Incidence and Risk Factors for Acute Kidney Injury after Allogeneic Stem Cell Transplantation: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Hematopoietic Stem Cell Transplantation
2.3. Collected Data and Follow-Up
2.4. Definitions
2.5. Statistical Analysis
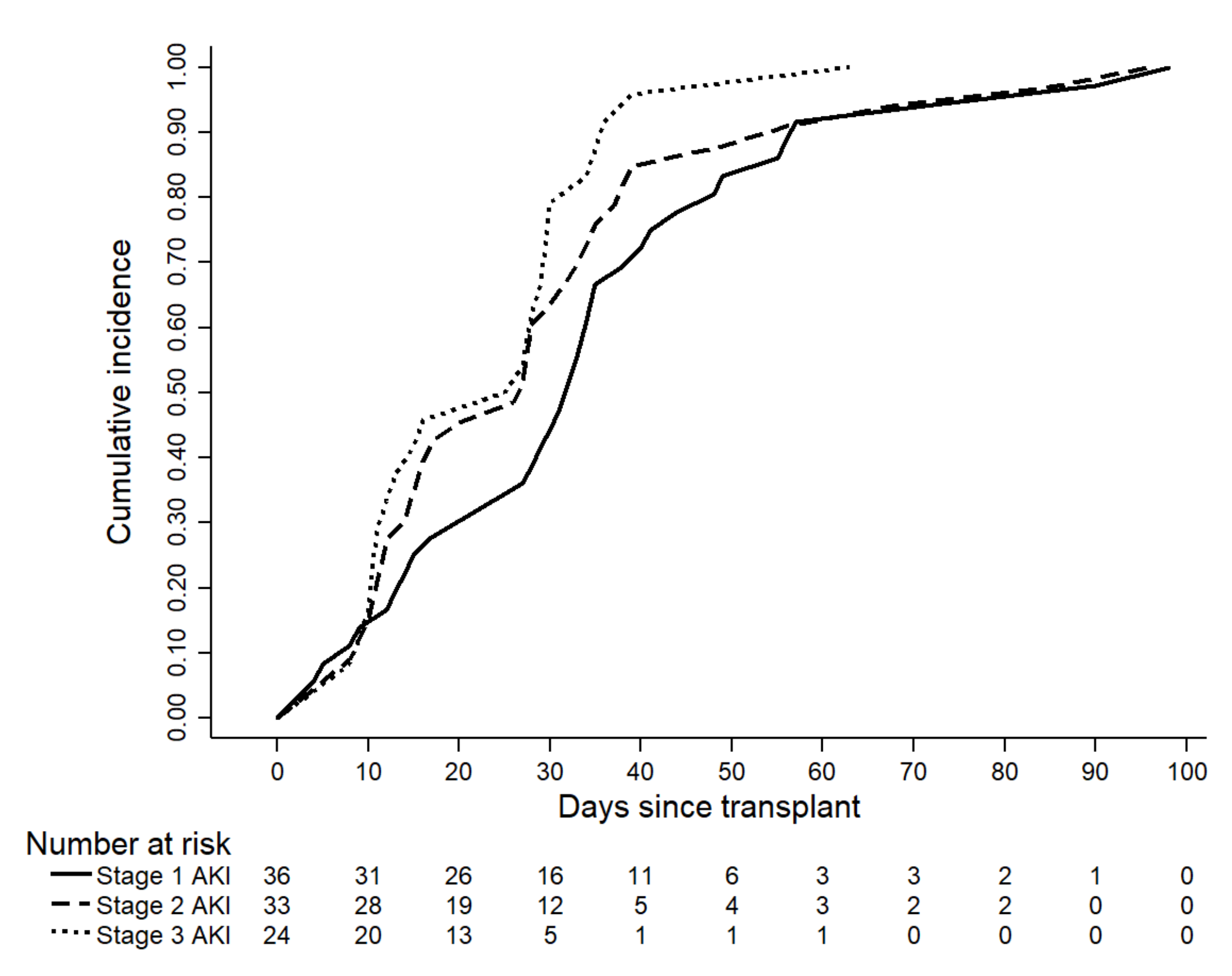
3. Results
3.1. Patients’ Characteristics and Incidence of Acute Kidney Injury
3.2. Comparison between AKI and Non-AKI Subgroups
3.3. Risk Factors for Acute Kidney Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niederwieser, D.; Baldomero, H.; Bazuaye, N.; Bupp, C.; Chaudhri, N.; Corbacioglu, S.; Elhaddad, A.; Frutos, C.; Galeano, S.; Hamad, N.; et al. One and a half million hema-topoietic stem cell transplants: Continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica 2021. e-pub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kanduri, S.R.; Cheungpasitporn, W.; Thongprayoon, C.; Bathini, T.; Kovvuru, K.; Garla, V.; Medaura, J.; Vaitla, P.; Kashani, K.B. Incidence and mortality of acute kidney injury in patients undergoing hematopoietic stem cell transplantation: A systematic review and meta-analysis. QJM Int. J. Med. 2020, 113, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.H.; Gutgarts, V.; Zheng, J.; Maloy, M.A.; Ruiz, J.D.; Scordo, M.; Jaimes, E.A.; Sathick, I.J. Acute Kidney Injury in the Modern Era of Allogeneic Hematopoietic Stem Cell Transplantation. Clin. J. Am. Soc. Nephrol. 2021, 16, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Guthrie, K.; Batchelder, A.; Schoch, G.; Aboulhosn, N.; Manchion, J.; Mcdonald, G.B. Acute renal failure after myeloablative hematopoietic cell transplant: Incidence and risk factors. Kidney Int. 2005, 67, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñana, J.L.; Valcarcel, D.; Martino, R.; Barba, P.; Moreno, E.; Sureda, A.; Vega, M.; Delgado, J.; Briones, J.; Brunet, S.; et al. Study of Kidney Function Impairment after Reduced-Intensity Conditioning Allogeneic Hematopoietic Stem Cell Transplantation. A Single-Center Experience. Biol. Blood Marrow Transplant. 2009, 15, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Cairo, M.S.; Cooke, K.R.; Lazarus, H.M.; Chao, N. Modified diagnostic criteria, grading classification and newly elucidated pathophysiology of hepatic SOS/VOD after haematopoietic cell transplantation. Br. J. Haematol. 2020, 190, 822–836. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Ruutu, T.; Barosi, G.; Benjamin, R.J.; Clark, R.E.; George, J.N.; Gratwohl, A.; Holler, E.; Iacobelli, M.; Kentouche, K.; Lämmle, B.; et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: Results of a consensus process by an International Working Group. Haematologica 2007, 92, 95–100. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R.; Baldomero, H.; Bader, P.; Bonini, M.C.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Dufour, C.; Kuball, J.; Farge-Bancel, D.; et al. Hematopoietic stem cell transplantation in Europe 2014: More than 40,000 transplants annually. Bone Marrow Transplant. 2016, 51, 786–792. [Google Scholar] [CrossRef]
- Hingorani, S. Renal Complications of Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2016, 374, 2256–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renaghan, A.D.; Jaimes, E.A.; Malyszko, J.; Perazella, M.A.; Sprangers, B.; Rosner, M.H. Acute Kidney Injury and CKD Associated with Hematopoietic Stem Cell Transplantation. Clin. J. Am. Soc. Nephrol. 2020, 15, 289–297. [Google Scholar] [CrossRef]
- Andronesi, A.G.; Tanase, A.D.; Sorohan, B.M.; Craciun, O.G.; Stefan, L.; Varady, Z.; Lipan, L.; Obrisca, B.; Truica, A.; Ismail, G. Incidence and risk factors for acute kidney injury following autologous stem cell transplantation for multiple myeloma. Cancer Med. 2019, 8, 3278–3285. [Google Scholar] [CrossRef]
- Ando, M.; Mori, J.; Ohashi, K.; Akiyama, H.; Morito, T.; Tsuchiya, K.; Nitta, K.; Sakamaki, H. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone Marrow Transplant. 2010, 45, 1427–1434. [Google Scholar] [CrossRef]
- Shingai, N.; Morito, T.; Najima, Y.; Kobayashi, T.; Doki, N.; Kakihana, K.; Ohashi, K.; Ando, M. Early-onset acute kidney injury is a poor prognostic sign for allogeneic SCT recipients. Bone Marrow Transplant. 2015, 50, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Kuypers, D.R.; Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, A.Q.; Humphreys, B.D. Onco-Nephrology: AKI in the Cancer Patient. Clin. J. Am. Soc. Nephrol. 2012, 7, 1692–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, M.S.; Yee, G.C.; Mcguire, T.R.; Leonard, T.M.; Crowley, J.J.; Deeg, H.J. Correlation of Serum Cyclosporine Concentration with Renal Dysfunction in Marrow Transplant Recipients. Transplantation 1985, 40, 249–252. [Google Scholar] [CrossRef]
- Naymagon, S.; Naymagon, L.; Wong, S.-Y.; Ko, H.M.; Renteria, A.; Levine, J.; Colombel, J.-F.; Ferrara, J. Acute graft-versus-host disease of the gut: Considerations for the gastroenterologist. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Perazella, M.A. Acute Kidney Injury in Patients with Cancer. N. Engl. J. Med. 2017, 376, 1770–1781. [Google Scholar] [CrossRef]
- Lopes, J.A.; Jorge, S. Acute kidney injury following HCT: Incidence, risk factors and outcome. Bone Marrow Transplant. 2011, 46, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, B.; Alchaqmaqchi, H.; Al-Hashmi, S.; Brodin, D.; Hassan, Z.M.; Abedi-Valugerdi, M.; Moshfegh, A.; Hassan, M. Early-phase GVHD gene expression profile in target versus non-target tissues: Kidney, a possible target? Bone Marrow Transplant. 2012, 48, 284–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Li, D.; Vasquez, H.G.; You, M.J.; Afshar-Kharghan, V. Kidney Injury in Murine Models of Hematopoietic Stem CellTransplantation. Biol. Blood Marrow Transplant. 2019, 25, 1920–1924. [Google Scholar] [CrossRef]
- Kitchlu, A.; McArthur, E.; Amir, E.; Booth, C.M.; Sutradhar, R.; Majeed, H.; Nash, D.M.; Silver, S.A.; Garg, A.X.; Chan, C.T.; et al. Acute Kidney Injury in Patients Receiving Systemic Treatment for Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2019, 111, 727–736. [Google Scholar] [CrossRef]
- Epperla, N.; Li, A.; Logan, B.; Fretham, C.; Chhabra, S.; Aljurf, M.; Chee, L.; Copelan, E.; Freytes, C.O.; Hematti, P.; et al. Incidence, Risk Factors for and Outcomes of Transplant-Associated Thrombotic Microangiopathy. Br. J. Haematol. 2020, 189, 1171–1181. [Google Scholar] [CrossRef]
- Lahoti, A.; Kantarjian, H.; Salahudeen, A.K.; Ravandi, F.; Cortes, J.; Faderl, S.; O’Brien, S.; Wierda, W.; Mattiuzzi, G.N. Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer 2010, 116, 4063–4068. [Google Scholar] [CrossRef]
- Kal, H.B.; van Kempen-Harteveld, M.L. Renal dysfunction after total body irradiation: Dose–effect relationship. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Igaki, H.; Karasawa, K.; Sakamaki, H.; Saito, H.; Nakagawa, K.; Ohtomo, K.; Tanaka, Y. Renal Dysfunction after Total-Body Irradiation. Significance of selective renal shielding blocks. Strahlenther. und Onkol. 2005, 181, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Wanchoo, R.; Bayer, R.L.; Bassil, C.; Jhaveri, K.D. Emerging Concepts in Hematopoietic Stem Cell Transplantation–Associated Renal Thrombotic Microangiopathy and Prospects for New Treatments. Am. J. Kidney Dis. 2018, 72, 857–865. [Google Scholar] [CrossRef]
- Wanchoo, R.; Stotter, B.R.; Bayer, R.L.; Jhaveri, K.D. Acute kidney injury in hematopoietic stem cell transplantation. Curr. Opin. Crit. Care 2019, 25, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Laskin, B.L.; Goebel, J.; Davies, S.M.; Jodele, S. Small vessels, big trouble in the kidneys and beyond: Hematopoietic stem cell transplantation–associated thrombotic microangiopathy. Blood 2011, 118, 1452–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.A.; Pallas, C.R.; Knovich, M.A. Transplant-associated thrombotic microangiopathy: Theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021, 56, 1805–1817. [Google Scholar] [CrossRef]
- Bonifazi, F.; Barbato, F.; Ravaioli, F.; Sessa, M.; DeFrancesco, I.; Arpinati, M.; Cavo, M.; Colecchia, A. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeker, L.; Kim, H.T.; Glotzbecker, B.; Nageshwar, P.; Nikiforow, S.; Koreth, J.; Armand, P.; Cutler, C.; Alyea, E.P.; Antin, J.H.; et al. Early Clinical Predictors of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome after Myeloablative Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Murashima, M.; Nishimoto, M.; Kokubu, M.; Hamano, T.; Matsui, M.; Eriguchi, M.; Samejima, K.-I.; Akai, Y.; Tsuruya, K. Inflammation as a predictor of acute kidney injury and mediator of higher mortality after acute kidney injury in non-cardiac surgery. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, R.V.; Szalai, A.J. Therapeutic Lowering of C-Reactive Protein. Front. Immunol. 2021, 11, 619564. [Google Scholar] [CrossRef]
- Lai, W.; Tang, Y.; Huang, X.R.; Tang, P.M.-K.; Xu, A.; Szalai, A.J.; Lou, T.-Q.; Lan, H.Y. C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int. 2016, 90, 610–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Chung, A.C.; Zhou, L.; Huang, X.R.; Liu, F.; Fu, P.; Fan, J.M.; Szalai, A.J.; Lan, H.Y. C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab. Investig. 2011, 91, 837–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarbock, A.; Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 2014, 20, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
| Overall (N = 135) | No AKI (N = 42) | AKI (N = 93) | p-Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age at transplant (mean, years) | 38.3 ± 11.9 | 39.7 ± 11.6 | 37.6 ± 12.1 | 0.34 |
| Male gender (%) | 64 (47.4 %) | 16 (38.1%) | 48 (51.6%) | 0.14 |
| Underlying hematologic disease (%) | 0.15 | |||
| AML/MDS ALL Lymphoma CML Aplastic anemia | 76 (56.3%) 36 (26.7%) 13 (9.6%) 4 (3.0%) 6 (4.4%) | 25 (59.5%) 7 (16.7%) 4 (9.5%) 2 (4.8%) 4 (9.5%) | 51 (54.8%) 29 (31.2%) 9 (9.6%) 2 (2.2%) 2 (2.2%) | |
| Hematologic disease length (median, months) | 9 (6–15) | 7 (5–14.2) | 10 (6–16.5) | 0.17 |
| Donor type (%) | 0.95 | |||
| Siblings Haploidentical 10/10 9/10 | 93 (68.9%) 6 (4.4%) 23 (17.0%) 13 (9.7%) | 30 (71.4%) 2 (4.8%) 6 (14.3%) 4 (9.5%) | 63 (67.7%) 4 (4.3%) 17 (18.3%) 9 (9.7%) | |
| Donor cell source (%) | 0.42 | |||
| Peripheral blood Bone marrow | 131 (97%) 4 (3%) | 40 (95.2%) 2 (4.8%) | 91 (97.8%) 2 (2.2%) | |
| Conditioning type (%) | 0.004 a | |||
| Myeloablative Non-myeloablative | 88 (65.2%) 47 (34.8%) | 20 (47.6%) 22 (52.4%) | 68 (73.1%) 25 (26.9%) | |
| TBI (%) | 12 (8.9%) | 1 (2.4%) | 11 (11.8%) | 0.04 a |
| Fludarabine-based regimen (%) | 77 (57.0%) | 29 (69.0%) | 48 (51.6%) | 0.05 |
| Aplasia recovery period (mean, days) | 18.2 ± 3.5 | 18.6 ± 3.8 | 17.9 ± 3.3 | 0.30 |
| Hospital length of stay (mean, days) | 24.9 ± 9.9 | 24.4 ± 4.8 | 25.2 ± 11.6 | 0.67 |
| Comorbidities (%) | ||||
| Hypertension Type 2 DM HBV infection HCV infection | 4 (3.0%) 4 (3.0%) 20 (14.8%) 3 (2.2%) | 1 (2.4%) 1 (2.4%) 7 (16.7%) 1 (2.4%) | 3 (3.2%) 3 (3.2%) 13 (14.0%) 2 (2.2%) | 0.78 0.78 0.68 0.93 |
| Kidney function at transplantation | ||||
| Serum creatinine (mean, mg/dL) eGFR (mean, mL/min) | 0.7 ± 0.1 110.8 ± 18.5 | 0.71 ± 0.1 109.8 ± 16.4 | 0.73 ± 0.1 111.3 ± 19.4 | 0.47 0.66 |
| Complications (%) | ||||
| SOS (%) | 22 (16.3%) | 2 (4.8%) | 20 (21.5%) | 0.01 a |
| GVHD (%) | 46 (34.1%) | 11 (26.2%) | 35 (37.6%) | 0.19 |
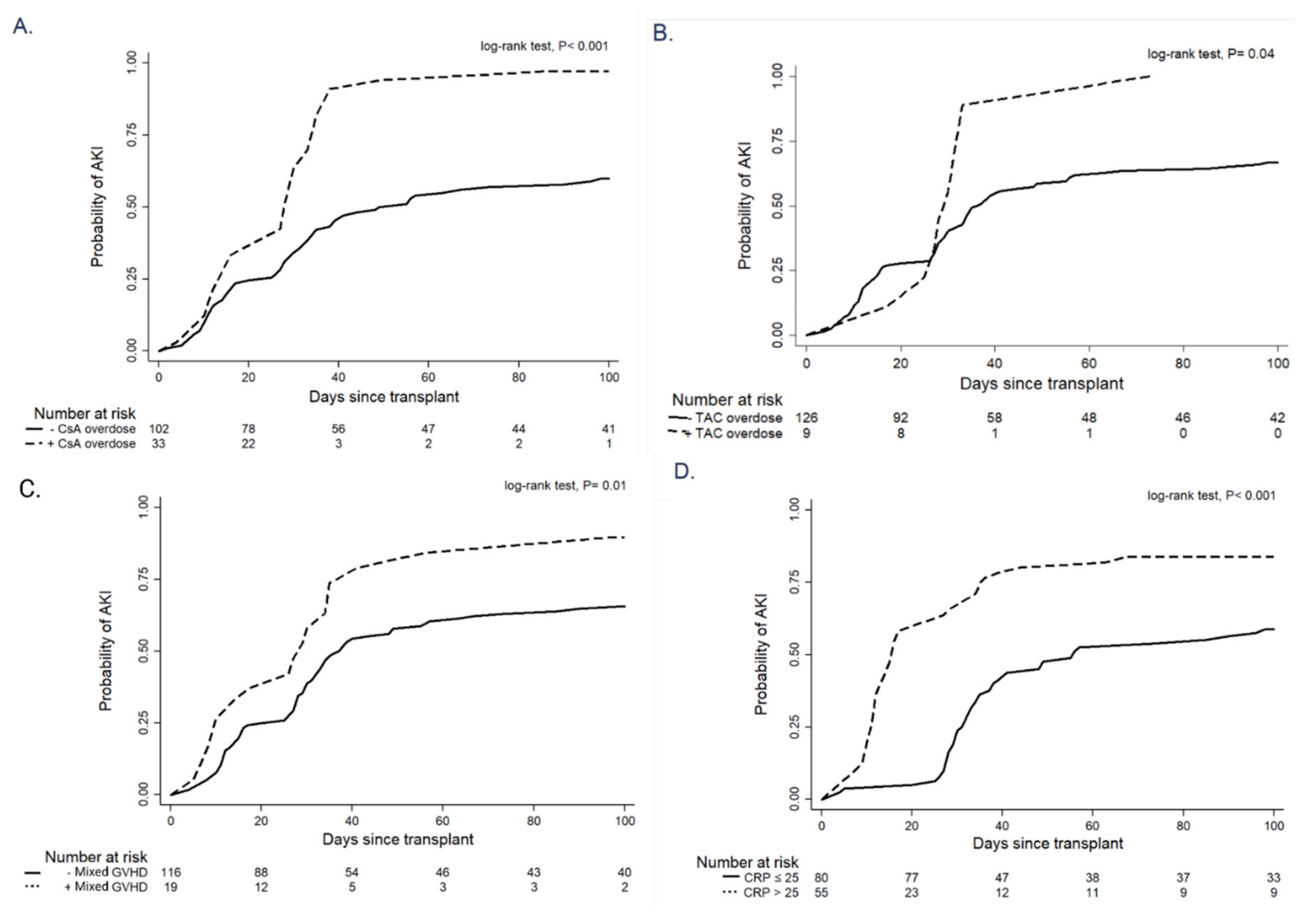
| Mixed GVHD (%) | 19 (14.1%) | 2 (4.8%) | 17 (18.3%) | 0.004 a |
| GVHD grading (%) | 0.10 | |||
| 1 | 15 (11.1%) | 6 (14.3%) | 9 (9.7%) | |
| 2 | 20 (14.8%) | 5 (11.9%) | 15 (16.1%) | |
| 3 | 11 (8.1%) | 0 (0%) | 11 (11.8%) | |
| TMA (%) | 38 (28.1%) | 6 (14.3%) | 32 (34.4%) | 0.01 a |
| Fever (%) | 73 (54.1%) | 19 (45.2%) | 54 (58.1%) | 0.16 |
| Sepsis (%) | 24 (17.8%) | 9 (21.4%) | 9 (21.4%) | 0.45 |
| Death (%) | 19 (14.1%) | 3 (7.1%) | 16 (17.25) | 0.12 |
| CRP serum level (median, mg/L) | 14.2 (3.0–80.0) | 10 (3–21) | 24.3 (2.9–123.5) | 0.02 a |
| Nephrotoxic drugs | ||||
| CNI type (%) | 0.61 | |||
| Cyclosporine | 109 (80.7%) | 35 (83.3%) | 74 (79.6%) | |
| Tacrolimus | 26 (19.3%) | 7 (16.7%) | 19 (20.4%) | |
| CsA overdosage (%) | 33 (24.4%) | 1 (2.4%) | 32 (34.4%) | <0.001 a |
| TAC overdosage (%) | 9 (6.7%) | 0 (0%) | 9 (9.7%) | 0.008 a |
| Contrast media (%) | 12 (8.9%) | 5 (11.9%) | 7 (7.5%) | 0.41 |
| MTX (%) | 65 (48.1%) | 36 (85.7%) | 29 (31.2%) | <0.001 a |
| Antibiotics (%) | 40 (29.6%) | 14 (33.3%) | 26 (28.0%) | 0.52 |
| Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Male gender | 1.25 | 0.83–1.88 | 0.27 | 1.46 | 0.96–2.23 | 0.07 |
| Acute lymphoblastic leukemia | 1.37 | 0.88–2.13 | 0.16 | 1.59 | 0.98–2.58 | 0.05 |
| Myeloablative conditioning regimens | 2.27 | 1.43–3.60 | <0.001 a | |||
| TBI | 2.49 | 1.31–4.72 | 0.005 a | |||
| Fludarabine | 0.57 | 0.38–0.86 | 0.008 a | |||
| MTX | 0.42 | 0.26–0.66 | <0.001 a | |||
| CsA overdose | 2.78 | 1.78–4.35 | <0.001 a | 2.36 | 1.45–3.85 | 0.001 a |
| TAC overdose | 2.02 | 1.01–4.06 | 0.04 a | 4.72 | 2.22–10.01 | <0.001 a |
| mGVHD | 1.89 | 1.11–3.21 | 0.01 a | 1.96 | 1.13–3.40 | 0.01 a |
| GVHD grading | ||||||
| I | 0.75 | 0.37–1.52 | 0.43 | |||
| II | 1.39 | 0.78–2.45 | 0.25 | |||
| III | 2.00 | 1.05–3.82 | 0.03 a | |||
| Sinusoidal obstruction syndrome | 2.42 | 1.46–4.00 | 0.001 a | |||
| Thrombotic microangiopathy | 1.77 | 1.15–2.72 | 0.009 a | |||
| CRP level | 1.008 | 1.006–1.010 | <0.001 a | 1.009 | 1.007–1.10 | <0.001 a |
| No AKI Stage 3 (N = 111) | AKI Stage 3 (N = 24) | p-Value | |
|---|---|---|---|
| Demographic data | |||
| Age at transplant (mean, years) | 38.9 ± 11.9 | 35.5 ± 11.8 | 0.19 |
| Male gender (%) | 55 (49.5%) | 9 (37.5%) | 0.28 |
| Underlying hematologic disease (%) | 0.007 | ||
| AML/MDS ALL Lymphoma CML Aplastic anemia | 69 (62.2%) 24 (21.6%) 10 (9%) 2 (1.8%) 6 (5.4%) | 7 (29.2%) 12 (50%) 3 (12.5%) 2 (8.3%) 0 (0%) | |
| Hematologic disease length (median, months) | 8 (6–14) | 10.5 (6.2–27.2) | 0.21 |
| Donor type (%) | 0.25 | ||
| Related Unrelated haploidentical 10/10 9/10 | 73 (65.8%) 5 (4.5%) 20 (18%) 13 (11.7%) | 20 (83.3%) 1 (4.2%) 3 (12.5%) 0 (0%) | |
| Donor cell source (%) | 1 | ||
| Peripheral blood Bone marrow | 107 (96.4%) 4 (3.6%) | 111 (100%) 0 (0%) | |
| Conditioning type (%) | 0.01 | ||
| Myeloablative Non-myeloablative | 67 (60.4%) 44 (39.6%) | 21 (87.5%) 3 (2.5%) | |
| TBI (%) | 7 (6.3%) | 5 (20.8%) | 0.03 |
| Fludarabine based regimen (%) | 70 (63.1%) | 7 (29.2%) | 0.002 |
| Aplasia recovery period (mean, days) | 18.3 ± 3.5 | 17.3 ± 3.6 | 0.20 |
| Hospital length of stay (mean, days) | 24.9 ± 8.2 | 25.2 ± 16.0 | 0.25 |
| Comorbidities (%) | |||
| Hypertension | 4 (3.6%) | 0 (0%) | 0.34 |
| Type 2 DM | 4 (3.6%) | 0 (0%) | 0.34 |
| HBV infection | 17 (15.3%) | 3 (12.5%) | 1 |
| HCV infection | 2 (1.8%) | 1 (4.2%) | 0.44 |
| Kidney function at transplantation | |||
| Serum creatinine (mean, mg/dL) eGFR (mean, mL/min) | 0.7 ± 0.1 108.8 ± 17.8 | 0.6 ± 0.1 120 ± 18.9 | 0.001 0.006 |
| Complications (%) | |||
| SOS (%) | 10 (9%) | 12 (50%) | <0.001 |
| GVHD (%) | 32 (28.8%) | 14 (58.3%) | 0.006 |
| mGVHD (%) | 13 (11.7%) | 6 (25%) | 0.11 |
| GVHD grading (%) | <0.001 | ||
| 1 | 15 (13.5%) | 0 (0%) | |
| 2 | 14 (12.6%) | 6 (25%) | |
| 3 | 3 (2.7%) | 8 (33.3%) | |
| TMA (%) | 26 (23.4%) | 12 (50%) | 0.009 |
| Fever (%) | 52 (46.8%) | 21 (87.5%) | <0.001 |
| Sepsis (%) | 17 (15.3%) | 7 (29.2) | 0.10 |
| Death (%) | 13 (11.7%) | 12 (50%) | <0.001 |
| CRP serum level (median, mg/L) | 7.8 (2.7–46) | 83.3 (22.9–218.6) | <0.001 |
| Nephrotoxic drugs | |||
| CNI type (%) | 0.56 | ||
| Cyclosporine | 88 (79.3%) | 21 (87.5%) | |
| Tacrolimus | 23 (20.7%) | 3 (12.5%) | |
| CsA overdosage (%) | 25 (22.5%) | 8 (33.3%) | 0.26 |
| TAC overdosage (%) | 7 (6.3%) | 2 (8.3%) | 0.71 |
| Contrast media (%) | 8 (7.2%) | 4 (16.7%) | 0.22 |
| MTX (%) | 59 (53.2%) | 6 (25%) | 0.01 |
| Antibiotics (%) | 30 (27%) | 10 (41.7%) | 0.15 |
| Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Acute lymphoblastic leukemia | 2.77 | 1.24–6.21 | 0.01 a | 1.59 | 0.98–2.58 | 0.05 |
| Myeloablative conditioning regimens | 2.27 | 1.43–3.60 | <0.001 a | |||
| TBI | 4.32 | 1.58–11.78 | 0.004 a | |||
| Fludarabine | 0.23 | 0.09–0.56 | 0.001 a | 0.34 | 0.11–1.01 | 0.06 |
| MTX | 0.37 | 0.14–0.94 | 0.03 a | 0.30 | 0.10–1.10 | 0.32 |
| Acute GVHD | 2.86 | 1.27–6.44 | 0.01 a | 2.19 | 0.87–5.49 | 0.09 |
| mGVHD | 2.67 | 1.06–6.76 | 0.03 a | |||
| GVHD grading | ||||||
| I | 1.34 | 0.36–1.97 | 0.55 | |||
| II | 3.12 | 1.13–8.61 | 0.02 a | |||
| III | 8.06 | 3.17–20.47 | <0.001 a | |||
| Sinusoidal obstruction syndrome | 2.42 | 1.46–4.00 | 0.001 a | 5.10 | 2.02–12.85 | 0.001 a |
| Thrombotic microangiopathy | 1.77 | 1.15–2.72 | 0.009 a | |||
| Sepsis | 2.58 | 1.06–6.25 | 0.03 a | 5.37 | 1.75–16.48 | 0.003 a |
| Baseline creatinine | 0.02 | 0.002–0.31 | 0.005 a | 0.05 | 0.003–1.10 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronesi, A.; Sorohan, B.; Burcea, A.; Lipan, L.; Stanescu, C.; Craciun, O.; Stefan, L.; Ranete, A.; Varady, Z.; Ungureanu, O.; et al. Incidence and Risk Factors for Acute Kidney Injury after Allogeneic Stem Cell Transplantation: A Prospective Study. Biomedicines 2022, 10, 262. https://doi.org/10.3390/biomedicines10020262
Andronesi A, Sorohan B, Burcea A, Lipan L, Stanescu C, Craciun O, Stefan L, Ranete A, Varady Z, Ungureanu O, et al. Incidence and Risk Factors for Acute Kidney Injury after Allogeneic Stem Cell Transplantation: A Prospective Study. Biomedicines. 2022; 10(2):262. https://doi.org/10.3390/biomedicines10020262
Chicago/Turabian StyleAndronesi, Andreea, Bogdan Sorohan, Andreea Burcea, Lavinia Lipan, Cristina Stanescu, Oana Craciun, Laura Stefan, Adela Ranete, Zsofia Varady, Oana Ungureanu, and et al. 2022. "Incidence and Risk Factors for Acute Kidney Injury after Allogeneic Stem Cell Transplantation: A Prospective Study" Biomedicines 10, no. 2: 262. https://doi.org/10.3390/biomedicines10020262






