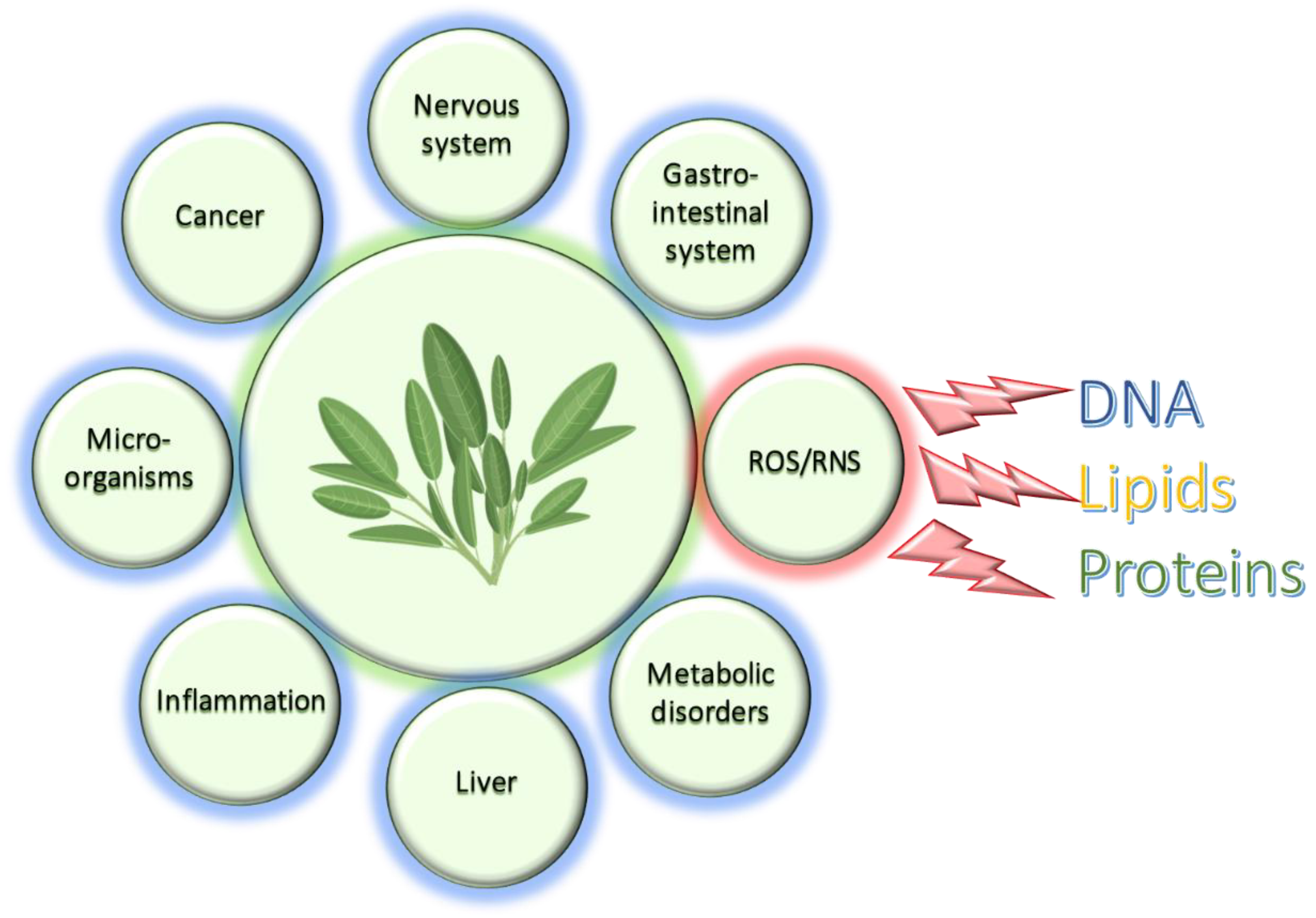
An Update on Recent Studies Focusing on the Antioxidant Properties of Salvia Species
Abstract
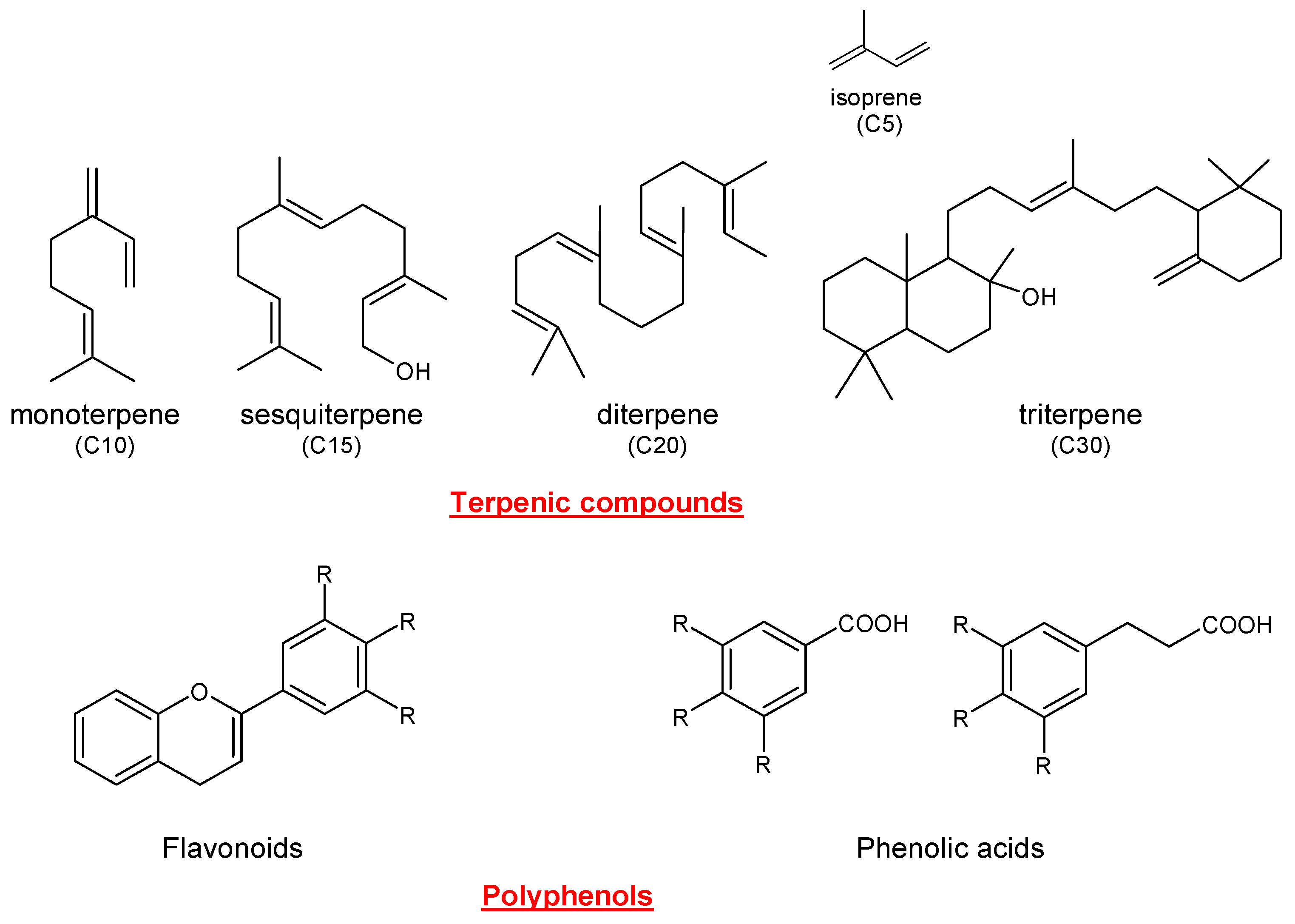
:1. Introduction
2. Extraction Methods
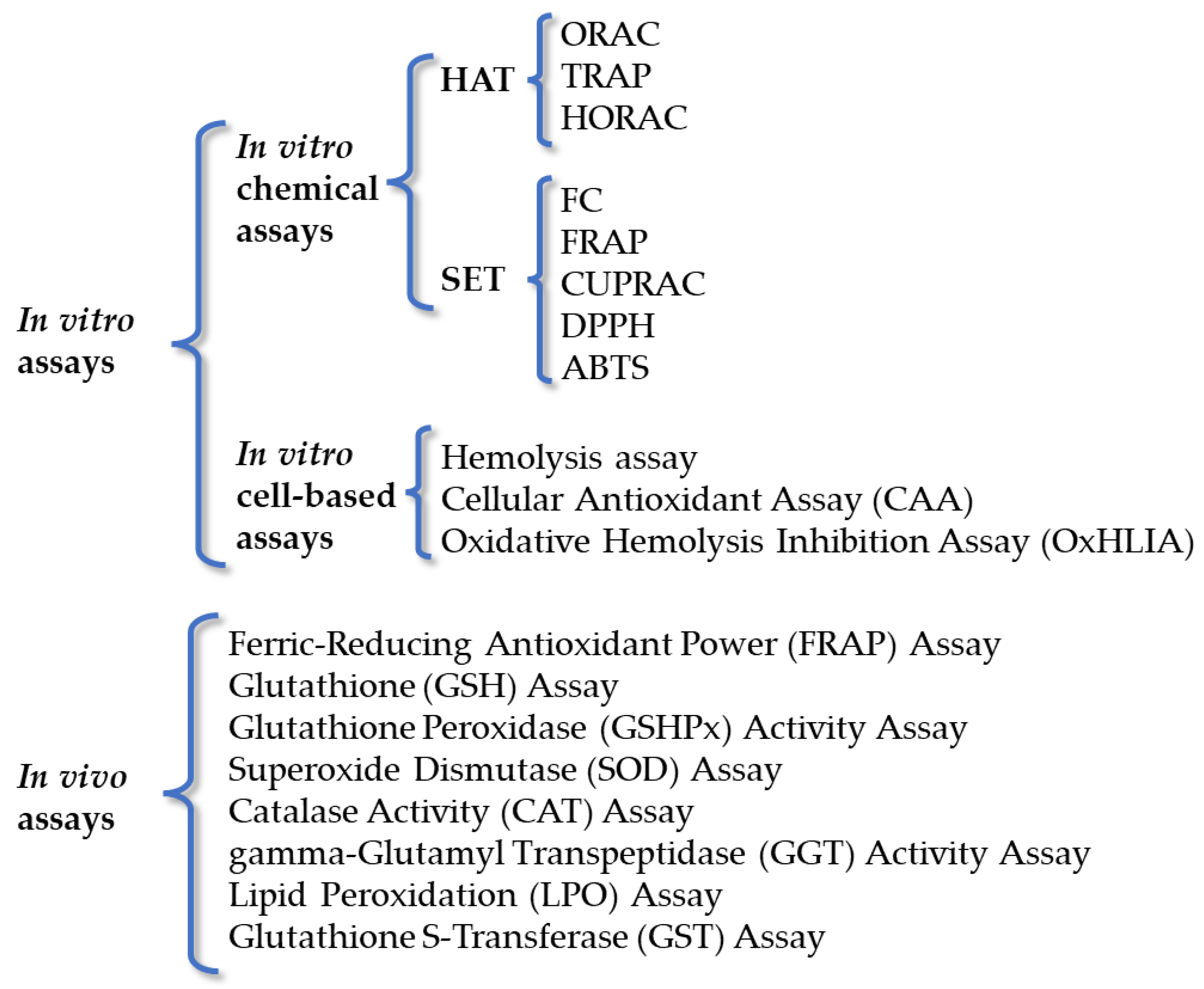
3. Methods for Evaluation of Antioxidant Activity
3.1. In Vitro Chemical Assays
3.2. In Vitro Cell-Based Assays
3.3. In Vivo Assays
4. Salvia spp. Extracts and the Evaluation of Antioxidant Activity
4.1. Antioxidant Activities of S. officinalis L. spp. Individually
4.2. Antioxidant Activities of S. officinalis L. and Other Species (S. elegans, S. greggii, S. sclarea, S. hispanica, S. africana, and S. mexicana)
4.3. Antioxidant Activities of S. miltiorrhiza, S. verbenaca, S. chamelaeagnea, S. bulleyana, S. multicaulis, and S. glutinosa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAPH | 2,2′–Azobis(2–Amidinopropane) Dihydrochloride |
| ABTS | 2,2′–Azinobis– (3–ethyl-benzothiazoline–6–sulfonic acid) |
| AgNPS | Silver Nanoparticles |
| CAA | Cellular Antioxidant Assay |
| CAT | Catalase |
| DCFH-DA | 2′,7′–Dichlorofluorescin Diacetate |
| DPPH | 2,2–Di(4–tert–octylphenyl)–1–picrylhydrazyl |
| FRAP | Ferric Reduction of Antioxidant Power |
| CUPRAC | Cupric Ion Reducing Antioxidant Capacity |
| DMPD | N,N–Dimethyl-p-phenylenediamine Dihydrochloride |
| EO | Essential Oil |
| ET | Electron Transfer |
| FC | Folin–Ciocalteu |
| FD | Freeze Drying |
| gGT | gamma–Glutamyl Transpeptidase |
| GR | Glutathione Reductase |
| GSH | Glutathione |
| GSHPx | Glutathione Peroxidase |
| GSSG | Glutathione Disulfide |
| GST | Glutathione–S–Transferase |
| HAT | Hydrogen Atom Transfer |
| HD | Hydrodistillation |
| HORAC | Hydroxyl Radical Antioxidant Capacity |
| LPO | Lipid Peroxidation |
| MAHD | Microwave–Assisted Hydrodistillation |
| MAE | Microwave–Assisted Extraction |
| MDA | Malondialdehyde |
| MHD | Microwave–Assisted Hydrodistillation |
| ORAC | Oxygen Radical Antioxidant Capacity |
| OxHLIA | Oxidative Hemolysis Inhibition Assay |
| qPCR | Quantitative PCR |
| ROS | Reactive Nitrogen Species |
| RNS | Reactive Oxygen Species |
| SC–CO2 | Supercritical CO2 Extraction |
| SCWE | Subcritical Water Extraction |
| SD | Steam Distillation |
| SFE | Supercritical Fluid Extraction |
| SFME | Solvent–Free Microwave-Assisted Extraction |
| SHD | Sonohydrodistillation |
| SE | Soxhlet Extraction |
| SET | Single Electron Transfer |
| SLE | Solid–Liquid Extraction |
| SOD | Superoxide Dismutase |
| TAC | Total Antioxidant Capacity |
| TBA | Thiobarbituric Acid |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| TF | Total Flavonoids |
| TP | Total Phenols |
| TPC | Total Phenolic Content |
| TPTZ | Tripyridyl Triazine |
| TRAP | Total Peroxyl Radical-Trapping Antioxidant Parameter |
| UAE | Ultrasound–Assisted Extraction |
References
- Noce, A.; Romani, A.; Bernini, R. Dietary Intake and Chronic Disease Prevention. Nutrients 2021, 13, 1358. [Google Scholar] [CrossRef]
- Taneja, N.K.; Singh, A.; Shivaprasad, D.P.; Taneja, P.; Sachdev, D. Nutraceuticals and Natural-Product Derivatives for Disease Prevention. In Handbook of Nutraceuticals and Natural Products; Wiley: England, UK, 2022; pp. 143–198. [Google Scholar]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current Advances on the Extraction and Identification of Bioactive Components of Sage (Salvia spp.). Curr. Pharm. Biotechnol. 2019, 20, 845–857. [Google Scholar] [CrossRef]
- Ertas, A.; Yigitkan, S.; Orhan, I.E. A Focused Review on Cognitive Improvement by the Genus Salvia, L. (Sage)—From Ethnopharmacology to Clinical Evidence. Pharmaceuticals 2023, 16, 171. [Google Scholar] [CrossRef]
- Uysal, I.; Koçer, O.; Mohammed, F.S.; Lekesiz, Ö.; Doğan, M.; Şabik, A.E.; Sevindik, E.; Gerçeker, F.Ö.; Sevindik, M. Pharmacological and Nutritional Properties: Genus Salvia. Adv. Pharmacol. Pharm. 2023, 11, 140–155. [Google Scholar] [CrossRef]
- Sazatornil, F.; Fornoni, J.; Fragoso-Martínez, I.; Pérez-Ishiwara, R.; Benitez-Vieyra, S. Did early shifts to bird pollination impose constraints on Salvia flower evolution? Evolution 2023, 77, 636–645. [Google Scholar] [CrossRef]
- Selim, S.; Almuhayawi, M.S.; Alqhtani, H.; Al Jaouni, S.K.; Saleh, F.M.; Warrad, M.; Hagagy, N. Anti-Salmonella and Antibiofilm Potency of Salvia officinalis L. Essential Oil against Antibiotic-Resistant Salmonella enterica. Antibiotics 2022, 11, 489. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef]
- Mervić, M.; Bival Štefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants 2022, 11, 625. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current State of the Art on the Antioxidant Activity of Sage (Salvia spp.) and Its Bioactive Components. Planta Med. 2020, 86, 224–238. [Google Scholar] [CrossRef]
- Luca, S.V.; Skalicka-Woźniak, K.; Mihai, C.-T.; Gradinaru, A.C.; Mandici, A.; Ciocarlan, N.; Miron, A.; Aprotosoaie, A.C. Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe. Antioxidants 2023, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Aydin, D.; Yalçin, E.; Çavuşoğlu, K. Metal chelating and anti-radical activity of Salvia officinalis in the ameliorative effects against uranium toxicity. Sci. Rep. 2022, 12, 15845. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Piątczak, E.; Owczarek, A.; Lisiecki, P.; Gonciarz, W.; Kozłowska, W.; Szemraj, M.; Chmiela, M.; Kiss, A.K.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Identification and quantification of phenolic compounds in Salvia cadmica Boiss. and their biological potential. Ind. Crops Prod. 2021, 160, 113113. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Margetts, G.; Kleidonas, S.; Zaibi, N.S.; Zaibi, M.S.; Edwards, K.D. Evidence for anti-inflammatory effects and modulation of neurotransmitter metabolism by Salvia officinalis L. BMC Complement. Med. Ther. 2022, 22, 131. [Google Scholar] [CrossRef]
- Wightman, E.L.; Jackson, P.A.; Spittlehouse, B.; Heffernan, T.; Guillemet, D.; Kennedy, D.O. The Acute and Chronic Cognitive Effects of a Sage Extract: A Randomized, Placebo Controlled Study in Healthy Humans. Nutrients 2021, 13, 218. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Menzies, R.; Hodgson, F.; Wedgewood, P.; Howes, M.J.R.; Brooker, H.J.; Wesnes, K.A.; Perry, E.K. A randomised double-blind placebo-controlled pilot trial of a combined extract of sage, rosemary and melissa, traditional herbal medicines, on the enhancement of memory in normal healthy subjects, including influence of age. Phytomedicine 2018, 39, 42–48. [Google Scholar] [CrossRef]
- Jiang, J.-S.; Gu, Q.-c.; Feng, Z.-M.; Yuan, X.; Zhang, X.; Zhang, P.-C.; Yang, Y.-N. The tanshinones from the plant of Salvia miltiorrhiza. Phytochemistry 2023, 210, 113673. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, M.; Cheng, W.; Yao, J.; Ma, L.; Wang, Y.; Wang, W.; Cao, J. A Pharmacological Review of Tanshinones, Naturally Occurring Monomers from Salvia miltiorrhiza for the Treatment of Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2023, 2023, 1–24. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Zhang, Y.; Wan, H.; Wan, H.; Yang, J.; Cao, J. Inhibition of Oxidative Stress: An Important Molecular Mechanism of Chinese Herbal Medicine (Astragalus membranaceus, Carthamus tinctorius L.; Radix Salvia miltiorrhizae, etc.) in the Treatment of Ischemic Stroke by Regulating the Antioxidant System. Oxidative Med. Cell. Longev. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Petitjean, S.J.L.; Lecocq, M.; Lelong, C.; Denis, R.; Defrère, S.; Mariage, P.-A.; Alsteens, D.; Pilette, C. Salvia miltiorrhiza Bunge as a Potential Natural Compound against COVID-19. Cells 2022, 11, 1311. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a Glance: An Up-to-Date Overview on Variants, Drug Design and Therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2018, 59, 953–964. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, H.; Peng, C.; Zhang, Z.; Yuan, Q.; Gao, H.; Tang, S.; Xie, C. Renoprotective Effects of Tanshinone IIA: A Literature Review. Molecules 2023, 28, 1990. [Google Scholar] [CrossRef]
- Elebeedy, D.; Badawy, I.; Elmaaty, A.A.; Saleh, M.M.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; Abd El Maksoud, A.I.; Al-karmalawy, A.A. In vitro and computational insights revealing the potential inhibitory effect of Tanshinone IIA against influenza A virus. Comput. Biol. Med. 2022, 141, 105149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, C.; Li, J.; Lu, Y.; Li, H.; Zhuang, J.; Ren, X.; Wang, M.; Sun, C. Tanshinone IIA: New Perspective on the Anti-Tumor Mechanism of a Traditional Natural Medicine. Am. J. Chin. Med. 2022, 50, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.S.M.; Samanta, A.; Uddin, F.; Ali, S.; Hoque, M. Tanshinone IIA targeting cell signaling pathways: A plausible paradigm for cancer therapy. Pharmacol. Rep. 2023, 75, 907–922. [Google Scholar] [CrossRef]
- Ni, H.; Ruan, G.; Sun, C.; Yang, X.; Miao, Z.; Li, J.; Chen, Y.; Qin, H.; Liu, Y.; Zheng, L.; et al. Tanshinone IIA inhibits gastric cancer cell stemness through inducing ferroptosis. Environ. Toxicol. 2021, 37, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Pachura, N.; Zimmer, A.; Grzywna, K.; Figiel, A.; Szumny, A.; Łyczko, J. Chemical investigation on Salvia officinalis L. Affected by multiple drying techniques—The comprehensive analytical approach (HS-SPME, GC–MS, LC-MS/MS, GC-O and NMR). Food Chem. 2022, 397, 133802. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Caron, M.M.; Chen, X.; Yu, D.; Niu, Y. Effect of temperature, light and storage on seed germination of Salvia plebeia R.Br., Leonurus japonicus Houtt., Mosla scabra (Thunb.) C.Y. Wu & H.W. Li and Perilla frutescens (L.) Britton. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100402. [Google Scholar] [CrossRef]
- Khoshsokhan, F.; Babalar, M.; Salami, S.A.; Sheikhakbari-Mehr, R.; Mirjalili, M.H. An efficient protocol for production of rosmarinic acid in Salvia nemorosa L. Vitr. Cell. Dev. Biol. Plant 2023, 59, 298–314. [Google Scholar] [CrossRef]
- Fan, Q.; Li, Y. Enrichment of rosmarinic acid from Salvia przewalskii Maxim. leaves using macroporous resin: Adsorption/desorption behavior, process optimization followed by scale-up. Ind. Crops Prod. 2023, 191, 115931. [Google Scholar] [CrossRef]
- Ozón, B.; Cotabarren, J.; Geier, F.R.; Kise, M.P.; García-Pardo, J.; Parisi, M.G.; Obregón, W.D. Development of Fortified Breads Enriched with Plant-Based Bioactive Peptides Derived from the Chia (Salvia hispanica L.) Expeller. Foods 2023, 12, 3382. [Google Scholar] [CrossRef]
- Domingo-Fernández, D.; Gadiya, Y.; Mubeen, S.; Bollerman, T.J.; Healy, M.D.; Chanana, S.; Sadovsky, R.G.; Healey, D.; Colluru, V. Modern drug discovery using ethnobotany: A large-scale cross-cultural analysis of traditional medicine reveals common therapeutic uses. iScience 2023, 26, 107729. [Google Scholar] [CrossRef]
- Pizani, R.S.; Viganó, J.; de Souza Mesquita, L.M.; Contieri, L.S.; Sanches, V.L.; Chaves, J.O.; Souza, M.C.; da Silva, L.C.; Rostagno, M.A. Beyond aroma: A review on advanced extraction processes from rosemary (Rosmarinus officinalis) and sage (Salvia officinalis) to produce phenolic acids and diterpenes. Trends Food Sci. Technol. 2022, 127, 245–262. [Google Scholar] [CrossRef]
- Tashani, F.; Karami, A.; Tahmasebi, A.; Maggi, F. Variability in chemical composition and antibacterial activity of Salvia majdae essential oil under various extraction techniques. J. Essent. Oil Res. 2022, 34, 279–289. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Cvetković, M.T.; Stanković Jeremić, J.M.; Pezo, L.L.; Varga, A.O.; Čabarkapa, I.S.; Kiprovski, B. Biological activity and profiling of Salvia sclarea essential oil obtained by steam and hydrodistillation extraction methods via chemometrics tools. Flavour Fragr. J. 2021, 37, 20–32. [Google Scholar] [CrossRef]
- Machado, C.A.; Oliveira, F.O.; de Andrade, M.A.; Hodel, K.V.S.; Lepikson, H.; Machado, B.A.S. Steam Distillation for Essential Oil Extraction: An Evaluation of Technological Advances Based on an Analysis of Patent Documents. Sustainability 2022, 14, 7119. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Mahdjoub, M.m.; Kadri, N.; Remini, H. Definitive screening design and I-optimal design for optimization of ultrasound-assisted extraction of phenolic content and antioxidant capacity from Salvia officinalis L. leaves. Sustain. Chem. Pharm. 2022, 29, 100820. [Google Scholar] [CrossRef]
- Benmoussa, H.; Béchohra, I.; He, S.; Elfalleh, W.; Chawech, R. Optimization of sonohydrodistillation and microwave assisted hydrodistillation by response surface methodology for extraction of essential oils from Cinnamomum cassia barks. Ind. Crops Prod. 2023, 192, 115995. [Google Scholar] [CrossRef]
- Peng, X.; Liu, N.; Wang, M.; Liang, B.; Feng, C.; Zhang, R.; Wang, X.; Hu, X.; Gu, H.; Xing, D. Recent advances of kinetic model in the separation of essential oils by microwave-assisted hydrodistillation. Ind. Crops Prod. 2022, 187, 115418. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Saleh, I.; Ali, S.; Hussien, T.; Hegazi, N.M.; Abdel-Halim, S.; Abd El-Razek, M.H.; El-Beih, A.; Marzouk, M.M.; Pare, P.W.; et al. A Comparative Evaluation of the Antimicrobial Activities of the Essential Oils of Three Salvia Species Growing in Egypt, Obtained by Hydrodistillation and Microwave-Assisted Hydro-distillation. J. Essent. Oil Bear. Plants 2022, 25, 1109–1121. [Google Scholar] [CrossRef]
- Nitthiyah, J.; Nour, A.H.; Ramesh, K.; Akindoyo, J.O. Microwave Assisted Hydrodistillation—An Overview of Mechanism and Heating Properties. Heliyon 2017, 6, e04893. [Google Scholar]
- Patrice Didion, Y.; Gijsbert Tjalsma, T.; Su, Z.; Malankowska, M.; Pinelo, M. What is next? The greener future of solid liquid extraction of biobased compounds: Novel techniques and solvents overpower traditional ones. Sep. Purif. Technol. 2023, 320. [Google Scholar] [CrossRef]
- Vieira, S.F.; Ferreira, H.; Neves, N.M. Antioxidant and Anti-Inflammatory Activities of Cytocompatible Salvia officinalis Extracts: A Comparison between Traditional and Soxhlet Extraction. Antioxidants 2020, 9, 1157. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Ilea, M.; Dias, M.I.; Calhelha, R.C.; Gavrilaș, L.; Rocchetti, G.; Crișan, G.; Mocan, A.; Barros, L.; et al. Potential therapeutic applications of infusions and hydroalcoholic extracts of Romanian glutinous sage (Salvia glutinosa L.). Front. Pharmacol. 2022, 13, 975800. [Google Scholar] [CrossRef] [PubMed]
- Mondor, M. Chia (Salvia hispanica) Seed Oil Extraction By-Product and Its Edible Applications. Food Rev. Int. 2023, 1–20. [Google Scholar] [CrossRef]
- Wang, S.; Su, Y.; Li, J.; Lu, Y.; Mei, X.; Wang, J. Integration of LC/MS-based molecular networking and molecular docking allows in-depth annotation and prediction of the metabolome: A study of Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2022, 186, 115298. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Zhu, Z.; Huang, D.; Qi, Y.; Ma, C.; Zou, Z.; Ni, H. Cinnamomum camphora fruit peel as a source of essential oil extracted using the solvent-free microwave-assisted method compared with conventional hydrodistillation. LWT 2022, 153, 112549. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, X.-h.; Jiang, W.-j. Theoretical models for supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Zainal Abidin, Z.; Yoshida, H.; Yunus, R.; Awang Biak, D.R. Towards Higher Oil Yield and Quality of Essential Oil Extracted from Aquilaria malaccensis Wood via the Subcritical Technique. Molecules 2020, 25, 3872. [Google Scholar] [CrossRef]
- Fikri, I.; Yulianah, Y.; Lin, T.-C.; Lin, R.-W.; Chen, U.-C.; Lay, H.-L. Optimization of supercritical fluid extraction of dihydrotanshinone, cryptotanshinone, tanshinone I, and tanshinone IIA from Salvia miltiorrhiza with a peanut oil modifier. Chem. Eng. Res. Des. 2022, 180, 220–231. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Abdelkader, M.; Ahcen, B.; Rachid, D.; Hakim, H. Phytochemical Study and Biological Activity of Sage (Salvia officinalis L.). World Acad. Sci. Eng. Technol. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015, 8, 1253–1257. [Google Scholar]
- Conde-Hernández, L.A.; Luna-Guevara, M.L.; Luna-Guevara, J.J.; Pérez-Vázquez, J.; Aranda-García, R.J.; Singh, A.K. Mexican Sage (Salvia officinalis) Extraction Using Factorial Design and Its Effect on Chemical and Antibacterial Properties. J. Chem. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Irakli, M.; Bouloumpasi, E.; Christaki, S.; Skendi, A.; Chatzopoulou, P. Modeling and Optimization of Phenolic Compounds from Sage (Salvia fruticosa L.) Post-Distillation Residues: Ultrasound- versus Microwave-Assisted Extraction. Antioxidants 2023, 12, 549. [Google Scholar] [CrossRef]
- Modi, P.I.; Parikh, J.K.; Desai, M.A. Sonohydrodistillation: Innovative approach for isolation of essential oil from the bark of cinnamon. Ind. Crops Prod. 2019, 142, 111838. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Ahmad Zaini, M.A.; Sulaiman, H. A comparative study of various oil extraction techniques from plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Nn, A. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Francik, S.; Francik, R.; Sadowska, U.; Bystrowska, B.; Zawiślak, A.; Knapczyk, A.; Nzeyimana, A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials 2020, 13, 5811. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chouhan, K.B.S.; Chandrakar, M.; Gupta, P.; Lal, K.; Mandal, V. A cross talk based critical analysis of solvent free microwave extraction to accentuate it as the new normal for extraction of essential oil: An attempt to overhaul the science of distillation through a comprehensive tutelage. Crit. Rev. Food Sci. Nutr. 2022, 63, 6960–6982. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Rodríguez, P.; Díaz-Reinoso, B.; Gaspar, M.C.; de Sousa, H.C.; Braga, M.E.M.; Domínguez, H. Supercritical fluid extraction as a suitable technology to recover bioactive compounds from flowers. J. Supercrit. Fluids 2022, 188, 105652. [Google Scholar] [CrossRef]
- Iacopetta, D.; Baldino, N.; Caruso, A.; Perri, V.; Lupi, F.R.; de Cindio, B.; Gabriele, D.; Sinicropi, M.S. Nutraceuticals Obtained by SFE-CO2 from Cladodes of Two Opuntia ficus-indica (L.) Mill Wild in Calabria. Appl. Sci. 2021, 11, 477. [Google Scholar] [CrossRef]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercritical fluid extraction from microalgae with high content of LC-PUFAs. A case of study: Sc-CO2 oil extraction from Schizochytrium sp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Zinnai, A. A Simplified Method to Estimate Sc-CO2 Extraction of Bioactive Compounds from Different Matrices: Chili Pepper vs. Tomato By-Products. Appl. Sci. 2017, 7, 361. [Google Scholar] [CrossRef]
- Rója, E.; Gagoś, M.; Dobrzyńska-Inger, A. Cost Optimization of Extract Production in Supercritical Extraction Process with the Use of CO2—A Novel Approach. Procedia Eng. 2012, 42, 323–328. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Demirci Çekiç, S.; Çetinkaya, A.; Avan, A.N.; Apak, R. Correlation of Total Antioxidant Capacity with Reactive Oxygen Species (ROS) Consumption Measured by Oxidative Conversion. J. Agric. Food Chem. 2013, 61, 5260–5270. [Google Scholar] [CrossRef]
- Čulina, P.; Cvitković, D.; Pfeifer, D.; Zorić, Z.; Repajić, M.; Elez Garofulić, I.; Balbino, S.; Pedisić, S. Phenolic Profile and Antioxidant Capacity of Selected Medicinal and Aromatic Plants: Diversity upon Plant Species and Extraction Technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Bentayeb, K.; Vera, P.; Rubio, C.; Nerín, C. The additive properties of Oxygen Radical Absorbance Capacity (ORAC) assay: The case of essential oils. Food Chem. 2014, 148, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Groo, A.-C.; Iacopetta, D.; Séguy, L.; Mariconda, A.; Puoci, F.; Saturnino, C.; Leroy, F.; Since, M.; Longo, P.; et al. A winning strategy to improve the anticancer properties of Cisplatin and Quercetin based on the nanoemulsions formulation. J. Drug Deliv. Sci. Technol. 2021, 66, 102907. [Google Scholar] [CrossRef]
- Marchica, A.; Lorenzini, G.; Papini, R.; Bernardi, R.; Nali, C.; Pellegrini, E. Signalling molecules responsive to ozone-induced oxidative stress in Salvia officinalis. Sci. Total Environ. 2019, 657, 568–576. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Jedidi, S.; Aloui, F.; Rtibi, K.; Sammari, H.; Selmi, H.; Rejeb, A.; Toumi, L.; Sebai, H. Individual and synergistic protective properties of Salvia officinalis decoction extract and sulfasalazine against ethanol-induced gastric and small bowel injuries. RSC Adv. 2020, 10, 35998–36013. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.; de Mello, J. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense, L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Ozdemir Olgun, F.A.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin–Ciocalteu Antioxidant Capacity Assay for Measuring Lipophilic Antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef]
- Dong, J.-W.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z.-T. Re-evaluation of ABTS•+ Assay for Total Antioxidant Capacity of Natural Products. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef]
- Ceramella, J.; La Torre, C.; De Luca, M.; Iacopetta, D.; Fazio, A.; Catalano, A.; Ragno, G.; Longo, P.; Sinicropi, M.S.; Rosano, C. Exploring the anticancer and antioxidant properties of Vicia faba L. pods extracts, a promising source of nutraceuticals. PeerJ 2022, 10, e13683. [Google Scholar] [CrossRef] [PubMed]
- Ovidi, E.; Laghezza Masci, V.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus nobilis, Salvia sclarea and Salvia officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef]
- Belkhiri, F.; Baghiani, A.; Zerroug, M.M.; Arrar, L. Investigation of Antihemolytic, Xanthine Oxidase Inhibition, Antioxidant and Antimicrobial Properties of Salvia verbenaca, L. Aerial Part Extracts. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 273–281. [Google Scholar] [CrossRef]
- Righi, N.; Boumerfeg, S.; Deghima, A.; Fernandes, P.A.R.; Coelho, E.; Baali, F.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Phenolic profile, safety assessment, and anti-inflammatory activity of Salvia verbenaca L. J. Ethnopharmacol. 2021, 272, 113940. [Google Scholar] [CrossRef]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M.R. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Graziano, A.; Avola, R.; Bruno, M.; Rigano, D. Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 292. [Google Scholar] [CrossRef]
- Iacopetta, D.; Fazio, A.; La Torre, C.; Barbarossa, A.; Ceramella, J.; Francomano, F.; Saturnino, C.; El-Kashef, H.; Alcaro, S.; Sinicropi, M.S. Annona cherimola Mill. Leaf Extracts Affect Melanoma Cells Growth and Progression. Foods 2022, 11, 2420. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Saturnino, C.; Malzert-Fréon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. 2019, 10, 4280–4290. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the Way to Fight Cancer Paved with Gold? Metal-Based Carbene Complexes with Multiple and Fascinating Biological Features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Souad, M.; Rachid, A.; Farid Boucif, L. Antioxidant Activity and Hemolytic Effect of Hydro-Methanolic Extract and its Phenolic Enriched Fractions from Leaves and Stems of Salvia officinalis L. from Algeria. J. Nat. Prod. Res. Appl. 2021, 1, 17–30. [Google Scholar] [CrossRef]
- Coelho-Fernandes, S.; Rodrigues, G.; Faria, A.S.; Caleja, C.; Pereira, E.; Pinela, J.; Carocho, M.; Barros, L.; Cadavez, V.; Gonzales-Barron, U. Effect of Sage (Salvia officinalis L.) Extract on the Survival of Staphylococcus aureus in Portuguese Alheira Sausage during Maturation. In Proceedings of the 2nd International Electronic Conference on Foods—”Future Foods and Food Technologies for a Sustainable World”. Biol. Life Sci. Forum 2021, 6, 125. [Google Scholar] [CrossRef]
- Khedher, M.R.B.; Khedher, S.B.; Chaieb, I.; Tounsi, S.; Hammami, M. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. EXCLI J. 2017, 16, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Patil, S. Evaluation of Traditional Herb Extract Salvia officinalis in Treatment of Alzheimers Disease. Pharmacogn. J. 2020, 12, 131–143. [Google Scholar] [CrossRef]
- Altay, A.; Kılıc Suloglu, A.; Sagdıcoglu Celep, G.; Selmanoglu, G.; Bozoglu, F. Anatolıan sage Salvıa frutıcosa ınhıbıts cytosolıc glutathıone-s-transferase actıvıty and colon cancer cell prolıferatıon. J. Food Meas. Charact. 2019, 13, 1390–1399. [Google Scholar] [CrossRef]
- Lieshchova, M.A.; Bohomaz, A.A.; Brygadyrenko, V.V. Effect of Salvia officinalis and S. sclarea on rats with a high-fat hypercaloric diet. Regul. Mech. Biosyst. 2021, 12, 554–563. [Google Scholar] [CrossRef]
- Alabdallat, N.G. Effect of orally administered aqueous extract of Salvia triloba L. in human volunteers. Ann. Phytomed. Int. J. 2021, 10, S111–S115. [Google Scholar] [CrossRef]
- Ripke Ferreira, C.S.; Figueiredo Saqueti, B.H.; Silva dos Santos, P.D.; Martins da Silva, J.; Matiucci, M.A.; Feihrmann, A.C.; Graton Mikcha, J.M.; Santos, O.O. Effect of Salvia (Salvia officinalis) on the oxidative stability of salmon hamburgers. LWT 2022, 154, 112867. [Google Scholar] [CrossRef]
- Hamrouni-Sellami, I.; Rahali, F.Z.; Rebey, I.B.; Bourgou, S.; Limam, F.; Marzouk, B. Total Phenolics, Flavonoids, and Antioxidant Activity of Sage (Salvia officinalis L.) Plants as Affected by Different Drying Methods. Food Bioprocess Technol. 2012, 6, 806–817. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Standiford, M.; Johnson, D.A.; Johnson, J.A. Structure activity relationship of phenolic diterpenes from Salvia officinalis as activators of the nuclear factor E2-related factor 2 pathway. Bioorg. Med. Chem. 2013, 21, 2618–2622. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Smach, M.A.; Hafsa, J.; Charfeddine, B.; Dridi, H.; Limem, K. Effects of sage extract on memory performance in mice and acetylcholinesterase activity. Ann. Pharm. Françaises 2015, 73, 281–288. [Google Scholar] [CrossRef]
- Reis, F.; Madureira, A.R.; Nunes, S.; Campos, D.; Fernandes, J.; Marques, C.; Zuzarte, M.; Gullón, B.; Rodríguez-Alcalá, L.M.; Calhau, C.; et al. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: In vitro and animal approaches. Int. J. Nanomed. 2016, 11, 3621–3640. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cindrić, M.; Zeković, Z. Subcritical water extraction of sage (Salvia officinalis L.) by-products—Process optimization by response surface methodology. J. Supercrit. Fluids 2016, 116, 36–45. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Composition and Antioxidant, Antienzymatic and Antimicrobial Activities of Volatile Molecules from Spanish Salvia lavandulifolia (Vahl) Essential Oils. Molecules 2017, 22, 1382. [Google Scholar] [CrossRef]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Salević, A.; Prieto, C.; Cabedo, L.; Nedović, V.; Lagaron, J. Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials 2019, 9, 270. [Google Scholar] [CrossRef]
- Tundis, R.; Leporini, M.; Bonesi, M.; Rovito, S.; Passalacqua, N.G. Salvia officinalis L. from Italy: A Comparative Chemical and Biological Study of Its Essential Oil in the Mediterranean Context. Molecules 2020, 25, 5826. [Google Scholar] [CrossRef] [PubMed]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of Plant Extracts on the Characteristics of Silver Nanoparticles for Topical Application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef]
- Ueda, J.M.; Pedrosa, M.C.; Fernandes, F.A.; Rodrigues, P.; Melgar, B.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Ivanov, M.; Soković, M.; et al. Promising Preserving Agents from Sage and Basil: A Case Study with Yogurts. Foods 2021, 10, 676. [Google Scholar] [CrossRef]
- Cvitković, D.; Lisica, P.; Zorić, Z.; Repajić, M.; Pedisić, S.; Dragović-Uzelac, V.; Balbino, S. Composition and Antioxidant Properties of Pigments of Mediterranean Herbs and Spices as Affected by Different Extraction Methods. Foods 2021, 10, 2477. [Google Scholar] [CrossRef]
- Đurović, S.; Micić, D.; Pezo, L.; Radić, D.; Bazarnova, J.G.; Smyatskaya, Y.A.; Blagojević, S. The effect of various extraction techniques on the quality of sage (Salvia officinalis L.) essential oil, expressed by chemical composition, thermal properties and biological activity. Food Chem. X 2022, 13. [Google Scholar] [CrossRef]
- Jedidi, S.; Aloui, F.; Selmi, S.; Selmi, H.; Sammari, H.; Ayari, A.; Abbes, C.; Sebai, H. Antioxidant Properties of Salvia officinalis Decoction Extract and Mechanism of Its Protective Effects on Ethanol-Induced Liver and Kidney Injuries. J. Med. Food 2022, 25, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Hrebień-Filisińska, A.M.; Bartkowiak, A. Antioxidative Effect of Sage (Salvia officinalis L.) Macerate as “Green Extract” in Inhibiting the Oxidation of Fish Oil. Antioxidants 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.; Catarino, M.; Afonso, A.; Silva, A.; Cardoso, S. Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant Activities and Inhibition of Carbohydrate and Lipid Metabolic Enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef]
- Gad, H.A.; Mamadalieva, R.Z.; Khalil, N.; Zengin, G.; Najar, B.; Khojimatov, O.K.; Al Musayeib, N.M.; Ashour, M.L.; Mamadalieva, N.Z. GC-MS Chemical Profiling, Biological Investigation of Three Salvia Species Growing in Uzbekistan. Molecules 2022, 27, 5365. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dziadek, M.; Sadowska, U.; Cholewa-Kowalska, K. The Changes in Bioactive Compounds and Antioxidant Activity of Chia (Salvia hispanica L.) Herb under Storage and Different Drying Conditions: A Comparison with Other Species of Sage. Molecules 2022, 27, 1569. [Google Scholar] [CrossRef]
- Fei, A.-h.; Cao, Q.; Chen, S.-y.; Wang, H.-r.; Wang, F.-l.; Pan, S.-m.; Lin, Z.-f. Salvianolate inhibits reactive oxygen species production in H2O2-treated mouse cardiomyocytes in vitro via the TGFβ pathway. Acta Pharmacologica Sinica 2013, 34, 496–500. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, J.; Dai, Z.; Lin, R.; Wang, G.; Ma, S. A Pair of New Antioxidant Phenolic Acid Stereoisomers Isolated from Danshen Injection (Lyophilized Powder). Molecules 2014, 19, 1786–1794. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Cen, Y.; Yang, D.; Qi, Z.; Hou, Z.; Han, S.; Cai, Z.; Liu, K. Bivariate Correlation Analysis of the Chemometric Profiles of Chinese Wild Salvia miltiorrhiza Based on UPLC-Qqq-MS and Antioxidant Activities. Molecules 2018, 23, 538. [Google Scholar] [CrossRef] [PubMed]
- Etsassala, N.G.E.R.; Adeloye, A.O.; El-Halawany, A.; Hussein, A.A.; Iwuoha, E.I. Investigation of In-Vitro Antioxidant and Electrochemical Activities of Isolated Compounds from Salvia chamelaeagnea P.J. Bergius Extract. Antioxidants 2019, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical Profile and Antioxidant Activity of Aerial and Underground Parts of Salvia bulleyana Diels. Plants. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Rowshan, V.; Najafian, S. Polyphenolic contents and antioxidant activities of aerial parts of Salvia multicaulis from the Iran flora. Nat. Prod. Res. 2019, 34, 2351–2353. [Google Scholar] [CrossRef]
| Method of Extraction | Acronym | Refs. |
|---|---|---|
| Hydrodistillation | HD | Aćimović et al., 2022 [44] |
| Steam Distillation | SD | Machado et al., 2022 [45] |
| Ultrasound-Assisted Extraction | UAE | Moussa et al., 2022 [46] |
| Sonohydrodistillation | SHD | Benmoussa et al., 2023 [47] |
| Microwave-Assisted Extraction | MAE | Peng et al., 2022 [48] |
| Microwave-Assisted Hydrodistillation | MHD or MAHD | Mohamed et al., 2022 [49] |
| Solid–Liquid Extraction | SLE | Didion et al., 2022 [51] |
| Soxhlet Extraction | SE | Vieira et al., 2020 [52] |
| Infusion | - | Nicolescu et al., 2022 [53] |
| Freeze-Drying | FD | Mondor et al., 2023 [54] Wang et al., 2022 [55] |
| Solvent-Free Microwave-Assisted Extraction | SFME | Liu et al., 2022 [56] |
| Supercritical Fluid Extraction | SFE | Huang et al., 2012 [57] |
| Subcritical Water Extraction | SCWE | Samadi et al., 2020 [58] |
| Supercritical CO2 Extraction | SC-CO2 | Fikri et al., 2022 [59] Alara et al., 2021 [60] |
| Species | Material | Country | Extract | Antioxidant Activity Determination Method | Refs. |
|---|---|---|---|---|---|
| S. officinalis L. | aerial parts | Tunisia | methanol extract | DPPH, β-carotene bleaching | Hamrouni-Sellami et al., 2013 [109] |
| S. officinalis L. | dried aerial parts | Netherlands | acetone extract | CAA | Fischedick et al., 2013 [110] |
| S. officinalis L. | flowering aerial parts | Spain | methanol/water (80:20, v/v) extract | DPPH, β-carotene bleaching, lipid peroxidation inhibition | Martins et al., 2015 [111] |
| S. officinalis L. | aerial parts | Tunisia | aqueous extract | DPPH, GSH | Smach et al., 2015 [112] |
| S. officinalis L. and savory (Satureja montana) | leaves | Portugal | solid-lipid NP aqueous extract | TBARS | Reis et al., 2016 [113] |
| S. officinalis L. | herbal dust | Montenegro | subcritical water extraction | FRAP | Pavlić et al., 2016 [114] |
| S. officinalis L. subsp. Lavandulifolia (Vahl) Gams or Spanish sage | aerial part of plants | Spain | EOs | ORAC, DPPH, ABTS, FRAP | Cutillas et al., 2017 [115] |
| S. officinalis L. | ground leaves | Bosnia and Herzegovina | CO2 extract | DPPH | Pavić et al., 2019 [116] |
| S. officinalis L. | plant | Serbia | solid dispersion | DPPH | Salević et al., 2019 [117] |
| S. officinalis L. | fresh aerial parts | Italy | EO | DPPH, ABTS, FRAP, β-carotene | Tundis et al., 2020 [118] |
| S. officinalis L. | commercial-grade cosmetics | Greece | AgNPs and hydroglycolic extracts | DPPH | Siakavella et al., 2020 [119] |
| S. officinalis L. variety Bona | leaves | Poland | water/ethanol (50% v/v) extract | DPPH, FRAP | Francik et al., 2020 [69] |
| S. officinalis L. | dried flowers | Tunisia | aqueous extract | β-carotene, SOD, CAT, GPx | Jedidi et al., 2020 [86] |
| S. officinalis L. | leaves | Croatia | ethyl acetate | FRAP | Cvitković et al., 2021 [121] |
| S. officinalis L. | leaves | Serbia | EO | DPPH, CUPRAC, FRAP, ABTS, HRSA, TBARS | Đurović et al., 2022 [94] |
| S. officinalis L. | flowers | Tunisia | aqueous extract | ABTS, SOD, CAT, GPx | Jedidi et al., 2022 [123] |
| S. officinalis L. var Bona | leaves | Poland | fish oil extract | DPPH | Hrebień-Filisińska & Bartkowiak 2022 [124] |
| S. officinalis L. | commercial EO | Romania | EO | DPPH, ABTS | Mot et al., 2022 [125] |
| Species | Material | Country | Extract | Antioxidant Activity Determination Method | Ref |
|---|---|---|---|---|---|
| Salvia elegans Vahl., Salvia greggii A. Gray, and S. officinalis L. | aerial parts (flowers, leaves, and stems) | Portugal | hexane extract | DPPH; FRAP | Pereira et al., 2018 [126] |
| S. africana, S. officinalis ‘Icterina’, and S. mexicana, | aerial parts (flowers, leaves, and stems) | Portugal | hexane extract | DPPH; TBARS; β-carotene | Afonso et al., 2019 [127] |
| Salvia sclarea and Salvia officinalis | inflorescences | Italy | EO | DPPH; ABTS | Ovidi et al., 2021 [92] |
| S. officinalis L., S. virgata, and S. sclarea. | aerial parts | Uzbekistan | EO | DPPH; ABTS; CUPRAC; FRAP | Gad et al., 2022 [128] |
| S. hispanica L. (Chia), in comparison with S. officinalis L. and S. sclarea L. | whole herb (leaves and stems) | Poland | methanolic extract | ABTS | Dziadek et al., 2022 [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacopetta, D.; Ceramella, J.; Scumaci, D.; Catalano, A.; Sinicropi, M.S.; Tundis, R.; Alcaro, S.; Borges, F. An Update on Recent Studies Focusing on the Antioxidant Properties of Salvia Species. Antioxidants 2023, 12, 2106. https://doi.org/10.3390/antiox12122106
Iacopetta D, Ceramella J, Scumaci D, Catalano A, Sinicropi MS, Tundis R, Alcaro S, Borges F. An Update on Recent Studies Focusing on the Antioxidant Properties of Salvia Species. Antioxidants. 2023; 12(12):2106. https://doi.org/10.3390/antiox12122106
Chicago/Turabian StyleIacopetta, Domenico, Jessica Ceramella, Domenica Scumaci, Alessia Catalano, Maria Stefania Sinicropi, Rosa Tundis, Stefano Alcaro, and Fernanda Borges. 2023. "An Update on Recent Studies Focusing on the Antioxidant Properties of Salvia Species" Antioxidants 12, no. 12: 2106. https://doi.org/10.3390/antiox12122106








