Associations of Prenatal Exposure to Phthalates with Measures of Cognition in 4.5-Month-Old Infants
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Phthalate Exposure Assessment
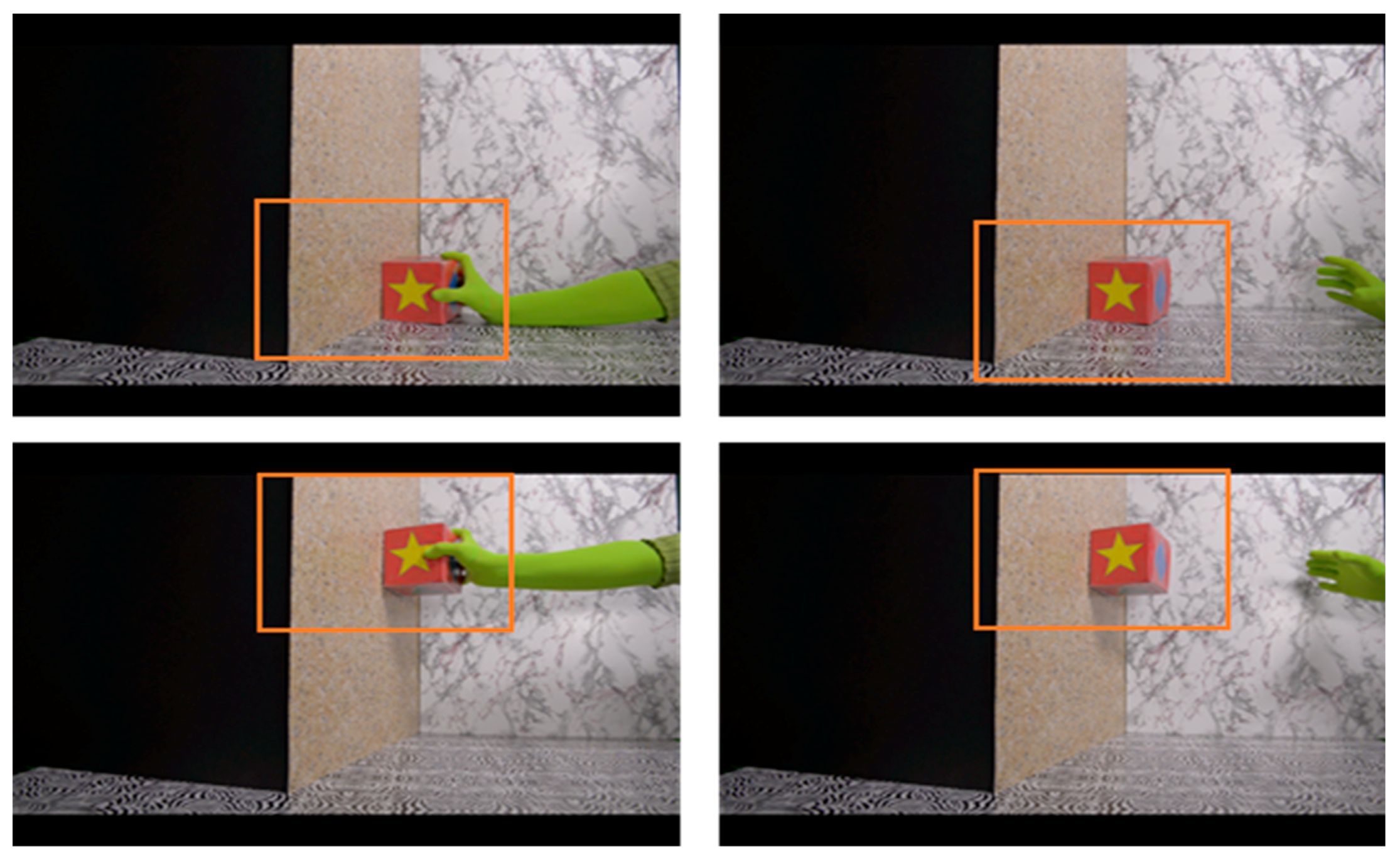
2.3. Physical Reasoning Task
2.4. Statistical Analysis
3. Results
3.1. Descriptive Data
3.2. Exposure Characterization
3.3. Phthalates and Physical Reasoning
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ejaredar, M.; Nyanza, E.C.; Ten Eycke, K.; Dewey, D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res. 2015, 142, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, S. Phthalates and children’s health. Curr. Probl. Pediatric Adolesc. Health Care 2008, 38, 34–49. [Google Scholar] [CrossRef]
- Miodovnik, A.; Edwards, A.; Bellinger, D.C.; Hauser, R. Developmental neurotoxicity of ortho-phthalate diesters: Review of human and experimental evidence. NeuroToxicology 2014, 41, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yolton, K.; Xu, Y.; Strauss, D.; Altaye, M.; Calafat, A.M.; Khoury, J. Prenatal exposure to bisphenol A and phthalates and infant behavior. Neurotoxicol. Teratol. 2011, 33, 558–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kim, E.; Park, H.; Ha, M.; Kim, J.; Hong, Y.; Chang, N.; Kim, B. Prenatal exposure to phthalates and infant development at 6 months: Prospective mothers and children’s environmental health (MOCEH) study. Environ. Health Perspect. 2011, 119, 1495–1500. [Google Scholar] [CrossRef] [Green Version]
- Doherty, B.T.; Engel, S.M.; Buckley, J.P.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Prenatal phthalate biomarker concentrations and performance on the Bayley Scale of Infant Development-II in a population of young urban children. Environ. Res. 2017, 152, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Ipapo, K.N.; Factor-Litvak, P.; Whyatt, R.M.; Calafat, A.M.; Diaz, D.; Perera, F.; Rauh, V.; Herbstman, J.B. Maternal prenatal urinary phthalate metabolite concentrations and visual recognition memory among infants at 27 weeks. Environ. Res. 2017, 155, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Eom, S.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Cho, G.J.; Kim, Y.D.; et al. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age—CHECK cohort study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef]
- Engel, S.M.; Zhu, C.; Berkowitz, G.S.; Calafat, A.M.; Silva, M.J.; Miodovnik, A.; Wolff, M.S. Prenatal phthalate exposure and performance on the neonatal behavioral assessment scale in a multiethnic birth cohort. NeuroToxicology 2009, 30, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Tellez-Rojo, M.M.; Cantoral, A.; Cantonwine, D.E.; Schnaas, L.; Peterson, K.; Hu, H.; Meeker, J.D. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci. Total Environ. 2013, 461–462, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Engel, S.M.; Miodovnik, A.; Canfield, R.L.; Zhu, C.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect. 2010, 118, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B. The intersection of neurotoxicology and endocrine disruption. NeuroToxicology 2012, 33, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Rovet, J. The role of thyroid hormones for brain development and cognitive function. Endoct Dev. 2014, 26, 26–43. [Google Scholar] [CrossRef]
- Huang, P.; Kuo, P.; Guo, Y.; Liao, P.; Lee, C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Wu, W.; Xu, Y.; Jin, Z.; Bao, H.; Zhu, P.; Su, P.; Sheng, J.; Hao, J.; Tao, F. Effects of prenatal phthalate exposure on thyroid hormone concentrations beginning at the embryonic stage. Nat. Sci. Rep. 2017, 7, 13106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moog, N.K.; Entringer, S.; Heim, C.; Wadwha, P.D.; Kathmann, N.; Buss, C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2017, 342, 68–100. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, K.K.; Peterson, K.E.; Lee, J.M.; Mercado-Garcia, A.; Blank-Goldenberg, C.; Tellez-Rojo, M.M.; Meeker, J.D. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod. Toxicol. 2014, 47, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowell, W.J.; Wrigth, R.J. Sex-specific effects of combined exposure to chemical and non-chemical stressors on neuroendocrine development: A review of recent findings and putative mechanisms. Curr. Environ. Health Rep. 2017, 4, 415–425. [Google Scholar] [CrossRef]
- Auyeung, B.; Lombardo, M.V.; Baron-Cohen, S. Prenatal and postnatal hormone effects on the human brain and development. Pflug. Arch. Eur. J. Physiol. 2013, 465, 557–571. [Google Scholar] [CrossRef]
- Brookmeyer, R. Analysis of multistage pooling studies of biological specimens for estimating disease incidence and prevalence. Biometrics 1999, 55, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, C.T.; Umbach, D.M. Using pooled exposure assessment to improve efficiency in case-control studies. Biometrics 1999, 55, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Sham, P.; Bader, J.S.; Craig, I.; O’Donovan, M.; Owen, M. DNA Pooling: A tool for larger-scale association studies. Nat. Rev. Genet. 2002, 3, 862–871. [Google Scholar] [CrossRef]
- Liu, A.; Schisterman, E.F. Comparison of diagnostic accuracy of biomarkers with pooled assessments. Biom. J. 2003, 45, 631–644. [Google Scholar] [CrossRef]
- Saha-Chaudhuri, P.; Weinberg, C.R. Specimen pooling for efficient used of biospecimens in studies of time to a common event. Am. J. Epidemiol. 2013, 178, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillargeon, R. A model of physical reasoning in infancy. In Advances in Infancy Research; Rovee-Collier, C., Lipsitt, L.P., Eds.; Ablex: Norwood, NJ, USA, 1995; Volume 9, pp. 305–371. [Google Scholar]
- Silva, M.J.; Samandar, E.; Preau, J.L., Jr.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B 2007, 860, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Automated on-line column switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal. Chem. 2005, 77, 5407–5413. [Google Scholar] [CrossRef]
- Duty, S.M.; Ackerman, R.M.; Calafat, A.M.; Hauser, R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect. 2005, 113, 1530–1535. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals; US Department of Health and Human Services: Atlanta, GA, USA, 2017; Volume 1. [Google Scholar]
- Shoaff, J.R.; Calafat, A.M.; Schantz, S.L.; Korrick, S.A. Endocrine disrupting chemical exposure and maladaptive behavior during adolescence. Environ. Res. 2019, 172, 231–241. [Google Scholar] [CrossRef]
- Hannas, B.R.; Lambright, C.S.; Furr, J.; Howdeshell, K.L.; Wilson, V.S.; Gray, L.E. Dose response assessment of fetal testosterone production and gene expression levels in rat testes following inutero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol. Sci. 2011, 123, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Howdeshell, K.L.; Rider, C.V.; Wilson, V.S.; Furr, J.R.; Lambright, C.R.; Gray, L.E. Dose addition models based on biologically relevant reductions in fetal testosterone accurately predict postnatal reproductive tract alterations by a phthalate mixture in rats. Toxicol. Sci. 2015, 148, 488–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merced-Nieves, F.M.; Aguiar, A.; Dzwilewski, K.L.C.; Musaad, S.; Korrick, S.A.; Schantz, S.L. Association of prenatal maternal perceived stress with a sexually dimorphic measure of cognition in 4.5-month-old infants. Neurotoxicol. Teratol. 2020, 77, 106850. [Google Scholar] [CrossRef] [PubMed]
- Hernan, M.A.; Hernandez-Diaz, S.; Werler, M.M.; Robins, J.M.; Mitchell, A.A. Causal knowledge as a prerequisite for confounding evaluation. Examples from birth defects epidemiology. Am. J. Epidemiol. 2000, 151, S43. [Google Scholar]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables; US Department of Health and Human Services: Atlanta, GA, USA, 2019. [Google Scholar]
- Houston-Price, C.; Nakai, S. Distinguish novelty and familiarity effect in infant preference procedures. Inf. Child. Dev. 2004, 13, 341–348. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
| Full Cohort (n = 558) | Participants with Phthalate Data (n = 481) | Current Analysis (n = 159) | |||||
|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | ||
| Maternal race | |||||||
| White, Non-Hispanic | 446 (79.9) | 388 (80.7) | 127 (80) | ||||
| Other | 111 (19.9) | 92 (19.1) | 32 (20) | ||||
| Unknown/Missing | 1 (0.2) | 1 (0.2) | |||||
| Maternal age (years) | 30.2 (4.3) | 30.3 (4.1) | 30.4 (3.9) | ||||
| Maternal education | |||||||
| Some college or less | 123 (22.0) | 90 (18.7) | 29 (18) | ||||
| College degree or higher | 435 (78.0) | 391 (81.3) | 130 (82) | ||||
| Annual household income | |||||||
| <$60,000 | 171 (30.6) | 138 (28.7) | 48 (30) | ||||
| ≥$60,000 | 382 (68.5) | 340 (70.7) | 109 (69) | ||||
| Unknown/Missing | 5 (0.9) | 3 (0.6) | 2 (1) | ||||
| Maternal smoking 1 | 30 (5.4) | 23 (4.8) | 6 (4) | ||||
| Maternal drinking 2 | 225 (40.3) | 199 (41.4) | 37 (23) | ||||
| Mode of delivery (% cesarean) | 127 (22.8) | 125 (26) | 34 (22) | ||||
| Gestational age (weeks) | 39.3 (1.5) | 39.3 (1.4) | 39.5 (1.1) | ||||
| Infant sex | |||||||
| Female | 237 (42.5) | 247 (51.2) | 78 (49) | ||||
| Male | 259 (46.4) | 234 (48.7) | 81 (51) | ||||
| Unknown/Missing | 62 (11.1) | 0 (0) | 0 (0) | ||||
| 16–18 Weeks Gestation Sample (n = 158) | Pooled Sample (n = 159) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure 1 | Units | Median | IQR 2 | Minimum | Maximum | Median | IQR | Minimum | Maximum |
| MEP | μg\L | 24.34 | 33.09 | 0 | 576.43 | 29.05 | 32.62 | 4.8 | 672.76 |
| Σ DEHP | µmol/L | 0.06 | 0.05 | 0.01 | 0.76 | 0.07 | 0.05 | 0.03 | 0.5 |
| Σ DINP | µmol/L | 0.02 | 0.04 | 0.002 | 2.97 | 0.03 | 0.04 | 0.01 | 0.82 |
| Σ AA | µmol/L | 0.21 | 0.18 | 0.03 | 2.97 | 0.23 | 0.17 | 0.09 | 4.17 |
| Σ All phthalates | µmol/L | 0.43 | 0.42 | 0.08 | 3.83 | 0.50 | 0.38 | 0.14 | 5.42 |
| Main Effect | Females (n = 78) | Males (n = 81) | |||||
|---|---|---|---|---|---|---|---|
| Exposure Measure | N | β Estimate | 95% Confidence Interval | β Estimate | 95% Confidence Interval | β Estimate | 95% Confidence Interval |
| Phthalates (16–18 weeks gestation sample) 1 | |||||||
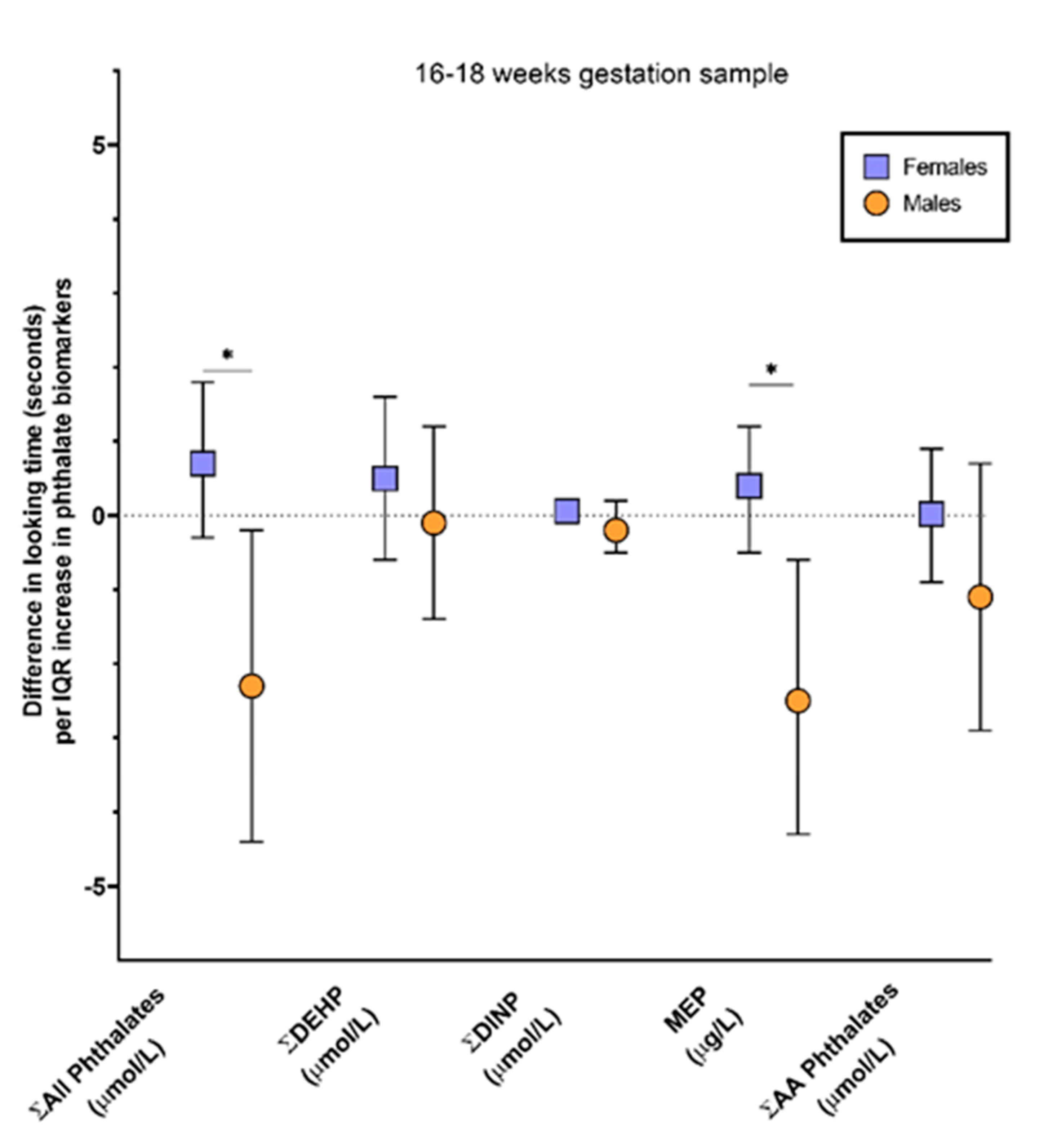
| MEP (μg\L) | 158 | 0.3 | −0.3, 0.9 | 0.4 | −0.5, 1.2 | −2.5 | −4.4, −0.6 |
| Σ DEHP (µmol/L) | 158 | 0.2 | −0.6, 1.1 | 0.5 | −0.6, 1.6 | −0.1 | −1.4, 1.2 |
| Σ DINP (µmol/L) | 158 | 0.004 | −0.2, 0.2 | 0.06 | −0.1, 0.2 | −0.2 | −0.5, 0.2 |
| Σ Anti-Androgenic (µmol/L) | 158 | −0.2 | −1.0, 0.6 | 0.02 | −0.9, 0.9 | −1.1 | −2.9, 0.7 |
| Σ All phthalates (µmol/L) | 158 | 0.2 | −0.8, 1.1 | 0.7 | −0.3, 1.8 | −2.3 | −4.4, −0.2 |
| Phthalates (Pooled sample) | |||||||
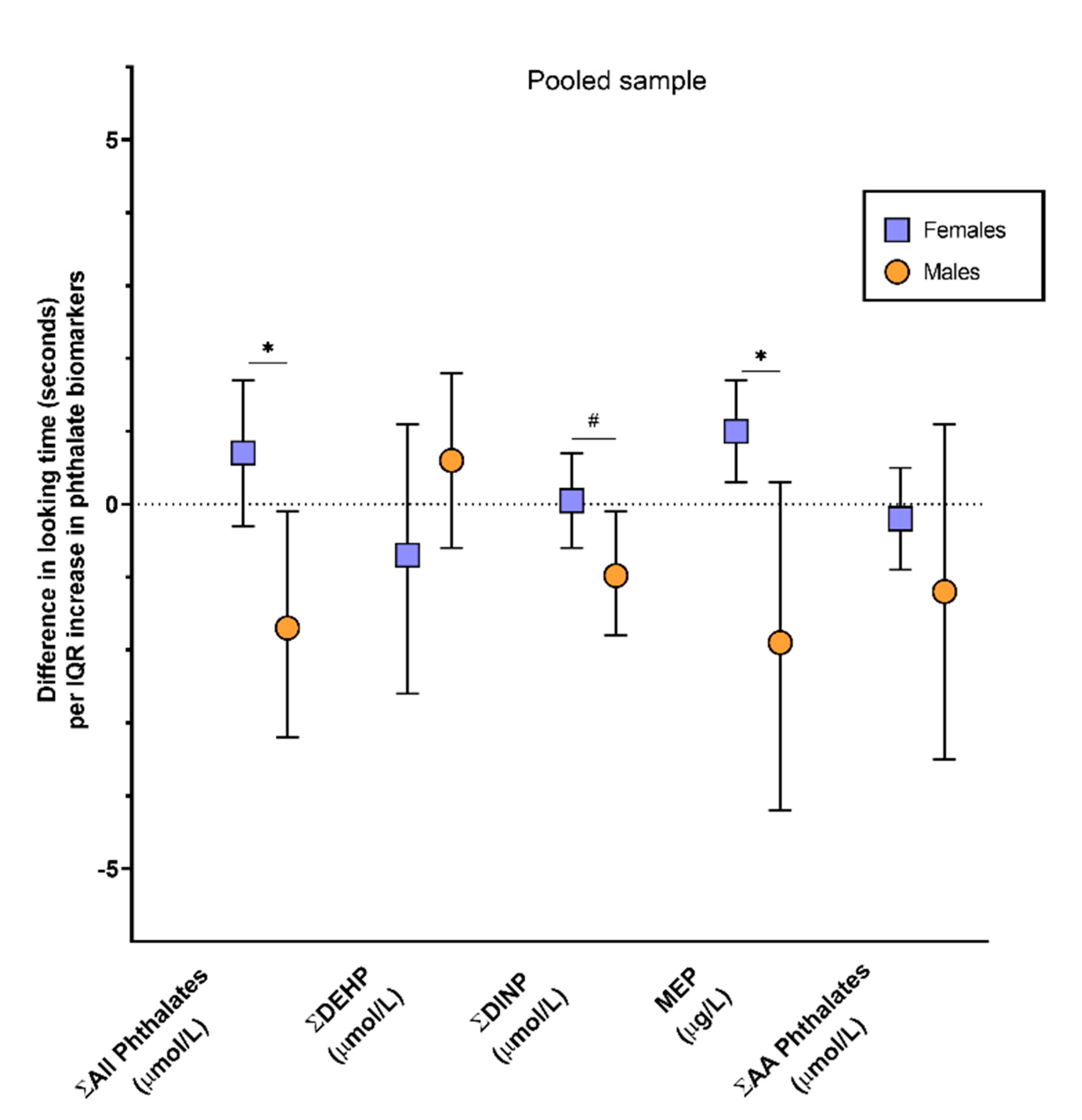
| MEP (μg\L) | 159 | 0.3 | −0.3, 0.8 | 1 | 0.3, 1.7 | −1.9 | −4.2, 0.3 |
| Σ DEHP (µmol/L) | 159 | 0.2 | −0.8, 1.2 | −0.7 | −2.6, 1.1 | 0.6 | −0.6, 1.7 |
| Σ DINP 2 (µmol/L) | 158 | −0.3 | −0.7, 0.2 | 0.05 | −0.6, 0.7 | −1 | −1.8, −0.1 |
| Σ Anti-Androgenic (µmol/L) | 159 | −0.3 | −1.0, 0.4 | −0.2 | −0.9, 0.5 | −1.2 | −3.5, 1.1 |
| Σ All phthalates (µmol/L) | 159 | 0.04 | −0.8, 0.9 | 0.7 | −0.3, 1.7 | −1.7 | −3.2, −0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merced-Nieves, F.M.; Dzwilewski, K.L.C.; Aguiar, A.; Musaad, S.; Korrick, S.A.; Schantz, S.L. Associations of Prenatal Exposure to Phthalates with Measures of Cognition in 4.5-Month-Old Infants. Int. J. Environ. Res. Public Health 2021, 18, 1838. https://doi.org/10.3390/ijerph18041838
Merced-Nieves FM, Dzwilewski KLC, Aguiar A, Musaad S, Korrick SA, Schantz SL. Associations of Prenatal Exposure to Phthalates with Measures of Cognition in 4.5-Month-Old Infants. International Journal of Environmental Research and Public Health. 2021; 18(4):1838. https://doi.org/10.3390/ijerph18041838
Chicago/Turabian StyleMerced-Nieves, Francheska M., Kelsey L. C. Dzwilewski, Andrea Aguiar, Salma Musaad, Susan A. Korrick, and Susan L. Schantz. 2021. "Associations of Prenatal Exposure to Phthalates with Measures of Cognition in 4.5-Month-Old Infants" International Journal of Environmental Research and Public Health 18, no. 4: 1838. https://doi.org/10.3390/ijerph18041838






