A Comparison of Acute Effects of Climbing Therapy with Nordic Walking for Inpatient Adults with Mental Health Disorder: A Clinical Pilot Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
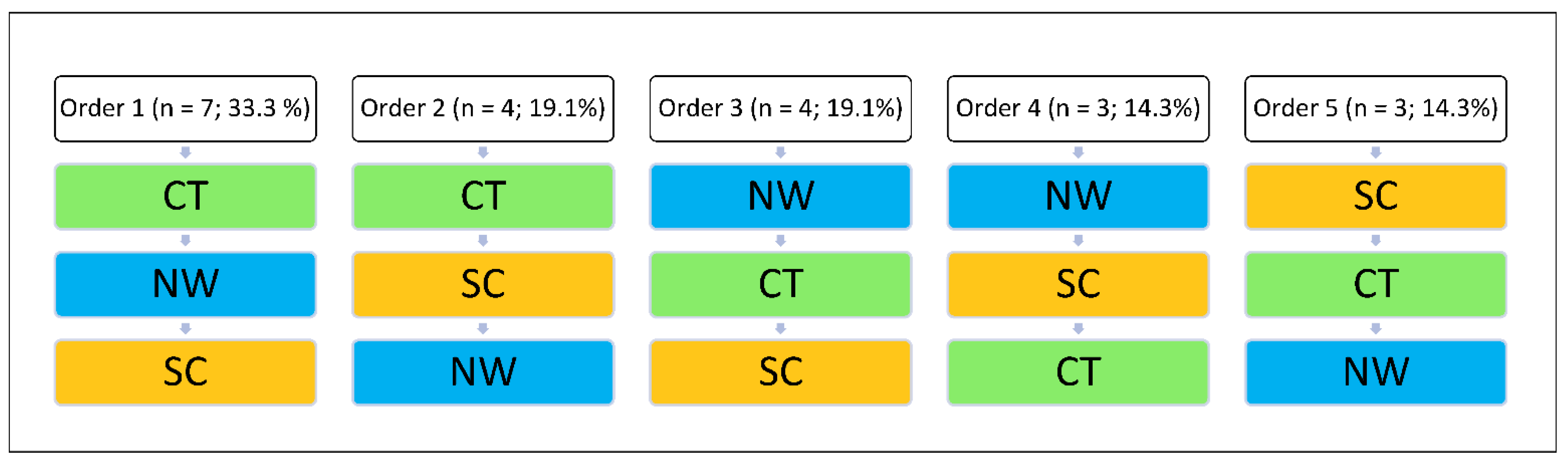
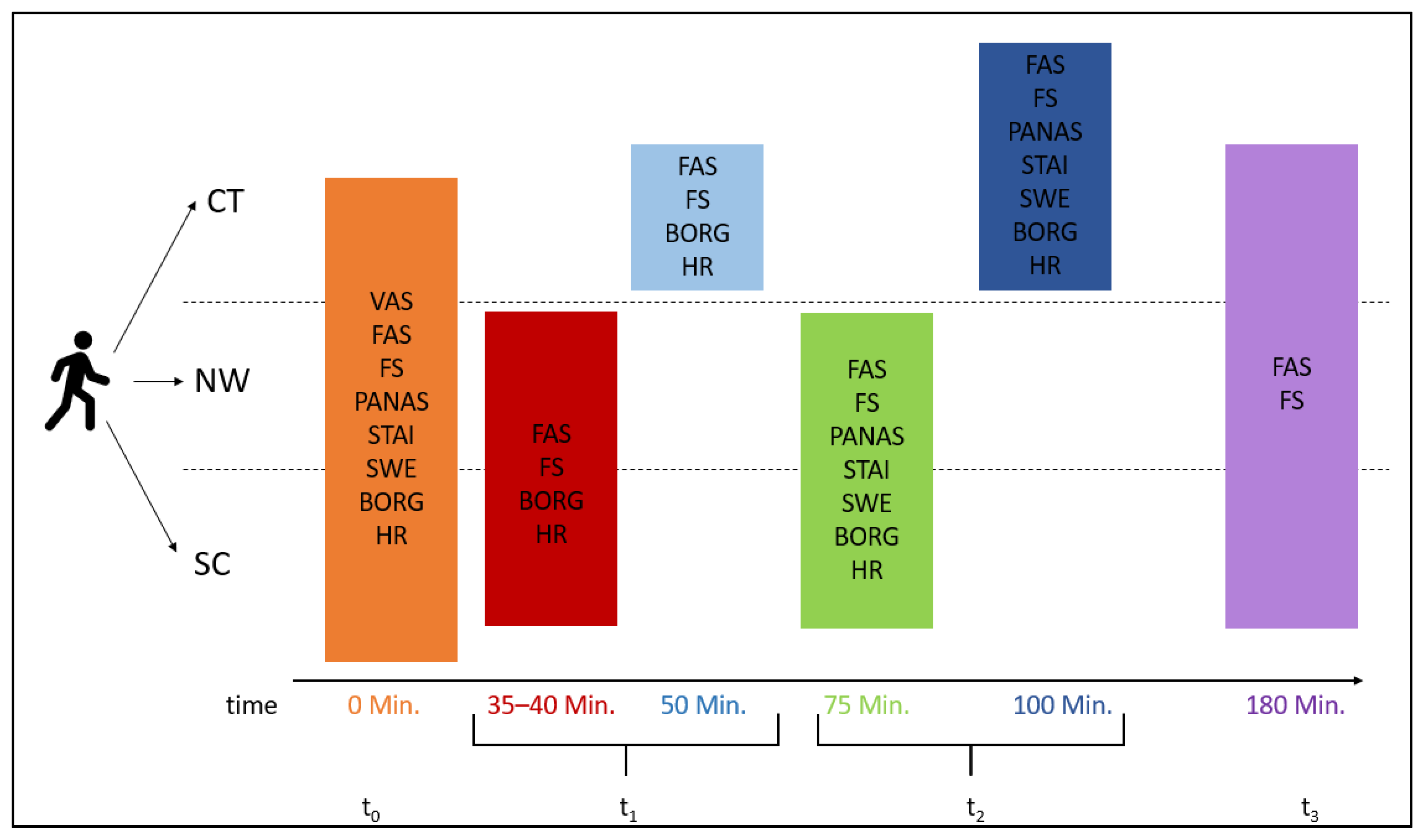
2.2. Procedure
2.3. Interventions
2.3.1. Climbing Therapy
2.3.2. Nordic Walking
2.3.3. Sedentary Control Condition
2.4. Measurements
2.4.1. Affective Responses
Affective Valence
Perceived Activation
Positive and Negative Affect Schedule
2.4.2. State Anxiety
2.4.3. Self-Efficacy
2.4.4. Exercise Variables and Daily Well-Being
2.5. Statistical Analyses
3. Results
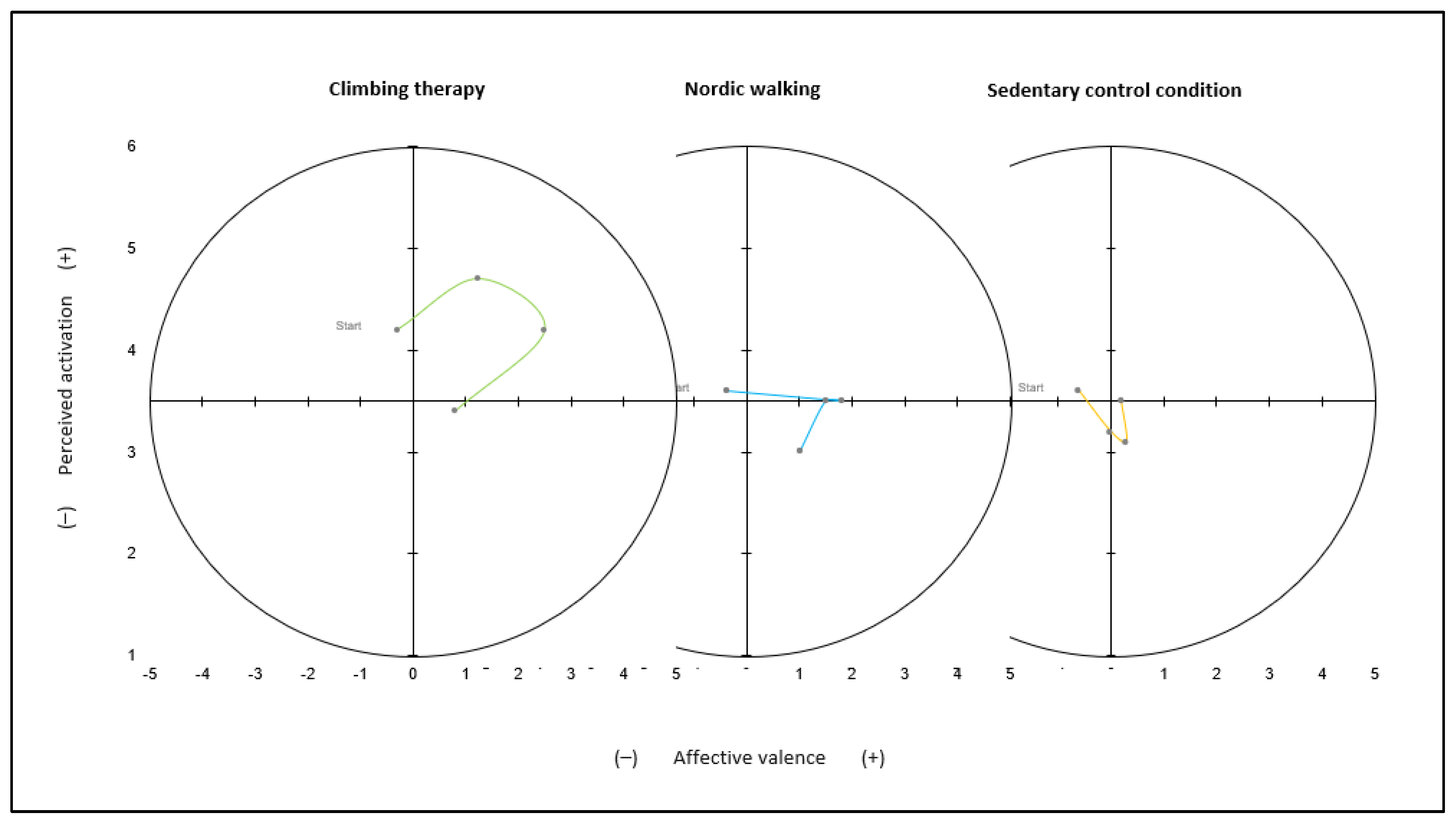
3.1. Affective Responses
3.2. State Anxiety
3.3. Self-Efficacy
3.4. Exercise Variables and Daily Well-Being
4. Discussion
4.1. Main Findings
4.2. Affective Responses
4.3. State Anxiety
4.4. Self-Efficacy
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Wittchen, H.-U.; Jacobi, F. Size and burden of mental disorders in Europe—A critical review and appraisal of 27 studies. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2005, 15, 357–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slade, T.; Johnston, A.; Oakley Browne, M.A.; Andrews, G.; Whiteford, H. 2007 National Survey of Mental Health and Wellbeing: Methods and key findings. Aust. N. Z. J. Psychiatry 2009, 43, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Chiu, W.T.; Demler, O.; Merikangas, K.R.; Walters, E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobi, F.; Höfler, M.; Strehle, J.; Mack, S.; Gerschler, A.; Scholl, L.; Busch, M.A.; Maske, U.; Hapke, U.; Gaebel, W.; et al. Psychische Störungen in der Allgemeinbevölkerung: Studie zur Gesundheit Erwachsener in Deutschland und ihr Zusatzmodul Psychische Gesundheit (DEGS1-MH). Der Nervenarzt 2014, 85, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; Ponce De Leon, A.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Faulkner, G. Inaugural editorial. Ment. Health Phys. Act. 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Chekroud, S.R.; Gueorguieva, R.; Zheutlin, A.B.; Paulus, M.; Krumholz, H.M.; Krystal, J.H.; Chekroud, A.M. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry 2018, 5, 739–746. [Google Scholar] [CrossRef]
- Mead, G.E.; Morley, W.; Campbell, P.; Greig, C.A.; McMurdo, M.; Lawlor, D.A. Exercise for depression. Cochrane Database Syst. Rev. 2010, 2010, CD004366. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, B.M.; Babyak, M.A.; Craighead, W.E.; Sherwood, A.; Doraiswamy, P.M.; Coons, M.J.; Blumenthal, J.A. Exercise and pharmacotherapy in patients with major depression: One-year follow-up of the SMILE study. Psychosom. Med. 2011, 73, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Babyak, M.; Blumenthal, J.A.; Herman, S.; Khatri, P.; Doraiswamy, M.; Moore, K.; Craighead, W.E.; Baldewicz, T.T.; Krishnan, K.R. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosom. Med. 2000, 62, 633–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, J.A.; Babyak, M.A.; Doraiswamy, P.M.; Watkins, L.; Hoffman, B.M.; Barbour, K.A.; Herman, S.; Craighead, W.E.; Brosse, A.L.; Waugh, R.; et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med. 2007, 69, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, J.A.; Babyak, M.A.; Moore, K.A.; Craighead, W.E.; Herman, S.; Khatri, P.; Waugh, R.; Napolitano, M.A.; Forman, L.M.; Appelbaum, M.; et al. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999, 159, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Frühauf, A.; Niedermeier, M.; Elliott, L.R.; Ledochowski, L.; Marksteiner, J.; Kopp, M. Acute effects of outdoor physical activity on affect and psychological well-being in depressed patients—A preliminary study. Ment. Health Phys. Act. 2016, 10, 4–9. [Google Scholar] [CrossRef]
- Bartholomew, J.B.; Morrison, D.; Ciccolo, J.T. Effects of acute exercise on mood and well-being in patients with major depressive disorder. Med. Sci. Sports Exerc. 2005, 37, 2032–2037. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, S.; Tiedemann, A.; Sherrington, C.; Curtis, J.; Ward, P.B. Physical Activity Interventions for People with Mental Illness: A systematic Review and Meta-Analysis. J. Sci. Med. Sport 2014, 75, 964–974. [Google Scholar] [CrossRef]
- Rethorst, C.D.; Wipfli, B.M.; Landers, D.M. The antidepressive effects of exercise: A meta-analysis of randomized trials. Sports Med. 2009, 39, 491–511. [Google Scholar] [CrossRef]
- Ensari, I.; Greenlee, T.A.; Motl, R.W.; Petruzzello, S.J. Meta-analysis of acute exercise effects on state anxiety: An update of randomized controlled trials over the past 25 years. Depress. Anxiety 2015, 32, 624–634. [Google Scholar] [CrossRef]
- Wipfli, B.M.; Rethorst, C.D.; Landers, D.M. The anxiolytic effects of exercise: A meta-analysis of randomized trials and dose-response analysis. J. Sport Exerc. Psychol. 2008, 30, 392–410. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, B.; Vancampfort, D.; Rosenbaum, S.; Firth, J.; Cosco, T.; Veronese, N.; Salum, G.A.; Schuch, F.B. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: A meta-analysis. Psychiatry Res. 2017, 249, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.; Klaperski, S. Stressregulation durch Sport und Bewegung. In Handbuch Stressregulation und Sport; Fuchs, R., Gerber, M., Eds.; Springer: Berlin, Germany, 2018; pp. 205–226. [Google Scholar] [CrossRef]
- McAuley, E.; Blissmer, B. Self-Efficacy Determinants and Consequences of Physical Activity. Exerc. Sport Sci. Rev. 2000, 28, 85–88. [Google Scholar] [PubMed]
- McAuley, E.; Elavsky, S.; Motl, R.W.; Konopack, J.F.; Hu, L.; Marquez, D.X. Physical activity, self-efficacy, and self-esteem: Longitudinal relationships in older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2005, 60, P268–P275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonstroem, R.J.; Morgan, W.P. Exercise and self-esteem. Med. Sci. Sports Exerc. 1989, 21, 329–337. [Google Scholar] [CrossRef]
- Randall, J.; Ellis, N.J.; Gidlow, C.; Jones, M. Comparing mental health diagnoses: Changes in mood and self esteem following a single bout of exercise. J. Psychol. Ther. Prim. Care 2014, 3, 34–46. [Google Scholar]
- Ellis, N.J.; Randall, J.A.; Punnett, G. The Effects of a Single Bout of Exercise on Mood and Self-Esteem in Clinically Diagnosed Mental Health Patients. Open J. Med. Psychol. 2013, 2, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, D.L.; Butki, B.D. Self-efficacy and affective responses to short bouts of exercise. J. Appl. Sport Psychol. 1998, 10, 268–280. [Google Scholar] [CrossRef]
- Katula, J.A.; McAuley, E. The mirror does not lie: Acute exercise and self-efficacy. Int. J. Behav. Med. 2001, 8, 319–326. [Google Scholar] [CrossRef]
- Bodin, T.; Martinsen, E.W. Mood and Self-Efficacy during Acute Exercise in Clinical Depression. A Randomized, Controlled Study. J. Sport Exerc. Psychol. 2004, 26, 623–633. [Google Scholar] [CrossRef]
- Luttenberger, K.; Karg-Hefner, N.; Berking, M.; Kind, L.; Weiss, M.; Kornhuber, J.; Dorscht, L. Bouldering psychotherapy is not inferior to cognitive behavioural therapy in the group treatment of depression: A randomized controlled trial. Br. J. Clin. Psychol. 2022, 61, 465–493. [Google Scholar] [CrossRef]
- Frühauf, A.; Sevecke, K.; Kopp, M. Ist-Stand der Fachliteratur zu Effekten des therapeutischen Kletterns auf die psychische Gesundheit—Fazit: Viel zu tun. Neuropsychiatr. Klin. Diagn. Ther. Rehabil. Organ Ges. Osterr. Nervenärzte Psychiater 2019, 33, 1–7. [Google Scholar] [CrossRef]
- Luttenberger, K.; Stelzer, E.-M.; Först, S.; Schopper, M.; Kornhuber, J.; Book, S. Indoor rock climbing (bouldering) as a new treatment for depression: Study design of a waitlist-controlled randomized group pilot study and the first results. BMC Psychiatry 2015, 15, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karg, N.; Dorscht, L.; Kornhuber, J.; Luttenberger, K. Bouldering psychotherapy is more effective in the treatment of depression than physical exercise alone: Results of a multicentre randomised controlled intervention study. BMC Psychiatry 2020, 20, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelzer, E.-M.; Book, S.; Graessel, E.; Hofner, B.; Kornhuber, J.; Luttenberger, K. Bouldering psychotherapy reduces depressive symptoms even when general physical activity is controlled for: A randomized controlled trial. Heliyon 2018, 4, e00580. [Google Scholar] [CrossRef] [PubMed]
- Soravia, L.; Stocker, E.; Schläfli, K.; Schönenberger, N.; Schreyer, M.; Dittrich, T.; Grossniklaus, C. Klettern als Chance in der Suchtbehandlung. Suchttherapie 2016, 17, 34–39. [Google Scholar] [CrossRef]
- Kowald, A.-C.; Zajetz, A.K. (Eds.) Therapeutisches Klettern: Anwendungsfelder in Psychotherapie und Pädagogik; Schattauer ein Imprint von J. G. Cotta’sche Buchhandlung Nachfolger: Stuttgart, Germany, 2015. [Google Scholar]
- Kratzer, A.; Luttenberger, K.; Karg-Hefner, N.; Weiss, M.; Dorscht, L. Bouldering psychotherapy is effective in enhancing perceived self-efficacy in people with depression: Results from a multicenter randomized controlled trial. BMC Psychol. 2021, 9, 126. [Google Scholar] [CrossRef]
- Wolf, M.; Mehl, K. Experiential learning in psychotherapy: Ropes course exposures as an adjunct to inpatient treatment. Clin. Psychol. Psychother. 2011, 18, 60–74. [Google Scholar] [CrossRef]
- Kleinstäuber, M.; Reuter, M.; Doll, N.; Fallgatter, A.J. Rock climbing and acute emotion regulation in patients with major depressive disorder in the context of a psychological inpatient treatment: A controlled pilot trial. Psychol. Res. Behav. Manag. 2017, 10, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, E.R.; Purves, P.L.; Southward, J.; Rhodes, R.E.; Temple, V.A. Effect of Indoor Wall Climbing on Self-Efficacy and Self-Perception of Children with special needs. Adapt. Phys. Act. Q. 2009, 26, 259–273. [Google Scholar] [CrossRef]
- Tschentscher, M.; Niederseer, D.; Niebauer, J. Health benefits of Nordic walking: A systematic review. Am. J. Prev. Med. 2013, 44, 76–84. [Google Scholar] [CrossRef]
- Heimbeck, A.; Süttinger, B. Bewegungstherapie bei depressiven Patienten—Ein Interventionsvergleich. Beweg. Gesundh. 2007, 23, 52–57. [Google Scholar] [CrossRef]
- Suija, K.; Pechter, U.; Kalda, R.; Tähepõld, H.; Maaroos, J.; Maaroos, H.-I. Physical activity of depressed patients and their motivation to exercise: Nordic Walking in family practice. International journal of rehabilitation research. Internationale Zeitschrift für Rehabilitationsforschung. Rev. Int. Rech. Readapt. 2009, 32, 132–138. [Google Scholar] [CrossRef]
- Stark, R.; Schöny, W.; Kopp, M. Auswirkungen einer moderaten Bewegungseinheit auf die psychische Befindlichkeit bei PatientInnen mit affektiven Störungen in stationär psychiatrischer Behandlung. Neuropsychiatr. Klin. Diagn. Ther. Rehabil. Organ Ges. Osterr. Nervenärzte Psychiater 2012, 26, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Frühauf, A.; Niedermeier, M.; Sevecke, K.; Haid-Stecher, N.; Albertini, C.; Richter, K.; Schipflinger, S.; Kopp, M. Affective responses to climbing exercises in children and adolescents during in-patient treatment for mental health disorders a pilot study on acute effects of different exercise interventions. Psychiatry Res. 2020, 291, 113245. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.J.; Rejeski, W.J. Not What, but How One Feels: The Measurement of Affect during Exercise. J. Sport Exerc. Psychol. 1989, 11, 304–317. [Google Scholar] [CrossRef]
- Van Landuyt, L.M.; Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. Throwing the Mountains into the Lakes: On the Perils of Nomothetic Conceptions of the Exercise-Affect Relationship. J. Sport Exerc. Psychol. 2000, 22, 208–234. [Google Scholar] [CrossRef]
- Maibach, M.; Niedermeier, M.; Sudeck, G.; Kopp, M. Erfassung unmittelbarer affektiver Reaktionen auf körperliche Aktivität. Z. Sportpsychol. 2020, 27, 4–12. [Google Scholar] [CrossRef]
- Rose, E.A.; Parfitt, G. Can the feeling scale be used to regulate exercise intensity? Med. Sci. Sports Exerc. 2008, 40, 1852–1860. [Google Scholar] [CrossRef]
- Svebak, S.; Murgatroyd, S. Metamotivational dominance: A multimethod validation of reversal theory constructs. J. Personal. Soc. Psychol. 1985, 48, 107–116. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Krohne, H.W.; Egloff, B.; Kohlmann, C.-W.; Tausch, A. Untersuchungen mit einer deutschen Version der “Positive and Negative Affect Schedule” (PANAS). Diagnostica 1996, 42, 139–156. [Google Scholar] [CrossRef]
- Breyer, B.; Bluemke, M. Deutsche Version der Positive and Negative Affect Schedule PANAS (GESIS Panel); GESIS—Leibniz-Institut für Sozialwissenschaften: Mannheim, Germany, 2016. [Google Scholar] [CrossRef]
- Laux, L.; Glanzmann, P.; Schaffner, P.; Spielberger, C.D. Das State-Trait-Angstinventar, 1st ed.; Beltz Verlag: Weinheim, Germany, 1981. [Google Scholar]
- Kopp, M.; Niedermeier, M. Sport, Krankheit und Verletzungen. In Sportpsychologie: Grundlagen und Anwendung, 1st ed.; Schüler, J., Wegner, M., Plessner, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 607–638. [Google Scholar] [CrossRef]
- Schwarzer, R.; Jerusalem, M. Skalen zur Erfassung von Lehrer- und Schülermerkmalen. Dokumentation der Psychometrischen Verfahrenim Rahmen der Wissenschaftlichen Begleitung des Modellversuchs Selbstwirksame Schulen; Freie Universität Berlin: Berlin, Germany, 1999. [Google Scholar]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, M.; Schwarzer, R. SWE—Skala zur Allgemeinen Selbstwirksamkeitserwartung; ZPID: Trier, Germany, 2003. [Google Scholar] [CrossRef]
- Fuchs, R.; Hahn, A.; Schwarzer, R. Effekte sportlicher Aktivität auf Selbstwirksamkeitserwartung und Gesundheit in einer stressreichen Lebenssituation. Sportwissenschaft 1994, 24, 67–81. [Google Scholar]
- Buffart, L.M.; Ros, W.J.G.; Chinapaw, M.J.M.; Brug, J.; Knol, D.L.; Korstjens, I.; van Weert, E.; Mesters, I.; van den Borne, B.; Hoekstra-Weebers, J.E.H.M.; et al. Mediators of physical exercise for improvement in cancer survivors’ quality of life. Psychooncology 2014, 23, 330–338. [Google Scholar] [CrossRef]
- Borg, G. An introduction to Borg’s RPE-Scale; Movement Publications: New York, NY, USA, 1985. [Google Scholar]
- Fähndrich, E.; Linden, M. Zur Reliabilität und Validität der Stimmungsmessung mit der Visuellen Analog-Skala (VAS). Pharmacopsychiatria 1982, 15, 90–94. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar] [CrossRef]
- Rhodes, R.E.; Kates, A. Can the Affective Response to Exercise Predict Future Motives and Physical Activity Behavior? A Systematic Review of Published Evidence. Ann. Behav. Med. 2015, 49, 715–731. [Google Scholar] [CrossRef]
- Williams, D.M.; Dunsiger, S.; Ciccolo, J.T.; Lewis, B.A.; Albrecht, A.E.; Marcus, B.H. Acute Affective Response to a Moderate-intensity Exercise Stimulus Predicts Physical Activity Participation 6 and 12 Months Later. Psychol. Sport Exerc. 2008, 9, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Florange, C.; Göhler, F. Funktion der Sport- und Bewegungstherapie in der stationären Psychosomatik und Psychotherapie. Beweg. Gesundh. 2014, 30, 290–293. [Google Scholar] [CrossRef] [Green Version]
- Ekkekakis, P.; Parfitt, G.; Petruzzello, S.J. The pleasure and displeasure people feel when they exercise at different intensities: Decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med. 2011, 41, 641–671. [Google Scholar] [CrossRef]
- Kern, C. Den Flow erleben: Therapeutisches Klettern bei Multipler Sklerose. Erfahrungsheilkunde 2019, 68, 199–204. [Google Scholar] [CrossRef]
- Gallotta, M.C.; Emerenziani, G.P.; Monteiro, M.D.; Iasevoli, L.; Iazzoni, S.; Baldari, C.; Guidetti, L. Psychophysical benefits of rock-climbing activity. Percept. Mot. Ski. 2015, 121, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Petruzzello, S.J.; Landers, D.M.; Hatfield, B.D.; Kubitz, K.A.; Salazar, W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Outcomes and mechanisms. Sports Med. 1991, 11, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Byś, A.; Baszczowski, M.; Ginszt, M.; Suwała, M.; Majcher, P. The Influence of Sport Climbing on Depression and Anxiety Levels—Literature Review. J. Educ. Health Sport 2018, 8, 336–344. [Google Scholar] [CrossRef]
- Anderson, E.; Shivakumar, G. Effects of exercise and physical activity on anxiety. Front. Psychiatry 2013, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Ströhle, A. Physical activity, exercise, depression and anxiety disorders. J. Neural Transm. 2009, 116, 777–784. [Google Scholar] [CrossRef]
- McAuley, E.; Szabo, A.; Gothe, N.; Olson, E.A. Self-efficacy: Implications for Physical Activity, Function, and Functional Limitations in Older Adults. Am. J. Lifestyle Med. 2011, 5, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Grzybowski, C.; Eils, E. Therapeutisches Klettern—Kaum erforscht und dennoch zunehmend eingesetzt. Sportverletz. Sportschaden 2011, 25, 87–92. [Google Scholar] [CrossRef]
| Variable | Intervention | Pre | Post | Time | Intervention | Time*Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | F (1,20) | p | η2 | F (2,40) | p | η2 | F (2,40) | p | η2 | ||
| Positive Affect | CT | 2.79 | (0.7) | 3.63 | (0.9) | 30.014 | <0.001 *** | 0.60 | 26.180 | <0.001 *** | 0.567 | 8.957 | 0.001 ** | 0.309 |
| NW | 2.46 | (0.6) | 2.98 | (0.7) | ||||||||||
| SC | 2.17 | (0.6) | 2.26 | (0.8) | ||||||||||
| Negative Affect | CT | 2.24 | (0.7) | 1.61 | (0.7) | 51.512 | <0.001 *** | 0.72 | 2.072 | 0.154 | 0.094 | 2.626 | 0.089 | 0.116 |
| NW | 2.09 | (1.0) | 1.58 | (0.8) | ||||||||||
| SC | 2.2 | (1.0) | 1.93 | (1.0) | ||||||||||
| State anxiety | CT | 53.90 | (10.9) | 43.95 | (11.0) | 27.128 | <0.001 *** | 0.576 | 5.020 | 0.011 * | 0.201 | 6.603 | 0.003 ** | 0.248 |
| NW | 53.29 | (13.4) | 44.86 | (10.6) | ||||||||||
| SC | 54.38 | (12.8) | 52.33 | (13.0) | ||||||||||
| Self-efficacy | CT | 22.29 | (5.9) | 26.76 | (6.1) | 27.200 | <0.001 *** | 0.576 | 8.496 | 0.001 ** | 0.298 | 6.046 | 0.005 ** | 0.232 |
| NW | 22.14 | (7.13) | 23.71 | (6.6) | ||||||||||
| SC | 21.67 | (6.3) | 22.67 | (7.1) | ||||||||||
| Variable | Intervention | t0 | t1 | t2 | Time | Intervention | Time*Intervention | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | F (2,40) | p | η2 | F (2,40) | p | η2 | F (4,80) | p | η2 | ||
| Heart rate (bpm) | CT | 90.5 | (14.8) | 105.5 | (22.1) | 102.7 | (18.0) | 7.837 | 0.002 ** | 0.359 | 20.004 | <0.001 *** | 0.588 | 2.970 | 0.027 * | 0.175 |
| NW | 85.7 | (15.8) | 93.0 | (15.6) | 85.9 | (10.9) | ||||||||||
| SC | 76.3 | (7.7) | 75.0 | (11.1) | 78.9 | (11.0) | ||||||||||
| RPE | CT | 5.95 | (3.3) | 9.76 | (2.7) | 9.48 | (2.7) | 11.550 | <0.001 *** | 0.366 | 22.404 | <0.001 *** | 0.528 | 12.041 | <0.001 *** | 0.376 |
| NW | 6.0 | (3.6) | 7.57 | (1.9) | 7.38 | (2.1) | ||||||||||
| SC | 5.1 | (3.6) | 4.48 | (3.1) | 4.57 | (3.2) | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaller, L.; Frühauf, A.; Heimbeck, A.; Voderholzer, U.; Kopp, M. A Comparison of Acute Effects of Climbing Therapy with Nordic Walking for Inpatient Adults with Mental Health Disorder: A Clinical Pilot Trial. Int. J. Environ. Res. Public Health 2022, 19, 6767. https://doi.org/10.3390/ijerph19116767
Thaller L, Frühauf A, Heimbeck A, Voderholzer U, Kopp M. A Comparison of Acute Effects of Climbing Therapy with Nordic Walking for Inpatient Adults with Mental Health Disorder: A Clinical Pilot Trial. International Journal of Environmental Research and Public Health. 2022; 19(11):6767. https://doi.org/10.3390/ijerph19116767
Chicago/Turabian StyleThaller, Lisa, Anika Frühauf, Alexander Heimbeck, Ulrich Voderholzer, and Martin Kopp. 2022. "A Comparison of Acute Effects of Climbing Therapy with Nordic Walking for Inpatient Adults with Mental Health Disorder: A Clinical Pilot Trial" International Journal of Environmental Research and Public Health 19, no. 11: 6767. https://doi.org/10.3390/ijerph19116767






