Evaluation of Inhibitory Activities of Sophora flavescens and Angelica gigas Nakai Root Extracts against Monoamine Oxidases, Cholinesterases, and β-Secretase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of SF and AG
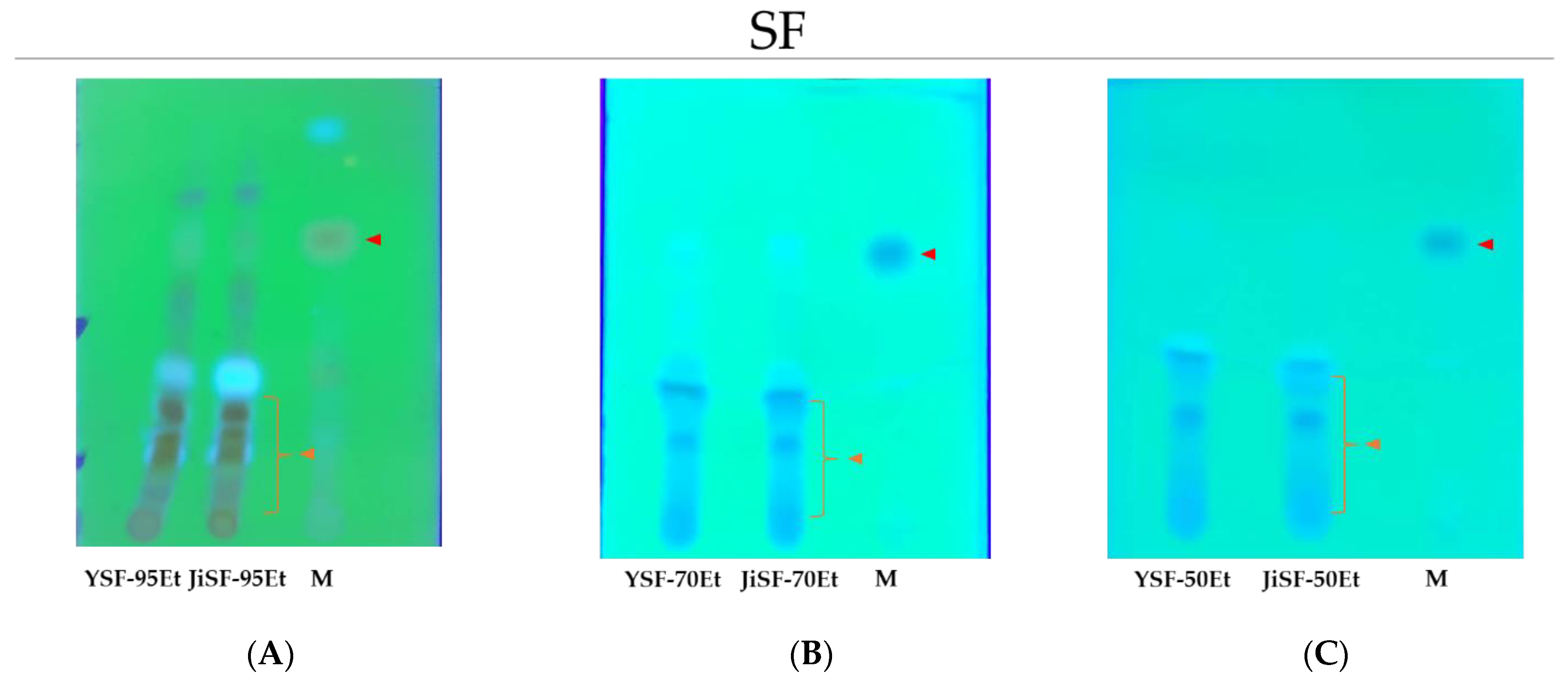
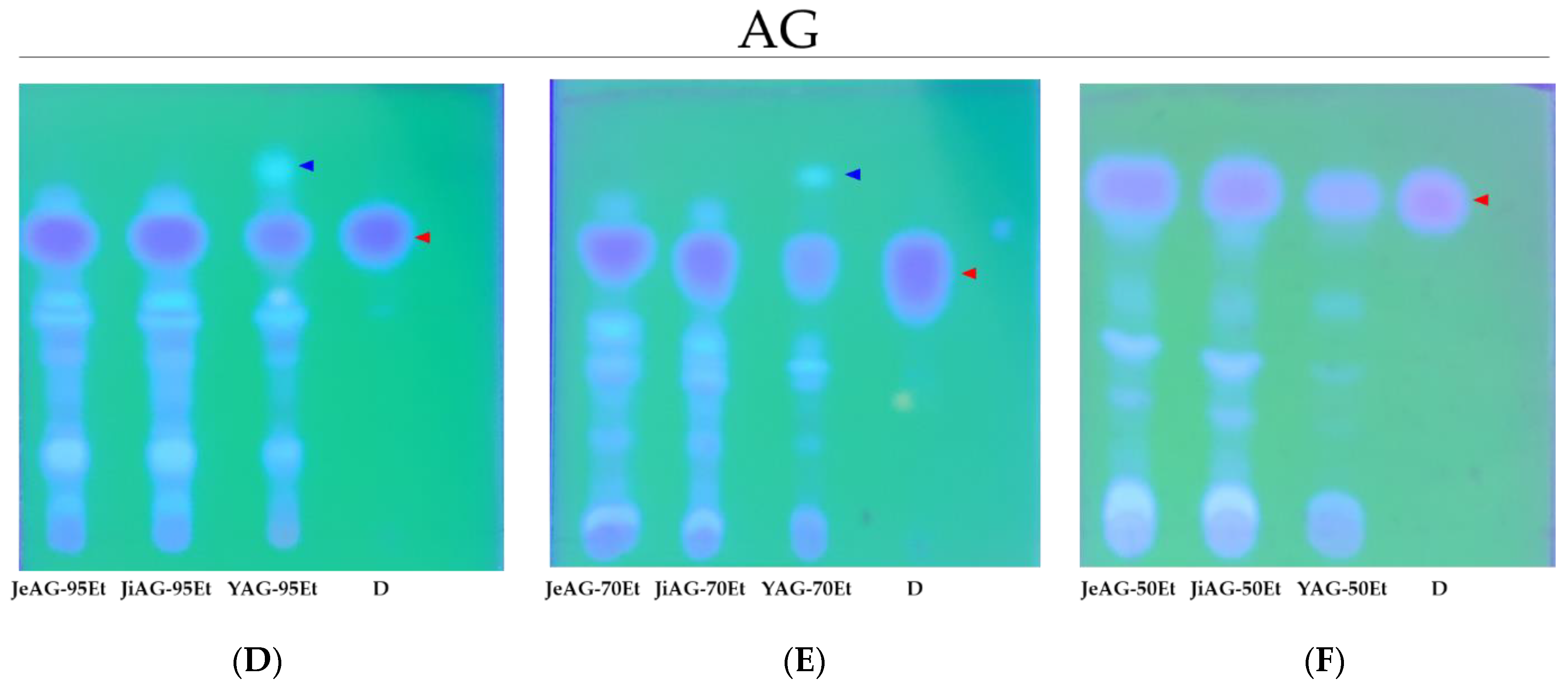
2.3. Thin-Layer Chromatography (TLC)
2.4. Enzyme Assays
2.5. DPPH Radical Scavenging Activity
2.6. Aβ42 Aggregation Assay
2.7. Cell Culture and Viability
3. Results
3.1. Yields of the Extracts
3.2. TLC Analysis of the Extracts
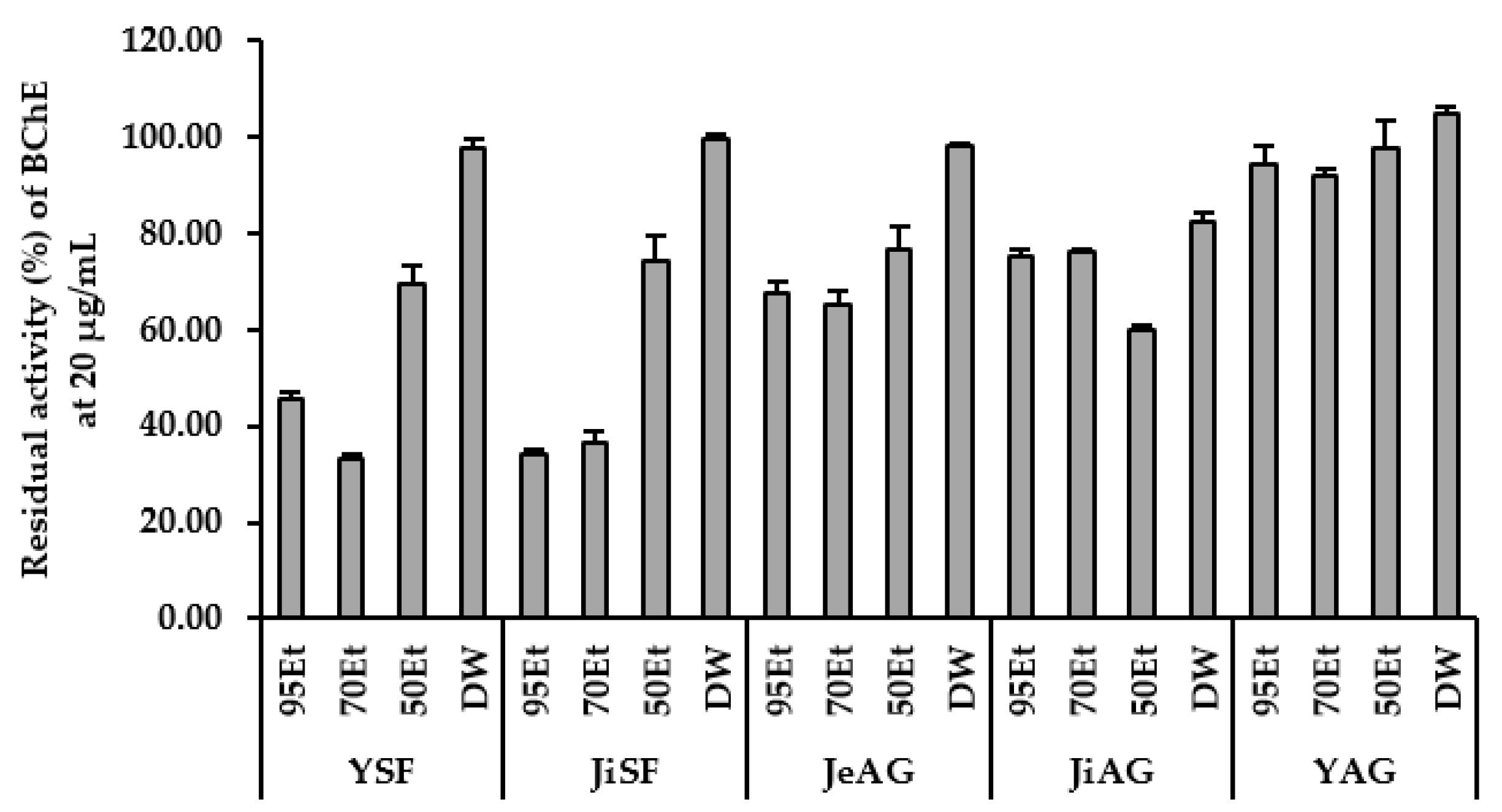
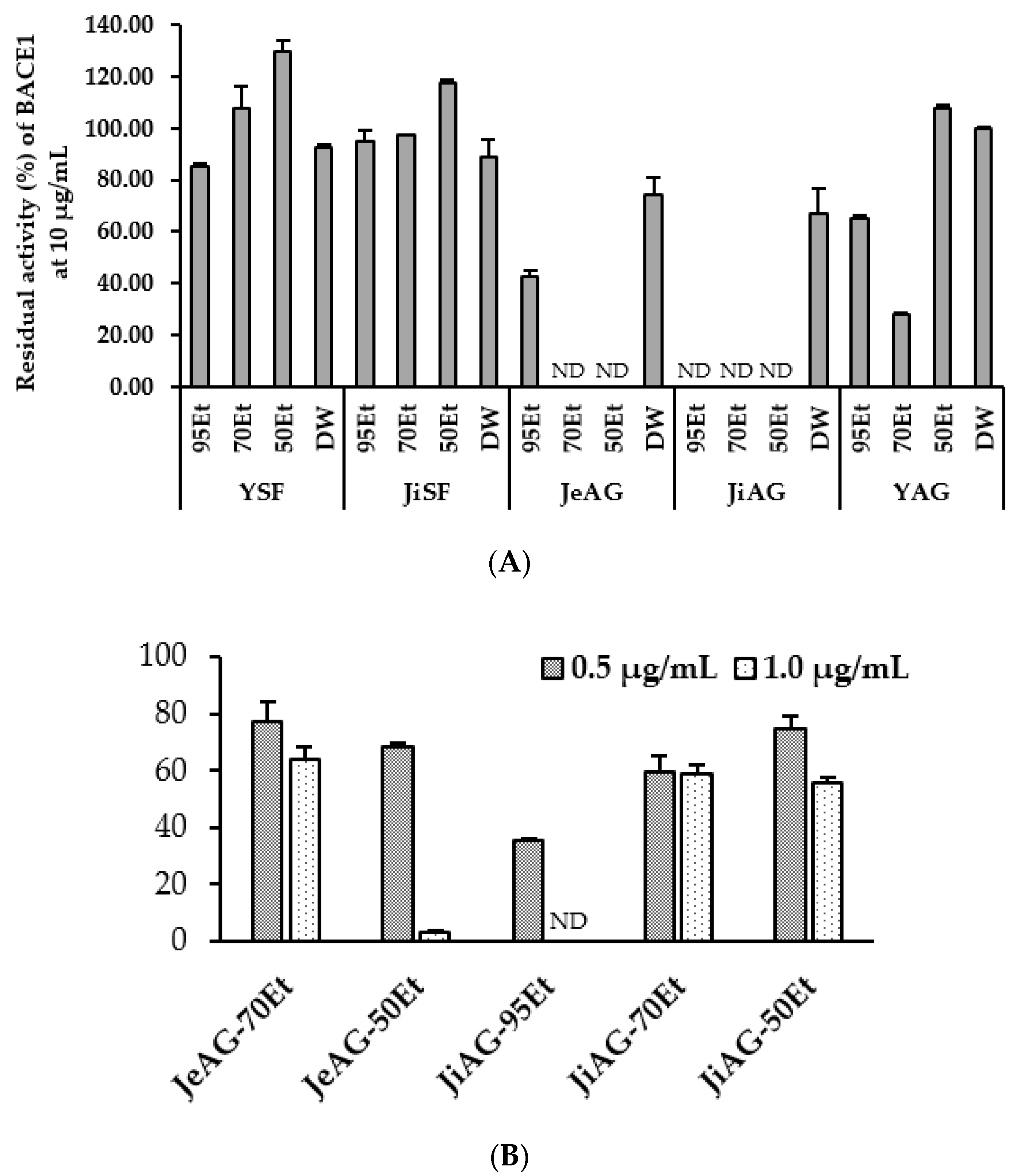
3.3. Inhibition of Enzymes by the Extracts
3.4. DPPH Radical Scavenging Activity and Aβ Aggregation Assay of the Extracts
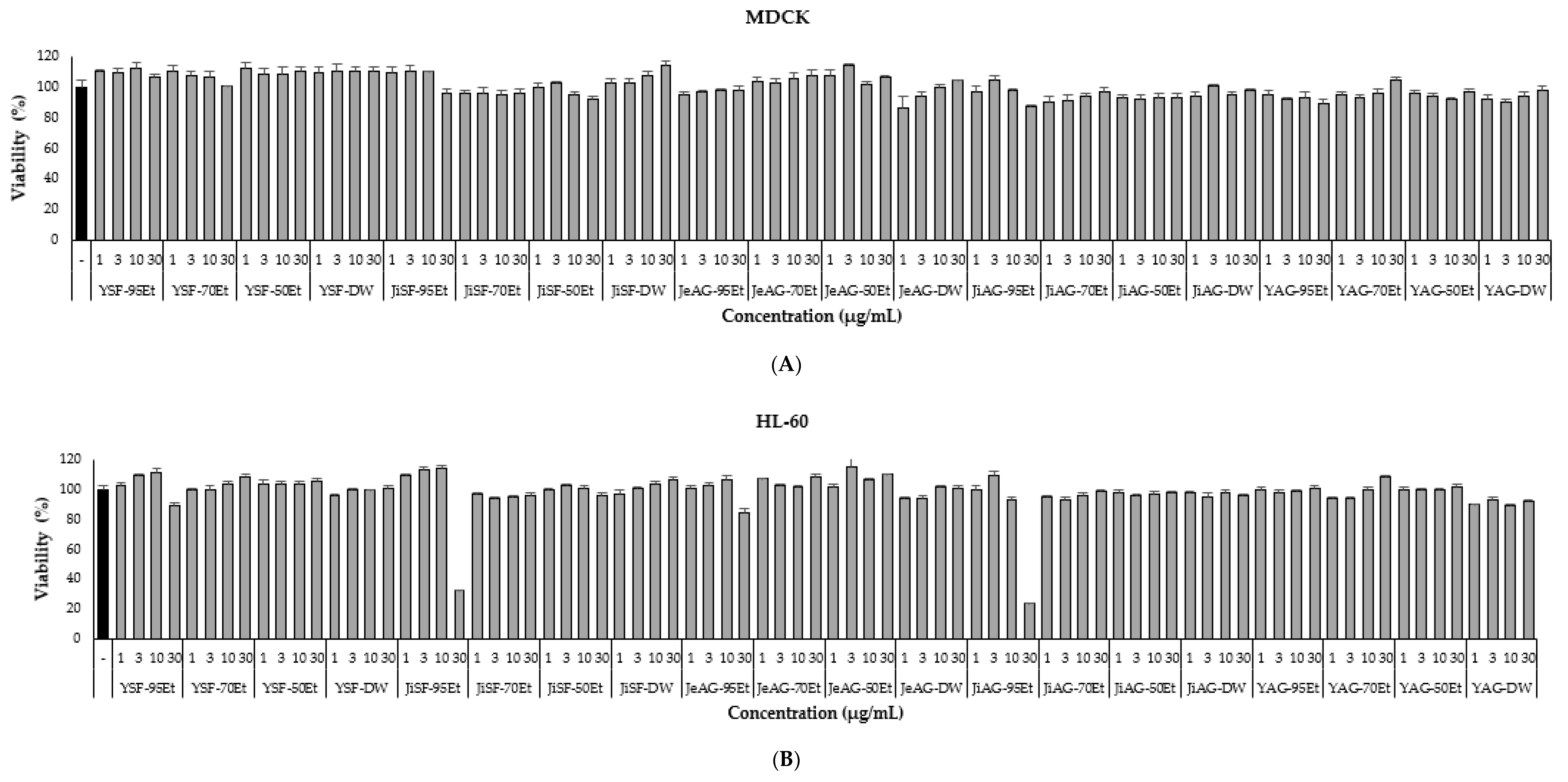
3.5. Cell Toxicity of the Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenk, G.L. Neuropathologic changes in Alzheimer’s disease. J. Clin. Psychiatry 2003, 64 (Suppl. 9), 7–10. [Google Scholar] [PubMed]
- Bloom, G.S. Amyloid-β and Tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Aducanumab: First approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimer’s Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Connolly, B.S.; Lang, A. Pharmacological treatment of parkinson disease. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.-M.; Poewe, W.; Rascol, O.; Goetz, C.G.; et al. The movement disorder society evidence-based medicine review update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26, S42–S80. [Google Scholar] [CrossRef]
- Ives, N.J.; Stowe, R.; Marro, J.; Counsell, C.; Macleod, A.; Clarke, C.; Gray, R.; Wheatley, K. Monoamine oxidase type B inhibitors in early Parkinson’s disease: Meta-analysis of 17 randomised trials involving 3525 patients. BMJ 2004, 329, 593. [Google Scholar] [CrossRef] [Green Version]
- De Zwart, P.L.; Jeronimus, B.; de Jonge, P. Empirical evidence for definitions of episode, remission, recovery, relapse and recurrence in depression: A systematic review. Epidemiol. Psychiatr. Sci. 2018, 28, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Daut, R.A.; Fonken, L.K. Circadian regulation of depression: A role for serotonin. Front. Neuroendocr. 2019, 54, 100746. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; Ginovart, N.; Boovariwala, A.; Sagrati, S.; Hussey, D.; Garcia, A.; Young, T.; Praschak-Rieder, N.; Wilson, A.A.; Houle, S. Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry 2006, 63, 1209–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsay, R.R. Monoamine Oxidases: The biochemistry of the proteins as targets in medicinal chemistry and drug discovery. Curr. Top. Med. Chem. 2012, 12, 2189–2209. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Georgieva, M.G.; Atanasov, A.G.; Tzvetkov, N.T. Monoamine oxidases (MAOs) as privileged molecular targets in neuroscience: Research literature analysis. Front. Mol. Neurosci. 2019, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, CD005593. [Google Scholar] [CrossRef]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2005, 9, 101–124. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Anderson, J.P.; Barbour, R.; Basi, G.S.; Caccavello, R.; Davis, D.; Doan, M.; Dovey, H.F.; Frigon, N.; Hong, J.; et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 1999, 402, 537–540. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2020, 167, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Osswald, H.L. BACE1 (β-secretase) inhibitors for the treatment of Alzheimer’s disease. Chem. Soc. Rev. 2014, 43, 6765–6813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, P.M.; KNV, R.; S, S.; Banji, D. A review on phytochemical, ethnomedical and pharmacological studies on genus Sophora, Fabaceae. Rev. Bras. Farm. 2012, 22, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.; Lee, J.; Jeon, E.; Ryu, H.W.; Oh, S.; Ahn, K.; Jun, H.S.; Ha, U. Maackiain, a compound derived from Sophora flavescens, increases IL-1β production by amplifying nigericin-mediated inflammasome activation. FEBS Open Biol. 2020, 10, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Wang, L.; Xiong, L.; Tang, H.; Du, J.; Peng, C. Maackiain modulates miR-374a/GADD45A axis to inhibit triple-negative breast cancer initiation and progression. Front. Pharmacol. 2022, 13, 806869. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Ryu, H.W.; Kang, M.-G.; Park, D.; Oh, S.-R.; Kim, H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens. Bioorg. Med. Chem. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef]
- Yan, J.-J.; Kim, D.-H.; Moon, Y.-S.; Jung, J.-S.; Ahn, E.-M.; Baek, N.-I.; Song, D.-K. Protection against β-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 25–30. [Google Scholar] [CrossRef]
- Ji, K.-Y.; Jung, D.H.; Pyun, B.-J.; Kim, Y.J.; Lee, J.Y.; Choi, S.; Jung, M.-A.; Song, K.H.; Kim, T. Angelica gigas extract ameliorates allergic rhinitis in an ovalbumin-induced mouse model by inhibiting Th2 cell activation. Phytomedicine 2021, 93, 153789. [Google Scholar] [CrossRef]
- Kwon, D.-A.; Kim, Y.S.; Kim, S.-K.; Baek, S.H.; Kim, H.K.; Lee, H.S. Antioxidant and antifatigue effect of a standardized fraction (HemoHIM) from Angelica gigas, Cnidium officinale, and Paeonia lactiflora. Pharm. Biol. 2021, 59, 389–398. [Google Scholar] [CrossRef]
- Joo, M.; Heo, J.B.; Kim, S.; Kim, N.; Jeon, H.J.; An, Y.; Song, G.-Y.; Kim, J.-M.; Lee, H.J. Decursin inhibits tumor progression in head and neck squamous cell carcinoma by downregulating CXCR7 expression in vitro. Oncol. Rep. 2021, 47, 39. [Google Scholar] [CrossRef]
- Lee, W.; Sim, H.; Choi, Y.-J.; Seo, J.Y.; Yun, M.-Y.; Song, G.Y.; Bae, J.-S. The decursin analog, CYJ-27, suppresses inflammation via the downregulation of NF-κB and STAT-J. Med. Food 2021, 24, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Ryu, H.W.; Kang, M.-G.; Park, D.; Lee, H.; Shin, H.M.; Oh, S.-R.; Kim, H. Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. Int. J. Biol. Macromol. 2017, 97, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Lee, H.-S.; Baek, S.C.; Lee, J.P.; Jeong, G.S.; Paik, M.-J.; Kim, H. Antidepressant-like activities of hispidol and decursin in mice and analysis of neurotransmitter Monoamines. Neurochem. Res. 2020, 45, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-S.; Kang, M.-G.; Han, S.-A.; Noh, J.-I.; Park, J.-E.; Nam, S.-J.; Park, D.; Yee, S.-T.; Kim, H. Selective inhibition of human monoamine oxidase B by 5-hydroxy-2-methyl-chroman-4-one isolated from an endogenous lichen fungus Daldinia fissa. J. Fungi 2021, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Lee, H.W.; Ryu, H.W.; Kang, M.-G.; Park, D.; Kim, S.H.; Cho, M.-L.; Oh, S.-R.; Kim, H. Selective inhibition of monoamine oxidase A by hispidol. Bioorganic Med. Chem. Lett. 2018, 28, 584–588. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lee, J.P.; Kang, M.-G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.-L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorganic Chem. 2019, 89, 103043. [Google Scholar] [CrossRef]
- Ali, S.; Bin Asad, M.H.H.; Maity, S.; Zada, W.; Rizvanov, A.; Iqbal, J.; Babak, B.; Hussain, I. Fluoro-benzimidazole derivatives to cure Alzheimer’s disease: In-silico studies, synthesis, structure-activity relationship and in vivo evaluation for β secretase enzyme inhibition. Bioorganic Chem. 2019, 88, 102936. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bolder, S.G.; Sagis, L.M.C.; Venema, P.; Van Der Linden, E. Thioflavin T and birefringence assays to determine the conversion of proteins into fibrils. Langmuir 2007, 23, 4144–4147. [Google Scholar] [CrossRef]
- Noh, J.-I.; Mun, S.-K.; Lim, E.; Kim, H.; Chang, D.-J.; Hur, J.-S.; Yee, S.-T. Induction of apoptosis in MDA-MB-231 cells treated with the methanol extract of lichen Physconia hokkaidensis. J. Fungi 2021, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chai, W.C.; Wang, Z.-Y.; Tang, K.-J.; Chen, J.-Y.; Venter, H.; Semple, S.J.; Xiang, L. Bioactivity-guided isolation of compounds from Sophora flavescens with antibacterial activity against Acinetobacter baumannii. Nat. Prod. Res. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Sun, Y.; Cheng, N. Liver metabolomic characterization of Sophora flavescens alcohol extract-induced hepatotoxicity in rats through UPLC/LTQ-Orbitrap mass spectrometry. Xenobiotica 2019, 50, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, Y.; Huang, H.; Ma, Y.; Lin, Q.; Yang, X.; Pang, K. Acute and 13 weeks subchronic toxicological evaluation of the flavonoid-rich extract of Sophora flavescens. Drug Chem. Toxicol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.-R.; Jiang, D.; He, Y.; Geng, L.; Ren, G.-X.; Bai, Z.-F.; Zhang, X.; Zhang, Z.-Y.; Liu, C.-S. Correlation between chemical composition, ecological factors and soil factors of Chinese herbal medicine Daphnes Cortex. Zhongguo Zhong Yao Za Zhi 2020, 45, 1059–1063. [Google Scholar]
- Hwang, J.-S.; Lee, S.A.; Hong, S.S.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch. Pharmacal Res. 2005, 28, 190–194. [Google Scholar] [CrossRef]
- Jung, H.A.; Yokozawa, T.; Kim, B.-W.; Jung, J.H.; Choi, J.S. Selective Inhibition of Prenylated Flavonoids from Sophora flavescens against BACE1 and Cholinesterases. Am. J. Chin. Med. 2010, 38, 415–429. [Google Scholar] [CrossRef]
- Mzezewa, S.C.; Omoruyi, S.I.; Zondagh, L.S.; Malan, S.F.; Ekpo, O.E.; Joubert, J. Design, synthesis, and evaluation of 3,7-substituted coumarin derivatives as multifunctional Alzheimer’s disease agents. J. Enzym. Inhib. Med. Chem. 2021, 36, 1606–1620. [Google Scholar] [CrossRef]
- Ali, Y.; Jannat, S.; Jung, H.A.; Choi, R.J.; Roy, A.; Choi, J.S. Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac. J. Trop. Med. 2016, 9, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Marumoto, S.; Miyazawa, M. Structure–activity relationships for naturally occurring coumarins as β-secretase inhibitor. Bioorganic Med. Chem. 2012, 20, 784–788. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Nuthakki, V.K.; Singh, L.; Singh, B.; Bharate, S.S.; Bhatti, R.; Bharate, S.B. Identification of plant-based multitargeted leads for Alzheimer’s disease: In-vitro and in-vivo validation of Woodfordia fruticosa (L.) Kurz. Phytomedicine 2021, 91, 153659. [Google Scholar] [CrossRef] [PubMed]
- Oyeniran, O.H.; Ademiluyi, A.O.; Oboh, G. Host–parasite relationship modulates the effect of African mistletoe leaves on the cholinergic, monoaminergic and carbohydrate hydrolyzing enzymes in fruit fly. J. Basic Clin. Physiol. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Hillman, P.F.; Kang, M.-G.; Hwang, S.; Park, J.-E.; Nam, S.-J.; Park, D.; Kim, H. Potent and Selective Inhibitors of Human Monoamine Oxidase A from an Endogenous Lichen Fungus Diaporthe mahothocarpus. J. Fungi 2021, 7, 876. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-S.; Lee, E.-Y.; Kang, M.-G.; Nam, S.-J.; Park, D.; Kim, H. (S)-5-Methylmellein isolated from an endogenous lichen fungus Rosellinia corticium as a potent inhibitor of human monoamine oxidase A. Processes 2022, 10, 166. [Google Scholar] [CrossRef]
- Oh, J.M.; Lee, C.; Nam, S.-J.; Kim, H. Chromenone derivatives as monoamine oxidase inhibitors from marine-derived MAR4 clade Streptomyces sp. CNQ-031. J. Microbiol. Biotechnol. 2021, 31, 1022–1027. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, H.; Nam, S.-J.; Fenical, W.; Kim, H. Potent inhibition of monoamine oxidase B by a piloquinone from marine-derived Streptomyces sp. CNQ-027. J. Microbiol. Biotechnol. 2017, 27, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Jeong, G.; Kang, M.-G.; Lee, J.; Lee, S.; Park, D.; Cho, M.; Kim, H. Inhibition of butyrylcholinesterase and human monoamine oxidase-B by the coumarin glycyrol and liquiritigenin isolated from Glycyrrhiza uralensis. Molecules 2020, 25, 3896. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.-J.; Kim, W.J.; Kang, M.-G.; Baek, S.C.; Lee, J.P.; Park, D.; Oh, S.-R.; Kim, H. Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int. J. Biol. Macromol. 2020, 151, 441–448. [Google Scholar] [CrossRef]
- Heo, J.H.; Eom, B.H.; Ryu, H.W.; Kang, M.-G.; Park, J.E.; Kim, D.-Y.; Kim, J.-H.; Park, D.; Oh, S.-R.; Kim, H. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of khellactone coumarin derivatives isolated from Peucedanum japonicum Thurnberg. Sci. Rep. 2020, 10, 21695. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.-J.; Kang, M.-G.; Song, S.; Kim, D.-Y.; Kim, J.; Noh, J.-I.; Park, J.E.; Park, D.; Yee, S.-T.; et al. Acetylcholinesterase and monoamine oxidase-B inhibitory activities by ellagic acid derivatives isolated from Castanopsis cuspidata var. sieboldii. Sci. Rep. 2021, 11, 13953. [Google Scholar] [CrossRef]




| Plant | Origin | Solvent | Yield (%) | Abbreviation |
|---|---|---|---|---|
| Sophora flavescens | Yeongcheon | 95% EtOH | 2.36 | YSF-95Et |
| 70% EtOH | 14.24 | YSF-70Et | ||
| 50% EtOH | 10.74 | YSF-50Et | ||
| DW | 12.82 | YSF-DW | ||
| Jiri | 95% EtOH | 5.09 | JiSF-95Et | |
| 70% EtOH | 12.37 | JiSF-70Et | ||
| 50% EtOH | 8.09 | JiSF-50Et | ||
| DW | 15.30 | JiSF-DW | ||
| Angelica gigas Nakai | Jecheon | 95% EtOH | 7.10 | JeAG-95Et |
| 70% EtOH | 28.00 | JeAG-70Et | ||
| 50% EtOH | 31.10 | JeAG-50Et | ||
| DW | 23.35 | JeAG-DW | ||
| Jiri | 95% EtOH | 8.00 | JiAG-95Et | |
| 70% EtOH | 33.82 | JiAG-70Et | ||
| 50% EtOH | 20.39 | JiAG-50Et | ||
| DW | 21.60 | JiAG-DW | ||
| Yeongcheon | 95% EtOH | 11.50 | YAG-95Et | |
| 70% EtOH | 33.78 | YAG-70Et | ||
| 50% EtOH | 31.24 | YAG-50Et | ||
| DW | 21.70 | YAG-DW |
| Extracts | Inhibitions at 20 μg/mL (%) | |
|---|---|---|
| DPPH | Aβ Aggregation | |
| YSF-95Et | 8.02 ± 0.97 | 28.63 ± 3.35 |
| YSF-70Et | 8.4 ± 2.39 | 12.45 ± 0.05 |
| YSF-50Et | 4.61 ± 1.73 | 0.08 ± 1.25 |
| YSF-DW | 12.25 ± 0.22 | 22.68 ± 1.26 |
| JiSF-95Et | 6.45 ± 2.13 | 28.19 ± 1.58 |
| JiSF-70Et | 18.83 ± 0.73 | −1.85 ± 0.00 |
| JiSF-50Et | 3.57 ± 2.66 | −8.69 ± 0.56 |
| JiSF-DW | 0.91 ± 1.73 | 4.64 ± 5.16 |
| JeAG-95Et | 2.44 ± 2.22 | 12.26 ± 1.21 |
| JeAG-70Et | 17.23 ± 2.22 | −2.36 ± 0.00 |
| JeAG-50Et | 4.89 ± 4.16 | 6.07 ± 6.12 |
| JeAG-DW | −4.51 ± 1.06 | 25.74 ± 1.11 |
| JiAG-95Et | 2.22 ± 4.21 | 12.96 ± 1.25 |
| JiAG-70Et | 3.13 ± 0.80 | 8.09 ± 5.46 |
| JiAG-50Et | 1.16 ± 3.50 | −7.85 ± 0.73 |
| JiAG-DW | 17.64 ± 2.35 | 9.21 ± 4.25 |
| YAG-95Et | 0.323 ± 2.70 | 6.62 ± 2.45 |
| YAG-70Et | 0.72 ± 0.31 | 2.35 ± 3.33 |
| YAG-50Et | 3.26 ± 0.97 | 17.10 ± 3.31 |
| YAG-DW | 3.60 ± 3.41 | 9.37 ± 1.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.E.; Mun, S.-K.; Yee, S.-T.; Kim, H. Evaluation of Inhibitory Activities of Sophora flavescens and Angelica gigas Nakai Root Extracts against Monoamine Oxidases, Cholinesterases, and β-Secretase. Processes 2022, 10, 880. https://doi.org/10.3390/pr10050880
Park JE, Mun S-K, Yee S-T, Kim H. Evaluation of Inhibitory Activities of Sophora flavescens and Angelica gigas Nakai Root Extracts against Monoamine Oxidases, Cholinesterases, and β-Secretase. Processes. 2022; 10(5):880. https://doi.org/10.3390/pr10050880
Chicago/Turabian StylePark, Jong Eun, Seul-Ki Mun, Sung-Tae Yee, and Hoon Kim. 2022. "Evaluation of Inhibitory Activities of Sophora flavescens and Angelica gigas Nakai Root Extracts against Monoamine Oxidases, Cholinesterases, and β-Secretase" Processes 10, no. 5: 880. https://doi.org/10.3390/pr10050880






