β-amino ester) |
| PBM | planetary ball milled |
| PBMC | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| PC-3 cells | human caucasian prostate adenocarcinoma cell line |
| PC-3–R cells | resistance human caucasian prostate adenocarcinoma cell line |
| PCL | poly(ε-caprolactone) |
| PEG | poly(ethylene glycol) |
| PDLA | poly(D,L-lactide) |
| PLA | poly(lactide) |
| PLGA | poly(lactide-co-glycolide) |
| PLGA-ATRA | poly(lactide-co-glycolide) all-trans-retinoic acid |
| PM | platelet membrane |
| PNT2 cells | normal human prostate cell line |
| PPI | poly(propylene imine) |
| PTT | photo-thermal therapy |
| Ptx | paclitaxel |
| Res | resveratrol |
| Res-AuNPs | Res-loaded gold nanoparticles |
| RLT | low-density lipoprotein receptor (LDLR)-binding peptide |
| ROS | reactive oxygen species |
| SA | stearic acid |
| SHBG | sex steroid hormone-binding globulin |
| SIRT1 | sirtuins signalling pathway |
| SKBR-3 cells | human breast adenocarcinoma cell line |
| SPC | phosphatidylcholine |
| SLNPs | solid lipid nanoparticles |
| STTP | chitosan and sodium tripolyphosphate |
| TF | transferrin |
| TNF-α | tumour necrosis factor |
| VEGF | vascular endothelial growth factor |
| Zol | zoledronate |
References
- IARC. World Cancer Report 2020; IARC: Lyon, France, 2020. [Google Scholar]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.-S.; Liu, H.-M. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med. Res. Rev. 2021, 41, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Dong, S.; Zhao, S.-C.; Liu, K.; Tan, Y.; Jiang, X.; Assaraf, Y.G.; Qin, B.; Chen, Z.-S.; Zou, C. Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist. Updates 2021, 58, 100777. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wu, Z.-X.; Assaraf, Y.G.; Chen, Z.-S.; Wang, L. Overcoming anti-cancer drug resistance via restoration of tumor suppressor gene function. Drug Resist. Updates 2021, 57, 100770. [Google Scholar] [CrossRef]
- Merlos Rodrigo, M.A.; Jimenez Jimemez, A.M.; Haddad, Y.; Bodoor, K.; Adam, P.; Krizkova, S.; Heger, Z.; Adam, V. Metallothionein isoforms as double agents—Their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist. Updates 2020, 52, 100691. [Google Scholar] [CrossRef]
- McLellan, L.I.; Wolf, C.R. Glutathione and glutathione-dependent enzymes in cancer drug resistance. Drug Resist. Updates 1999, 2, 153–164. [Google Scholar] [CrossRef]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist. Updates 2016, 24, 23–33. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Sharma, I.; Hannay, N.; Sridhar, S.; Ahmad, S.; Basha, R. Chapter 15—Future perspectives and new directions in chemosensitizing activities to reverse drug resistance in gynecologic cancers: Emphasis on challenges and opportunities. In Cancer Sensitizing Agents for Chemotherapy; Basha, R., Ahmad, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 17, pp. 339–355. ISBN 24683183. [Google Scholar]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Poltavets, Y.I.; Zhirnik, A.S.; Zavarzina, V.V.; Semochkina, Y.P.; Shuvatova, V.G.; Krasheninnikova, A.A.; Aleshin, S.V.; Dronov, D.O.; Vorontsov, E.A.; Balabanyan, V.Y.; et al. In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138. [Google Scholar] [CrossRef] [PubMed]
- Abed, S.N.; Deb, P.K.; Surchi, H.S.; Kokaz, S.F.; Jamal, S.M.; Bandopadhyay, S.; Tekade, R.K. Chapter 17—Nanocarriers in Different Preclinical and Clinical Stages. In Advances in Pharmaceutical Product Development and Research; Tekade, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 685–731. ISBN 978-0-12-817909-3. [Google Scholar]
- Chung, I.-M.; Subramanian, U.; Thirupathi, P.; Venkidasamy, B.; Samynathan, R.; Gangadhar, B.H.; Rajakumar, G.; Thiruvengadam, M. Resveratrol Nanoparticles: A Promising Therapeutic Advancement over Native Resveratrol. Processes 2020, 8, 458. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Liu, B. Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics 2019, 11, 381. [Google Scholar] [CrossRef]
- Arias, J.L.; Clares, B.; Morales, M.E.; Gallardo, V.; Ruiz, M.A. Lipid-based drug delivery systems for cancer treatment. Curr. Drug Targets 2011, 12, 1151–1165. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Tiwari, S.K.; Mohapatra, S.; Thomas, S. Chapter 1—Efficient Nanocarriers for Drug-Delivery Systems: Types and Fabrication. In Micro and Nano Technologies; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–41. ISBN 978-0-12-814033-8. [Google Scholar]
- Lorente, C.; Cabeza, L.; Clares, B.; Ortiz, R.; Halbaut, L.; Delgado, Á.V.; Perazzoli, G.; Prados, J.; Arias, J.L.; Melguizo, C. Formulation and in vitro evaluation of magnetoliposomes as a potential nanotool in colorectal cancer therapy. Colloids Surf. B Biointerfaces 2018, 171, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.; Keyikoglu, R.; Atalay Gengec, N.; Vatanpour, V.; Khataee, A. A review on dendrimers in preparation and modification of membranes: Progress, applications, and challenges. Mater. Today Chem. 2022, 23, 100683. [Google Scholar] [CrossRef]
- Arseneault, M.; Wafer, C.; Morin, J.-F. Recent Advances in Click Chemistry Applied to Dendrimer Synthesis. Molecules 2015, 20, 9263. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H. Advances in development of the dendrimers having natural saccharides in their structure for efficient and controlled drug delivery applications. Eur. Polym. J. 2021, 148, 110356. [Google Scholar] [CrossRef]
- Moughton, A.O.; Hillmyer, M.A.; Lodge, T.P. Multicompartment Block Polymer Micelles. Macromolecules 2012, 45, 2–19. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef]
- Di, Y.; Li, T.; Zhu, Z.; Chen, F.; Jia, L.; Liu, W.; Gai, X.; Wang, Y.; Pan, W.; Yang, X. pH-sensitive and folic acid-targeted MPEG-PHIS/FA-PEG-VE mixed micelles for the delivery of PTX-VE and their antitumor activity. Int. J. Nanomed. 2017, 12, 5863–5877. [Google Scholar] [CrossRef][Green Version]
- Hao, W.; Wang, T.; Liu, D.; Shang, Y.; Zhang, J.; Xu, S.; Liu, H. Folate-conjugated pH-controllable fluorescent nanomicelles acting as tumor targetable drug carriers. Mikrochim. Acta 2017, 184, 2881–2891. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhai, G.-X. Intelligent polymeric micelles: Development and application as drug delivery for docetaxel. J. Drug Target. 2017, 25, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Chokamonsirikun, A.; Phattaravorakarn, V.; Tiyaboonchai, W. Polymeric micelles for pulmonary drug delivery: A comprehensive review. J. Mater. Sci. 2021, 56, 2016–2036. [Google Scholar] [CrossRef]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Wu, X.; El Ghzaoui, A.; Li, S. Anisotropic self-assembling micelles prepared by the direct dissolution of PLA/PEG block copolymers with a high PEG fraction. Langmuir 2011, 27, 8000–8008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jackson, J.K.; Burt, H.M. Development of Amphiphilic Diblock Copolymers as Micellar Carriers of Taxol. Int. J. Pharm. 1996, 132, 195. [Google Scholar] [CrossRef]
- Burt, H.M.; Zhang, X.; Toleikis, P.; Embree, L.; Hunter, W.L. Development of copolymers of poly(d,l-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B Biointerfaces 1999, 16, 161–171. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yun, P.; Shen, X.; Su, F.; Chen, Y.; Li, S.; Song, D. Self-assembled filomicelles prepared from polylactide-poly(ethylene glycol) diblock copolymers for sustained delivery of cycloprotoberberine derivatives. Saudi Pharm. J. 2018, 26, 342–348. [Google Scholar] [CrossRef]
- Jelonek, K.; Li, S.; Wu, X.; Kasperczyk, J.; Marcinkowski, A. Self-assembled filomicelles prepared from polylactide/poly(ethylene glycol) block copolymers for anticancer drug delivery. Int. J. Pharm. 2015, 485, 357–364. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric Micelles: Recent Advancements in the Delivery of Anticancer Drugs. Pharm. Res. 2016, 33, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, K.; Zhu, W.; Deng, W.; Lam, K.S. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv. Drug Deliv. Rev. 2014, 66, 58–73. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Liu, G.; Luo, Z.; Zhou, L.; Xue, Y.; Liu, M. Recent progress in the applications of gold-based nanoparticles towards tumor-targeted imaging and therapy. RSC Adv. 2022, 12, 7635–7651. [Google Scholar] [CrossRef]
- Rand, D.; Ortiz, V.; Liu, Y.; Derdak, Z.; Wands, J.R.; Tatíček, M.; Rose-Petruck, C. Nanomaterials for X-ray Imaging: Gold Nanoparticle Enhancement of X-ray Scatter Imaging of Hepatocellular Carcinoma. Nano Lett. 2011, 11, 2678–2683. [Google Scholar] [CrossRef]
- Xu, J.-J.; Zhang, W.-C.; Guo, Y.-W.; Chen, X.-Y.; Zhang, Y.-N. Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv. 2022, 29, 664–678. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R. Solid lipid nanocarriers in drug delivery: Characterization and design. Expert Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Uchechi, O. Nanoparticles for Dermal and Transdermal Drug Delivery. In Application of Nanotechnology in Drug Delivery; Ogbonna, J.D.N., Ed.; IntechOpen: Rijeka, Croatia, 2014; Chapter 6. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Abraxane. Pharm. Times 2014, 80, 44.
- Barenholz, Y. (Chezy) Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Leonard, R.C.F.; Williams, S.; Tulpule, A.; Levine, A.M.; Oliveros, S. Improving the therapeutic index of anthracycline chemotherapy: Focus on liposomal doxorubicin (MyocetTM). Breast 2009, 18, 218–224. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef]
- de Weger, V.A.; Beijnen, J.H.; Schellens, J.H.M. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—A review. Anticancer. Drugs 2014, 25, 488–494. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A.; Fenton, C. Docetaxel. Drugs 2005, 65, 2513–2531. [Google Scholar] [CrossRef]
- Hitt, R.; Lopez-Martin, A. Docetaxel BT—Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1409–1412. ISBN 978-3-662-46875-3. [Google Scholar]
- Baker, S.D.; Sparreboom, A.; Verweij, J. Clinical Pharmacokinetics of Docetaxel. Clin. Pharmacokinet. 2006, 45, 235–252. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, N.; Liu, D.; Song, L.; Liu, T.; Li, S.; Zhao, Y. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo-and chemotherapy. Int. J. Nanomed. 2017, 12, 7869. [Google Scholar] [CrossRef]
- Eloy, J.O.; Ruiz, A.; de Lima, F.T.; Petrilli, R.; Raspantini, G.; Nogueira, K.A.B.; Santos, E.; de Oliveira, C.S.; Borges, J.C.; Marchetti, J.M.; et al. EGFR-targeted immunoliposomes efficiently deliver docetaxel to prostate cancer cells. Colloids Surf. B Biointerfaces 2020, 194, 111185. [Google Scholar] [CrossRef] [PubMed]
- Wenbo, Y.; Lan, H.A.O.; Zhigang2, W.; Ronggui, Z. CREKA peptide-modified docetaxel loaded liposomes for targeted treatment of prostate cancer in vitro. Di 3 Jun Yi Da Xue Xue Bao 2020, 42, 2017–2115. [Google Scholar]
- Aires Fernandes, M.O.; Eloy, J.; Tavares Luiz, M.; Ramos Junior, S.L.; Borges, J.C.; Rodríguez de la Fuente, L.; Ortega-de San Luis, C.; Maldonado Marchetti, J.; Santos-Martinez, M.J.; Chorilli, M. Transferrin-functionalized liposomes for docetaxel delivery to prostate cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125806. [Google Scholar] [CrossRef]
- Bi, X.; Yuan, Q.; Han, J.; Cong, W.; Ge, Y.; Ma, D.; Dai, Z.; Li, Y. Docetaxel-loaded solid lipid nanoparticles suppress breast cancer cells growth with reduced myelosuppression toxicity. Int. J. Nanomed. 2014, 9, 4829. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Maiti, S.; Lee, J.H.; Lee, J.H.; Kim, J.S. Rational design of biotin–disulfide–coumarin conjugates: A cancer targeted thiol probe and bioimaging. Chem. Commun. 2014, 50, 3044–3047. [Google Scholar] [CrossRef]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef]
- Kim, K.S.; Youn, Y.S.; Bae, Y.H. Immune-triggered cancer treatment by intestinal lymphatic delivery of docetaxel-loaded nanoparticle. J. Control. Release 2019, 311–312, 85–95. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, A.; Huang, X.; Ying, Y.; Shu, X.; Chen, X.; Yang, Y.; Ma, J.; Lin, G.; Wang, X.; et al. Docetaxel-loaded PAMAM-based poly (γ-benzyl-l-glutamate)-b-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles in human breast cancer and human cervical cancer therapy. J. Microencapsul. 2019, 36, 552–565. [Google Scholar] [CrossRef]
- Bai, S.-B.; Cheng, Y.; Liu, D.-Z.; Ji, Q.-F.; Liu, M.; Zhang, B.-L.; Mei, Q.-B.; Zhou, S.-Y. Bone-targeted PAMAM nanoparticle to treat bone metastases of lung cancer. Nanomedicine 2020, 15, 833–849. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent Conjugates of Anticancer Drugs and Monoclonal Antibody with PAMAM Dendrimers to Increase Efficacy of HER-2 Positive Breast Cancer Therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef]
- Song, S.Y.; Kim, K.-P.; Jeong, S.-Y.; Park, J.; Park, J.; Jung, J.; Chung, H.K.; Lee, S.-W.; Seo, M.H.; Lee, J.-S.; et al. Polymeric nanoparticle-docetaxel for the treatment of advanced solid tumors: Phase I clinical trial and preclinical data from an orthotopic pancreatic cancer model. Oncotarget 2016, 7, 77348–77357. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.I.; Ayub, A.D.; Mat Yusuf, S.N.; Yahaya, N.; Abd Kadir, E.; Lim, V. Docetaxel-Loaded Disulfide Cross-Linked Nanoparticles Derived from Thiolated Sodium Alginate for Colon Cancer Drug Delivery. Pharmaceutics 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, X.; Zhao, S.; Qiu, H.; Han, M.; Li, J.; Zhao, N.; Wang, R.; Guo, Y. The influence of nanocarrier architectures on antitumor efficacy of docetaxel nanoparticles. RSC Adv. 2020, 10, 11074–11078. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Ji, J.; Li, L.; Zhai, G. Redox-responsive nanoparticles based on Chondroitin Sulfate and Docetaxel prodrug for tumor targeted delivery of Docetaxel. Carbohydr. Polym. 2021, 255, 117393. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int. J. Biol. Macromol. 2019, 126, 662–672. [Google Scholar] [CrossRef]
- Kolluru, L.P.; Chandran, T.; Shastri, P.N.; Rizvi, S.A.A.; D’Souza, M.J. Development and evaluation of polycaprolactone based docetaxel nanoparticle formulation for targeted breast cancer therapy. J. Nanopart. Res. 2020, 22, 372. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Duan, J.; Yang, X.-D. Targeted Treatment of Colon Cancer with Aptamer-Guided Albumin Nanoparticles Loaded with Docetaxel. Int. J. Nanomed. 2020, 15, 6737–6748. [Google Scholar] [CrossRef] [PubMed]
- Scheetz, L.M.; Yu, M.; Li, D.; Castro, M.G.; Moon, J.J.; Schwendeman, A. Synthetic HDL Nanoparticles Delivering Docetaxel and CpG for Chemoimmunotherapy of Colon Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1777. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Madni, A.; Rehman, M.; Rahim, M.A.; Jabar, A. Ionically Cross-Linked Chitosan Nanoparticles for Sustained Delivery of Docetaxel: Fabrication, Post-Formulation and Acute Oral Toxicity Evaluation. Int. J. Nanomed. 2019, 14, 10035–10046. [Google Scholar] [CrossRef]
- Liu, N.; Ji, J.; Qiu, H.; Shao, Z.; Wen, X.; Chen, A.; Yao, S.; Zhang, X.; Yao, H.; Zhang, L. Improving radio-chemotherapy efficacy of prostate cancer by co-deliverying docetaxel and dbait with biodegradable nanoparticles. Artif. Cells Nanomed. Biotechnol. 2020, 48, 305–314. [Google Scholar] [CrossRef]
- Atrafi, F.; Dumez, H.; Mathijssen, R.H.J.; Menke van der Houven van Oordt, C.W.; Rijcken, C.J.F.; Hanssen, R.; Eskens, F.A.L.M.; Schöffski, P. A phase I dose-escalation and pharmacokinetic study of a micellar nanoparticle with entrapped docetaxel (CPC634) in patients with advanced solid tumours. J. Control. Release 2020, 325, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Thambiraj, S.; Shruthi, S.; Vijayalakshmi, R.; Ravi Shankaran, D. Evaluation of cytotoxic activity of docetaxel loaded gold nanoparticles for lung cancer drug delivery. Cancer Treat. Res. Commun. 2019, 21, 100157. [Google Scholar] [CrossRef] [PubMed]
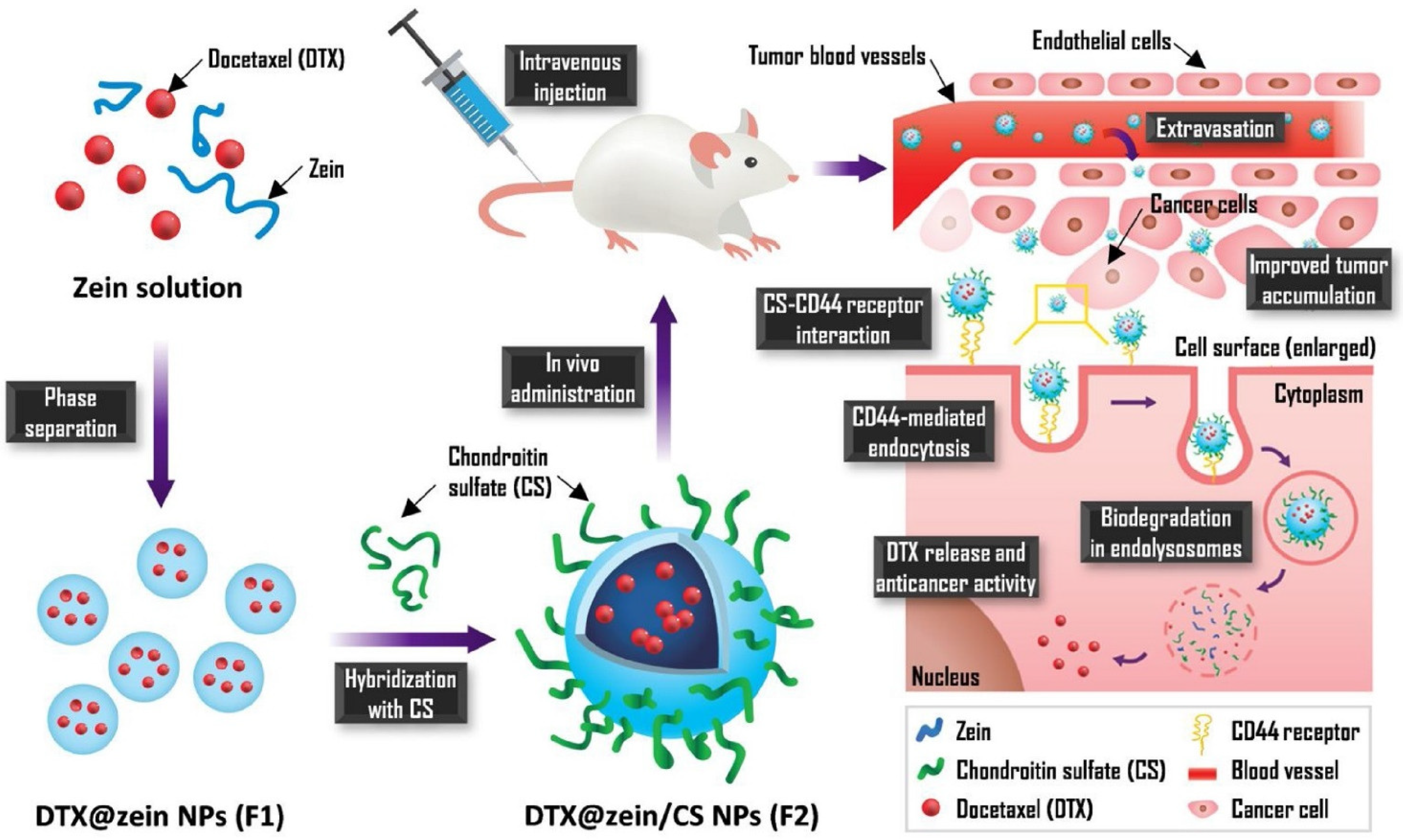
- Lee, H.S.; Kang, N.-W.; Kim, H.; Kim, D.H.; Chae, J.; Lee, W.; Song, G.Y.; Cho, C.-W.; Kim, D.-D.; Lee, J.-Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr. Polym. 2021, 253, 117187. [Google Scholar] [CrossRef]
- Karimi, N.; Soleiman-Beigi, M.; Fattahi, A. Co-delivery of all-trans-retinoic acid and docetaxel in drug conjugated polymeric nanoparticles: Improving controlled release and anticancer effect. Mater. Today Commun. 2020, 25, 101280. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, D.; Liu, M.; Ji, Q.; Mei, Q.; Cheng, Y.; Zhou, S. Bone-Targeted Calcium Phosphate-Polymer Hybrid Nanoparticle Co-Deliver Zoledronate and Docetaxel to Treat Bone Metastasis of Prostate Cancer. J. Pharm. Sci. 2021, 110, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Diao, L.; Chen, F.; Shen, A.; Wang, S.; Jin, H.; Cai, D.; Hu, Y. pH-Sensitive Nanoparticles Codelivering Docetaxel and Dihydroartemisinin Effectively Treat Breast Cancer by Enhancing Reactive Oxidative Species-Mediated Mitochondrial Apoptosis. Mol. Pharm. 2021, 18, 74–86. [Google Scholar] [CrossRef]
- Afsharzadeh, M.; Hashemi, M.; Babaei, M.; Abnous, K.; Ramezani, M. PEG-PLA nanoparticles decorated with small-molecule PSMA ligand for targeted delivery of galbanic acid and docetaxel to prostate cancer cells. J. Cell. Physiol. 2020, 235, 4618–4630. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, F.; Liu, H.; Feng, S.; Zhang, Y.; Zhou, D.; Zhang, R. Docetaxel-loaded biomimetic nanoparticles for targeted lung cancer therapy in vivo. J. Nanopart. Res. 2019, 21, 144. [Google Scholar] [CrossRef]
- Rodallec, A.; Brunel, J.-M.; Giacometti, S.; Maccario, H.; Correard, F.; Mas, E.; Orneto, C.; Savina, A.; Bouquet, F.; Lacarelle, B.; et al. Docetaxel-trastuzumab stealth immunoliposome: Development and in vitro proof of concept studies in breast cancer. Int. J. Nanomed. 2018, 13, 3451–3465. [Google Scholar] [CrossRef]
- Deeken, J.F.; Slack, R.; Weiss, G.J.; Ramanathan, R.K.; Pishvaian, M.J.; Hwang, J.; Lewandowski, K.; Subramaniam, D.; He, A.R.; Cotarla, I.; et al. A phase I study of liposomal-encapsulated docetaxel (LE-DT) in patients with advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 2013, 71, 627–633. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; LoRusso, P.M.; Burris, H.A.; Hart, L.L.; Low, S.C.; Parsons, D.M.; et al. Phase I Study of PSMA-Targeted Docetaxel-Containing Nanoparticle BIND-014 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Rijcken, C.J.; Bansal, R.; Hennink, W.E.; Storm, G.; Prakash, J. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials 2015, 53, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; de Bruijn, P.; Atrafi, F.; van Geijn, M.; Rijcken, C.J.F.; Mathijssen, R.H.J.; Koolen, S.L.W. A new method for the determination of total and released docetaxel from docetaxel-entrapped core-crosslinked polymeric micelles (CriPec®) by LC–MS/MS and its clinical application in plasma and tissues in patients with various tumours. J. Pharm. Biomed. Anal. 2018, 161, 168–174. [Google Scholar] [CrossRef]
- Ahmadi, R.; Ebrahimzadeh, M.A. Resveratrol—A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem. 2020, 200, 112356. [Google Scholar] [CrossRef]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 6, 2084. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011, 1215, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Das, D.K. Resveratrol and chemoprevention. Cancer Lett. 2009, 284, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araújo, G.R.; Vieira, A.; Chaves, M.M.; Lara, R.C. Antioxidant effect of Resveratrol: Change in MAPK cell signaling pathway during the aging process. Arch. Gerontol. Geriatr. 2021, 92, 104266. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, H.-m; Han, Y.; Zhang, Y. li Analgesic effect of resveratrol on colitis-induced visceral pain via inhibition of TRAF6/NF-κB signaling pathway in the spinal cord. Brain Res. 2019, 1724, 146464. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Milajerdi, A.; Sheikhi, A.; Kord-Varkaneh, H.; Feinle-Bisset, C.; Larijani, B.; Esmaillzadeh, A. Resveratrol supplementation significantly influences obesity measures: A systematic review and dose–response meta-analysis of randomized controlled trials. Obes. Rev. 2019, 20, 487–498. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Xie, Y.; Ding, Y.; Kong, D.; Yu, H. Research Progress on Alzheimer’s Disease and Resveratrol. Neurochem. Res. 2020, 45, 989–1006. [Google Scholar] [CrossRef]
- Xia, D.; Sui, R.; Zhang, Z. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J. Cell. Biochem. 2019, 120, 4942–4951. [Google Scholar] [CrossRef]
- Repossi, G.; Das, U.N.; Eynard, A.R. Molecular Basis of the Beneficial Actions of Resveratrol. Arch. Med. Res. 2020, 51, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, A.M.; Mahmoud, A.M.; El-Sherbiny, G.A.; El-Moselhy, M.A.; Nofal, S.M.; El-Latif, H.A.; El-Eraky, W.I.; El-Shemy, H.A. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Prolif. 2011, 44, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.-M.; Lee, J.-J.; Wang, Y.-S.; Chiang, H.-C.; Huang, C.-C.; Hsieh, P.-J.; Han, W.; Ke, C.-H.; Liao, A.T.C.; Lin, C.-S. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro- hyperthermia. BMC Cancer 2020, 20, 603. [Google Scholar] [CrossRef] [PubMed]
- Park Young, S.; Chae Yeong, S.; Park Oh, J.; Lee Jin, K.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-Loaded Nanoparticles Based on Poly(epsilon-caprolactone) and Poly(d,l-lactic-co-glycolic acid)–Poly(ethylene glycol) Blend for Prostate Cancer Treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef]
- Hsu, H.-T.; Tseng, Y.-T.; Wong, W.-J.; Liu, C.-M.; Lo, Y.-C. Resveratrol prevents nanoparticles-induced inflammation and oxidative stress via downregulation of PKC-α and NADPH oxidase in lung epithelial A549 cells. BMC Complement. Altern. Med. 2018, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Wu, M.; Feng, Z.; Deng, Y.; Zhong, C.; Zhao, X. Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: Preparation, characterization, bioavailability and targeting of liver tumors. Artif. Cells Nanomed. Biotechnol. 2019, 47, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 2021, 5, 113–124. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnol. 2016, 14, 27. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef]
- Jeon, Y.O.; Lee, J.-S.; Lee, H.G. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and γ-poly (glutamic acid). Colloids Surf. B Biointerfaces 2016, 147, 224–233. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar]
- Bunaciu, R.P.; Yen, A. Resveratrol and Malignancies. Curr. Pharmacol. Rep. 2015, 1, 266–271. [Google Scholar] [CrossRef][Green Version]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.-H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Rosso, F.; Grimaldi, A.; Barbarisi, A.; Avvisati, V.; De Chiaro, M.; Di Lazzaro, A.; Arra, C.; Barbieri, A.; Palma, G.; Iaffaioli, R. V 1219 Trans-resveratrol reverse drug resistance to docetaxel: A preliminary in vivo study. Eur. J. Cancer Suppl. 2009, 7, 126. [Google Scholar] [CrossRef]
- Vinod, B.S.; Nair, H.H.; Vijayakurup, V.; Shabna, A.; Shah, S.; Krishna, A.; Pillai, K.S.; Thankachan, S.; Anto, R.J. Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2–Akt axis. Cell Death Discov. 2015, 1, 15061. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, S.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21 WAF1/CIP1 and p27 KIP1 pathway. Oncotarget 2017, 8, 17216. [Google Scholar]
- Singh, S.K.; Lillard, J.W.; Singh, R. Reversal of drug resistance by planetary ball milled (PBM) nanoparticle loaded with resveratrol and docetaxel in prostate cancer. Cancer Lett. 2018, 427, 49–62. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, Y.-J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef]
- Aw, M.S.; Kurian, M.; Losic, D. Polymeric micelles for multidrug delivery and combination therapy. Chemistry 2013, 19, 12586–12601. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.; Tomoda, K.; Kwon, G. Polymeric Micelles for Multi-Drug Delivery in Cancer. AAPS PharmSciTech 2014, 16, 10–20. [Google Scholar] [CrossRef]
- Anwar, D.M.; El-Sayed, M.; Reda, A.; Fang, J.-Y.; Khattab, S.N.; Elzoghby, A.O. Recent advances in herbal combination nanomedicine for cancer: Delivery technology and therapeutic outcomes. Expert Opin. Drug Deliv. 2021, 18, 1609–1625. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef]
- Singh, S.K.; Gordetsky, J.B.; Bae, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Selective Targeting of the Hedgehog Signaling Pathway by PBM Nanoparticles in Docetaxel-Resistant Prostate Cancer. Cells 2020, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Li, S.; Kaczmarczyk, B.; Marcinkowski, A.; Orchel, A.; Musiał-Kulik, M.; Kasperczyk, J. Multidrug PLA-PEG filomicelles for concurrent delivery of anticancer drugs—The influence of drug-drug and drug-polymer interactions on drug loading and release properties. Int. J. Pharm. 2016, 510, 365–374. [Google Scholar] [CrossRef] [PubMed]

| DDS | Material | Size [nm] | EE [%] | Drug | Preparation Method | Location | Status | Ref. |
|---|---|---|---|---|---|---|---|---|
| GNRs/liposomes | DSPE-PEG2000, CHOL, SPC, HSPC, RLT, PEG | 163.15 ± 1.83 | 98.45 ± 0.37 | Dtx | Film hydration | Prostate | In vitro-PC-3 cells In vivo-mice | [76] |
| Liposomes | DSPE-PEG2000, CHOL, SPC, cetuximab | 67.47 ± 4.32 | 99.95 | Dtx | Film hydration | Prostate | In vitro–PC-3, DU145 cells | [77] |
| Liposomes | DSPE-PEG2000, CHOL, SPC, transferrin | 220.23 ± 3.95 | 37 ± 3.15 | Dtx | Film hydration | Prostate | In vitro–PC-3, PNT2 cells | [79] |
| Dendrimers | PAMAM | n/d | n/d | Dtx/Ptx | Covalent method | Breast | In vitro–SKBR-3 cells | [86] |
| NPs | PLGA, FA | 227.6 ± 5.9 | n/d | Dtx | Solvent-evaporation | Breast | In vitro–HeLa, MCF-7 cells In vivo–mice | [14] |
| NPs | Au | 18 | n/d | Dtx | Chemical reduction | Lung | In vitro–H520 cells | [98] |
| NPs | GX1, PEG, DA, DGC | 150.9 ± 3.5 | 52.7 ± 4.4 | Dtx | Dialysis | Gastric | In vitro–co-HUVEC In vivo-mice | [91] |
| NPs | PCL, Pluronic F108 | 216 ± 3.4 | 86.0 ± 3.9 | Dtx | Nanoprecipitation | Breast | In vitro–BT-474 cells In vivo–mice | [92] |
| NPs | Albumin, aptamer | 62 ± 0.6 | 90.0 ± 0.7 | Dtx | Salting-out method | Colon | In vitro–CT26 cells In vivo–mice | [93] |
| SLNPs | Span 80, Pluronic F127 | 128 ± 2.2 | 86.0 ± 2.4 | Dtx | N/d | Breast | In vitro–4T1 cells In vivo–mice | [82] |
| NPs | Chondroitin sulphate, zein | 157.8 ± 3.6 | 64.2 ± 1.9 | Dtx | Solvent displacement | Prostate | In vitro–PC-3 cells In vivo-mice | [99] |
| NPs | fluorescein-labelled wheat germ agglutinin (fWGA)-conjugated disulfide cross-linked sodium alginate | 289 | 17.8 | Dtx | N/d | Colon | In vitro–HT-29 cells | [88] |
| Synthetic high-density lipoprotein nanoparticles | Egg sphingomyelin (eSM), apolipoprotein A-1 mimetic peptide 22A | 11.3 | n/d | Dtx + Cho-CpG | Co-lyophilisation | Colon | In vitro–MC-38 cells In vivo-mice | [94] |
| NPs | PBAE | 137.9 ± 2.09 | 20.36 ± 0.01 | Dtx + ATRA | Solvent displacement | Breast | In vitro-HUVEC and MCF-7 cells | [100] |
| micelles | Cap, HA, PLA | 144 | n/d | Dtx + Zol | Dialysis | Prostate | In vitro–PC-3 cells In vivo-mice | [101] |
| NPs | PEG | 153.1 | n/d | Dtx + Dha | Dialysis | Breast | In vitro–4T1 cells In vivo-mice | [102] |
| NPs | H1 nanopolymer (folate–-polyethylenimine600–cyclodextrin) | 117 ± 12.9 | n/d | Dtx + dbait | N/d | Prostate | In vitro–CRPC, PC-3, DU145, LNCaP cells In vivo-mice | [96] |
| NPs | PEG, PLA, ACUPA | 135 ± 15 | 45 ± 5 | Dtx + Gba | Solvent-evaporation | Prostate | In vitro–PC-3, LNCaP cells | [103] |
| NPs | PLGA, PM | 98.2 | 92.4 | Dtx | Dialysis | Lung cancer | In vitro–A549 cells In vivo-mice | [104] |
| Nr | Study Title | Cancer | DDS | Phase | Status |
|---|---|---|---|---|---|
| NCT01300533 | A Study of BIND-014 Given to Patients with Advanced or Metastatic Cancer | Metastatic cancer, solid tumours | NPs | 1 | C |
| NCT02479178 | A Study of BIND-014 in Patients with Urothelial Carcinoma, Cholangiocarcinoma, Cervical Cancer and Squamous Cell Carcinoma of the Head and Neck (iNSITE2) | Urothelial carcinoma cholangiocarcinoma, cervical cancer, squamous cell carcinoma of head and neck | NPs | 2 | T |
| NCT02283320 | A Study of BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) as Second-line Therapy for Patients with KRAS Positive or Squamous Cell Non-Small Cell Lung Cancer | KRAS-positive patients with non-small cell lung cancer, squamous cell non-small cell lung cancer | NPs | 2 | C |
| NCT01792479 | A Phase 2 Study to Determine the Safety and Efficacy of BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) as Second-line Therapy to Patients with Non-Small Cell Lung Cancer | Non-small cell lung cancer | NPs | 2 | C |
| NCT01812746 | A Phase 2 Study to Determine the Safety and Efficacy of BIND-014 (Docetaxel Nanoparticles for Injectable Suspension), Administered to Patients with Metastatic Castration-Resistant Prostate Cancer | Castration-resistant prostate cancer, prostate cancer | NPs | 2 | C |
| NCT01151384 | Liposome Encapsulated Docetaxel (LE-DT) in Patients with Solid Tumours (LE-DT) | Solid tumours | Liposomes | 1 | C |
| NCT01186731 | Efficacy and Safety Study of LE-DT to Treat Locally Advanced or Metastatic Pancreatic Cancer | Pancreatic cancer | Liposomes | 2 | C |
| NCT01188408 | Efficacy and Safety Study of LE-DT to Treat Metastatic Castrate Resistant Prostate Cancer | Prostate cancer | Liposomes | 2 | W |
| NCT01103791 | A Trial to Determine the Maximum Tolerated Dose and Evaluate the Safety and Pharmacokinetics of Docetaxel-PNP, Polymeric Nanoparticle Formulation of Docetaxel, in Subjects with Advanced Solid Malignancies | Advanced solid malignancies | NPs | 1 | C |
| NCT03712423 | PET Study With [89Zr]-Df-CriPec® Docetaxel | Solid tumour | CCL-PMs | 1 | C |
| NCT03742713 | Efficacy Study of CPC634 (CriPec® Docetaxel) in Platinum Resistant Ovarian Cancer (CINOVA) | Cancer, ovarian cancer | CCL-PMs | 2 | C |
| NCT02442531 | A Study of CriPec® Docetaxel Given to Patients with Solid Tumours (NAPOLY) | Cancer, metastatic cancer, solid tumours | CCL-PMs | 1 | C |
| DDS | Material | Size [nm] | EE [%] | Preparation Method | Location | Status | Ref. |
|---|---|---|---|---|---|---|---|
| NPs | Au | 30.75 ± 3.41 | n/d | Reduction with chloroauric acid | Breast | In vitro—MCF-7 cells | [133] |
| NPs | PCL | 132 ± 4 a | 98.4 ± 0.3 a | Interfacial deposition | Skin | In vitro—B16F10 cells In vivo—mice | [134] |
| NPs | PLC, PLGA, PEG | 150 | 83.30 ± 13.47 | Nanoprecipitation | Prostate | In vitro—DU-145, PC-3 and LNCaP cells | [135] |
| NPs | PLGA | 202.8 ± 2.64 | 89.32 ± 3.51 | Solvent displacement | Prostate | In vitro—LNCaP cells | [136] |
| NPs | CB | n/d | n/d | N/d | Lung | In vitro—A549 cells | [137] |
| NPs | FA-HSA | 102.1 ± 4.9 | 98.36 | High pressure fluid nano-homogeneous emulsification | Liver | In vitro—HepG2 cells In vivo—mice | [138] |
| NPs | Pluronic F127 block copolymer, vitamin E-TPGS | 179 ± 22 | 73 ± 0.9 | Emulsification | Breast | In vitro—MCF-7, MDA-MB-231, MCF-10A cells | [139] |
| SLNPs | SA, saturated monoacid, triglyceride, Myrj52 | 168 ± 10.7 | n/d | Emulsification and low-temperature solidification | Breast | In vitro—MDA-MB-231 cells | [140] |
| SLNPs | Apolipoprotein E, DSPE, palmitic acid | 217.1 ± 5.8 | 98.9 ± 0.6 | High shear homogenization | Brain | hCMEC/D3 cells | [141] |
| DDS | Material | Preparation Method | Location | Status | Ref. |
|---|---|---|---|---|---|
| micelles | mPEG-PDLA | Thin film hydration-ultrasound method | Breast | In vitro—MCF-7 cells In vivo—rats | [71] |
| PBM NPs | FA-PCL-PEG | Planetary ball milling | Prostate | In vitro—PC3 and PC3-R cells | [151] |
| LPNPs | Lipid-polymer | Nanoprecipitation method | Lung | In vitro—HCC827, NCIH2135 and HUVEC cells In vivo—mice | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines 2022, 10, 1187. https://doi.org/10.3390/biomedicines10051187
Jurczyk M, Kasperczyk J, Wrześniok D, Beberok A, Jelonek K. Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines. 2022; 10(5):1187. https://doi.org/10.3390/biomedicines10051187
Chicago/Turabian StyleJurczyk, Magdalena, Janusz Kasperczyk, Dorota Wrześniok, Artur Beberok, and Katarzyna Jelonek. 2022. "Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy" Biomedicines 10, no. 5: 1187. https://doi.org/10.3390/biomedicines10051187
APA StyleJurczyk, M., Kasperczyk, J., Wrześniok, D., Beberok, A., & Jelonek, K. (2022). Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines, 10(5), 1187. https://doi.org/10.3390/biomedicines10051187







