Exploring Morpho-Physiological Variation for Heat Stress Tolerance in Tomato
Abstract
:1. Introduction
2. Results
2.1. First Open-Field Screening
2.2. Growth Chamber and Greenhouse Screening
2.2.1. Chlorophyll Fluorescence (CF)
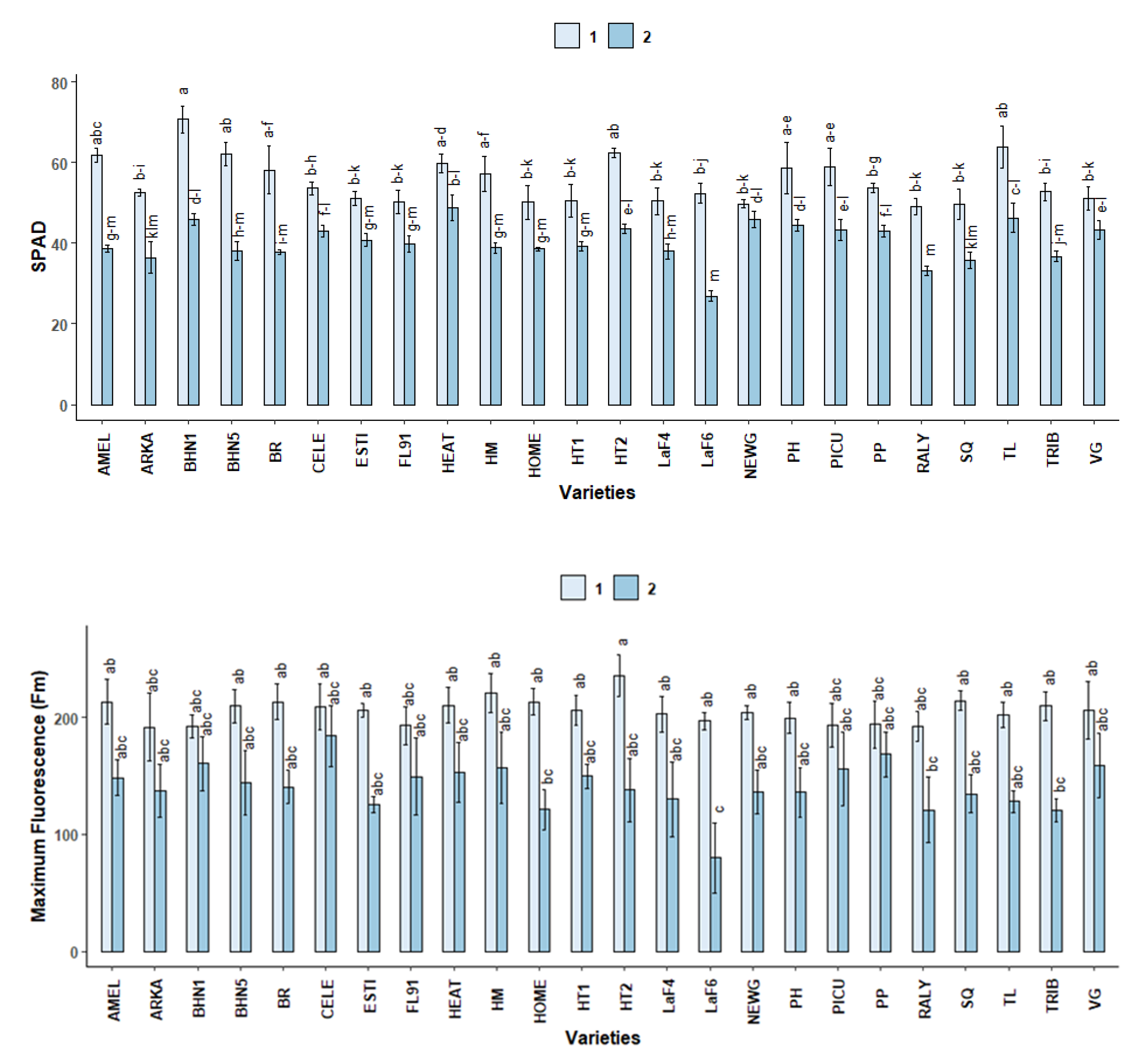
2.2.2. SPAD
2.2.3. Plant Height (Ht)
2.2.4. Stem Diameter (D)
2.2.5. Heat Injury Index (HII)
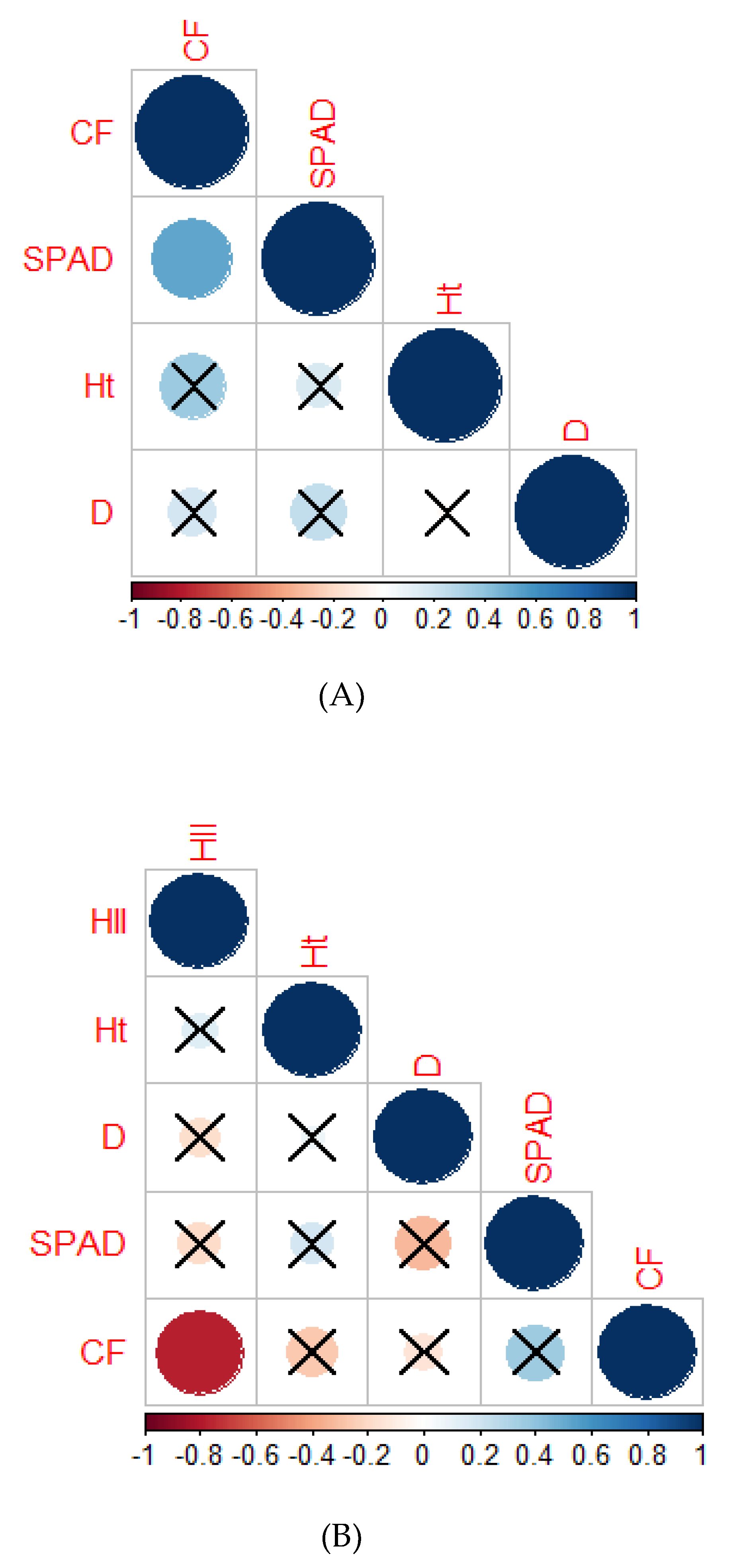
2.2.6. Correlation Analysis
2.3. Second Open-Field Screening
2.3.1. SPAD
2.3.2. Chlorophyll Fluorescence
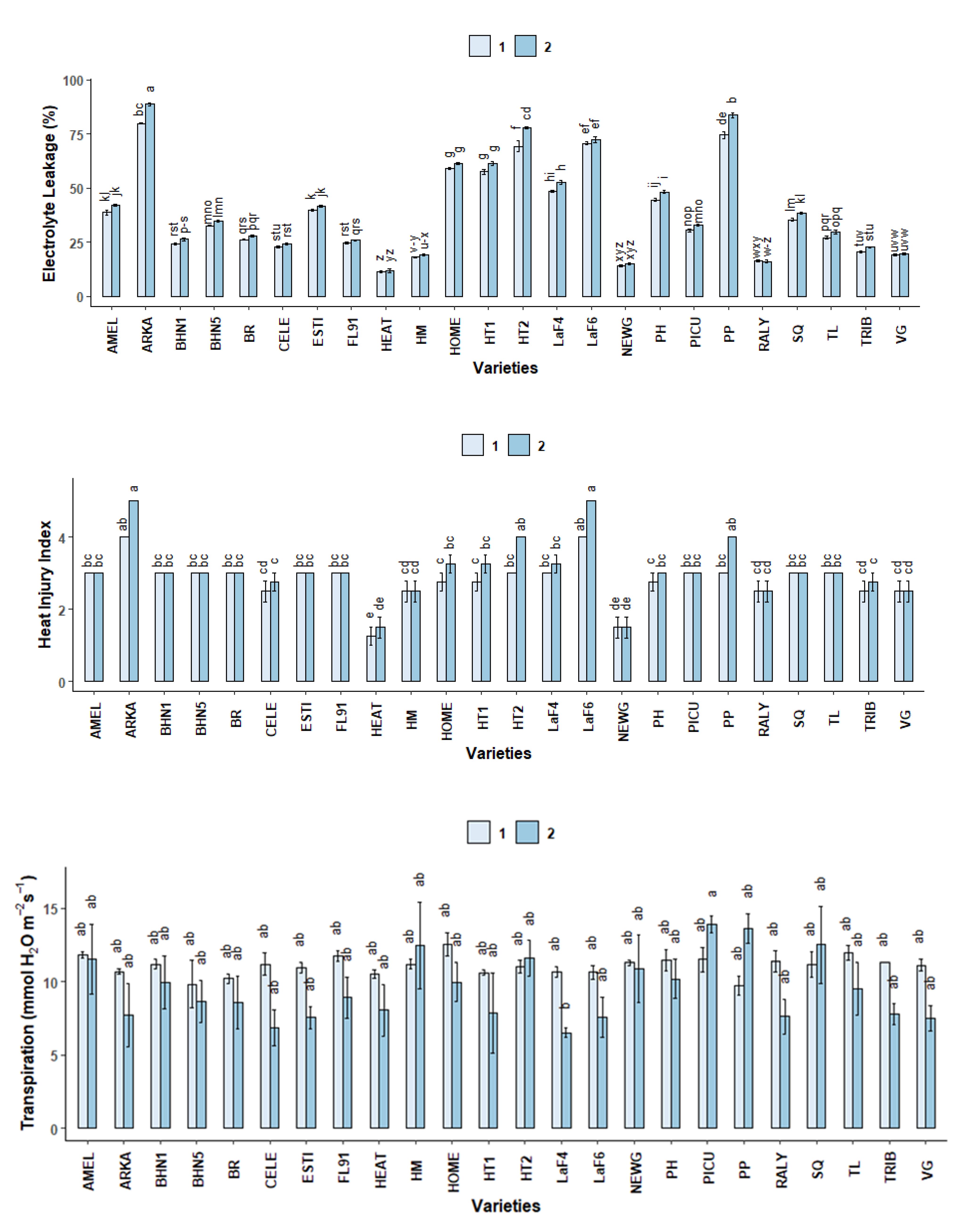
2.3.3. Electrolyte Leakage (EL)
2.3.4. Heat Injury Index (HII)
2.3.5. Gas Exchange
2.3.6. Leaf Temperature (LT)
2.3.7. Water Use Efficiency
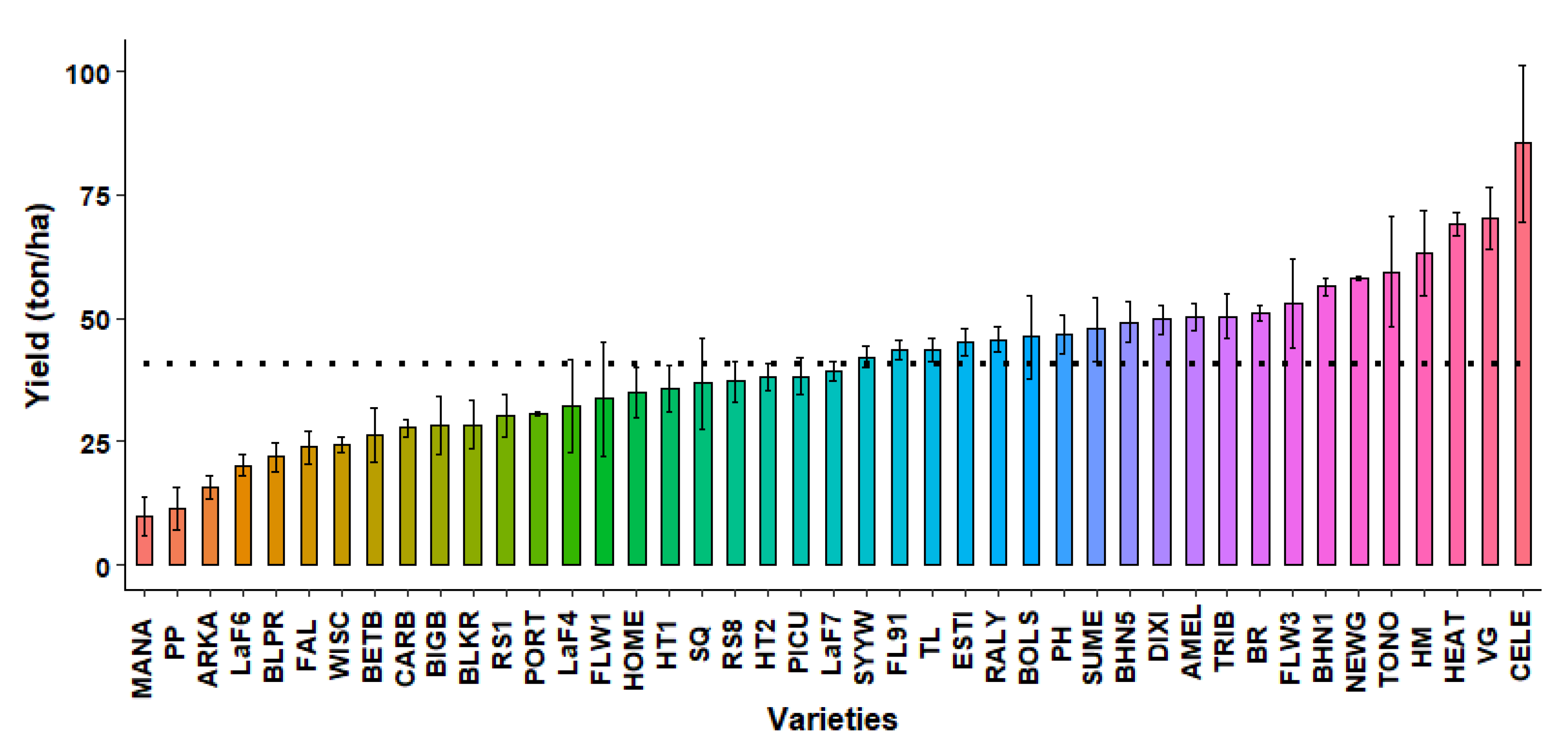
2.3.8. Marketable Yield
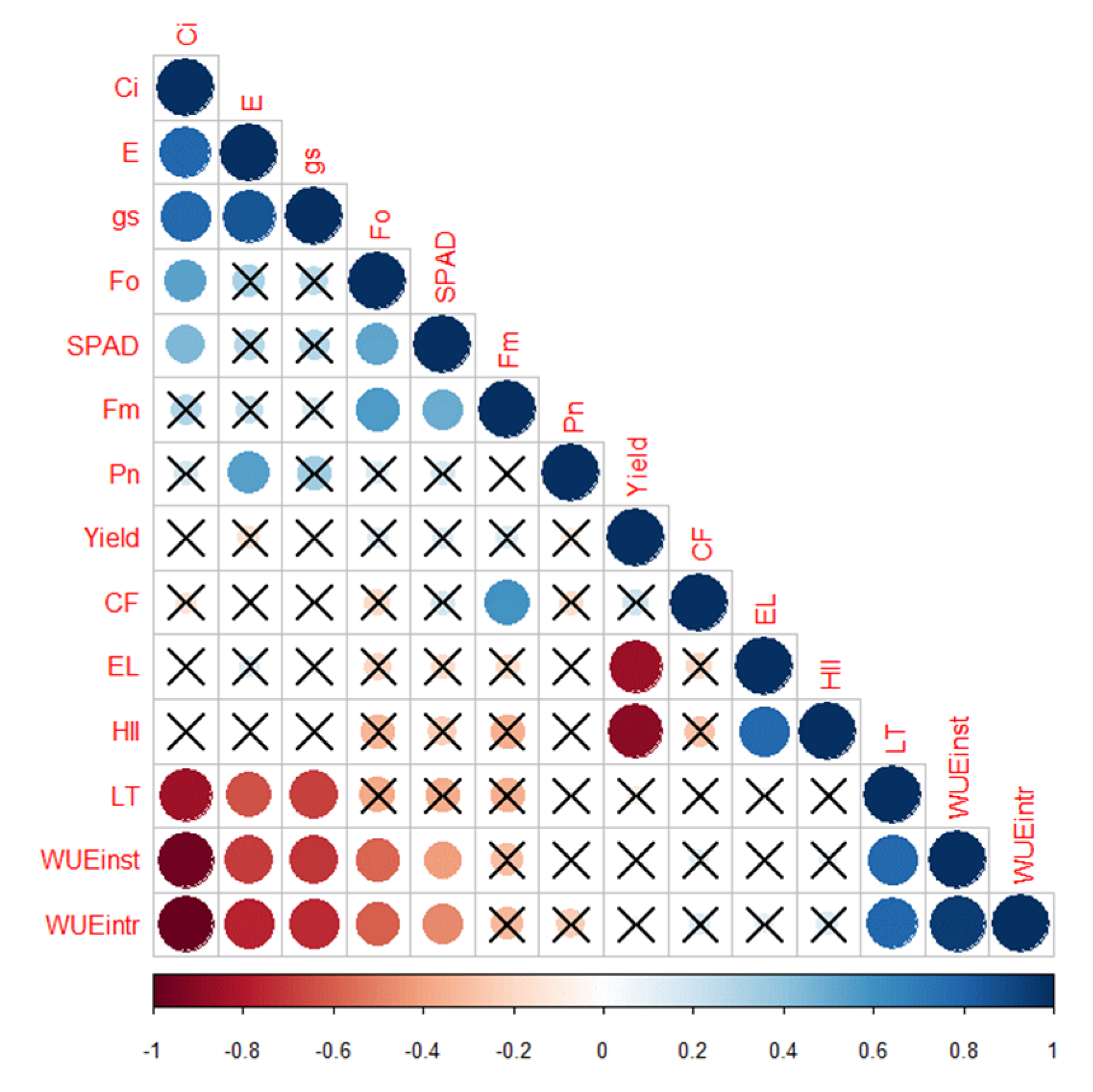
2.3.9. Correlation Analysis
3. Discussion
3.1. Growth Chamber and Greenhouse Experiment
3.2. Field Experiments
4. Materials and Methods
4.1. Plant Material and Field Growing Conditions in 2019
4.2. Plant Materials and Growth Chamber and Greenhouse Conditions in 2019
4.2.1. Chlorophyll Content
4.2.2. Chlorophyll Fluorescence
4.2.3. Plant Height and Stem Diameter
4.2.4. Heat Injury Index (HII)
- = no injury
- = yellow and mildly dehydrated margins of old leaflets
- = mildly dehydrated plants with the middle and crinkled bottom leaflets
- = severely dehydrated plants with upper leaflets crinkled
- = plants with most leaves withered
4.3. Plant Materials and Field Growing Conditions in 2020
4.3.1. Chlorophyll Content
4.3.2. Leaf Gas Exchange, Chlorophyll Fluorescence and Leaf Temperature
4.3.3. Intrinsic and Instantaneous Leaf Water Use Efficiency
4.3.4. Electrolyte Leakage
4.3.5. Average Total Yield
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Chlorophyll Fluorescence |
| SPAD | Soil Plant Analysis Development |
| Ht | Plant Height |
| D | Stem Diameter |
| HII | Heat Injury Index |
| Ci | Intercellular CO2 Concentration |
| E | Transpiration Rate |
| gs | Stomatal Conductance |
| Fo | Initial Chlorophyll Fluorescence |
| Fm | Maximum Chlorophyll Fluorescence |
| Pn | Net Photosynthetic Rate |
| EL | Electrolyte Leakage |
| LT | Leaf Temperature |
| WUEinst | Instantaneous Water Use Efficiency |
| WUEintr | Intrinsic Water Use Efficiency |
Appendix A
| Initial Chlorophyll Fluorescence (Fo) | |||
|---|---|---|---|
| Variety | Stage 1 | Stage 2 | Increase (%) |
| PH | 40.7 ± 2.4 b | 79.0 ± 8.30 a | 93.8 |
| BR | 42.7 ± 1.4 b | 80.5 ± 9.10 a | 88.3 |
| PICU | 39.7 ± 1.7 b | 74.7 ± 1.40 a | 88.0 |
| BHN1 | 40.5 ± 1.3 b | 73.7 ± 8.60 a | 82.0 |
| LaF6 | 41.7 ± 2.6 b | 73.2 ± 7.40 a | 75.4 |
| ESTI | 43.5 ± 2.0 b | 74.7 ± 11.7 a | 71.8 |
| AMEL | 44.5 ± 0.6 b | 75.5 ± 7.50 a | 69.6 |
| ARKA | 36.2 ± 4.1 b | 61.5 ± 7.50 a | 69.6 |
| CELE | 42.7 ± 1.8 a | 72.2 ± 12.0 a | |
| HT2 | 44.0 ± 1.2 b | 74.2 ± 3.50 a | 68.7 |
| FL91 | 40.0 ± 1.0 b | 66.7 ± 5.40 a | 66.8 |
| PP | 41.5 ± 0.9 b | 66.7 ± 4.50 a | 60.8 |
| HEAT | 41.2 ± 1.4 a | 65.5 ± 10.5 a | |
| NEWG | 45.0 ± 2.0 a | 71.2 ± 12.2 a | |
| BHN5 | 42.7 ± 1.4 b | 66.2 ± 7.70 a | 54.9 |
| SQ | 43.5 ± 1.1 a | 65.2 ± 11.2 a | |
| VG | 41.0 ± 1.8 b | 61.5 ± 3.30 a | 50.0 |
| HM | 45.2 ± 1.2 b | 65.7 ± 6.40 a | 45.3 |
| LaF4 | 43.2 ± 0.9 a | 62.7 ± 11.1 a | |
| TRIB | 41.2 ± 1.2 b | 59.0 ± 4.60 a | 43.0 |
| RALY | 43.0 ± 2.9 a | 59.5 ± 13.5 a | |
| HT1 | 40.2 ± 2.0 b | 55.5 ± 3.30 a | 37.9 |
| HOME | 45.2 ± 1.8 a | 60.0 ± 8.40 a | |
| TL | 43.2 ± 1.6 a | 57.0 ± 9.40 a | |
| Chlorophyll Fluorescence (CF) | |||
|---|---|---|---|
| Variety | Stage 1 | Stage 2 | Decrease (%) |
| LaF6 | 0.79 ± 0.01 a | 0.37 ± 0.10 b | 53.4 |
| PH | 0.79 ± 0.01 a | 0.40 ± 0.04 b | 49.1 |
| HT2 | 0.81 ± 0.01 a | 0.42 ± 0.08 b | 48.6 |
| ESTI | 0.79 ± 0.01 a | 0.41 ± 0.07 b | 48.4 |
| BR | 0.79 ± 0.01 a | 0.43 ± 0.02 b | 46.5 |
| PICU | 0.79 ± 0.01 a | 0.47 ± 0.09 b | 41.1 |
| NEWG | 0.78 ± 0.01 a | 0.47 ± 0.06 b | 39.7 |
| HOME | 0.79 ± 0.01 a | 0.48 ± 0.10 b | 38.9 |
| BHN5 | 0.79 ± 0.02 a | 0.49 ± 0.05 b | 38.5 |
| LaF4 | 0.78 ± 0.01 a | 0.48 ± 0.06 b | 38.0 |
| AMEL | 0.79 ± 0.02 a | 0.49 ± 0.01 b | 37.6 |
| TRIB | 0.80 ± 0.00 a | 0.51 ± 0.01 b | 36.5 |
| FL91 | 0.79 ± 0.02 a | 0.50 ± 0.07 b | 36.0 |
| SQ | 0.79 ± 0.01 a | 0.51 ± 0.08 b | 35.9 |
| RALY | 0.77 ± 0.02 a | 0.50 ± 0.06 b | 35.1 |
| HEAT | 0.80 ± 0.01 a | 0.53 ± 0.03 b | 34.5 |
| ARKA | 0.80 ± 0.01 a | 0.53 ± 0.05 b | 34.0 |
| HM | 0.79 ± 0.01 a | 0.56 ± 0.06 b | 29.9 |
| TL | 0.78 ± 0.01 a | 0.56 ± 0.05 b | 28.1 |
| BHN1 | 0.79 ± 0.01 a | 0.58 ± 0.01 b | 26.6 |
| VG | 0.79 ± 0.02 a | 0.59 ± 0.04 b | 25.5 |
| PP | 0.78 ± 0.02 a | 0.59 ± 0.02 b | 23.3 |
| CELE | 0.79 ± 0.01 a | 0.61 ± 0.02 b | 23.3 |
| HT1 | 0.80 ± 0.01 a | 0.62 ± 0.03 b | 22.2 |
| Stomatal Conductance (gs, mol H2O m−2 s−1) | |||
|---|---|---|---|
| Variety | Stage 1 | Stage 2 | Decrease (%) |
| LaF4 | 0.72 ± 0.0813 a | 0.1195 ± 0.0114 b | 83.340 |
| CELE | 0.72 ± 0.0239 a | 0.1405 ± 0.0333 b | 80.372 |
| RALY | 0.79 ± 0.1043 a | 0.1579 ± 0.0356 b | 80.093 |
| BHN1 | 0.80 ± 0.0307 a | 0.1749 ± 0.0127 b | 78.112 |
| ESTI | 0.67 ± 0.0361 a | 0.1496 ± 0.0268 b | 77.762 |
| VG | 0.70 ± 0.0402 a | 0.1611 ± 0.0313 b | 77.067 |
| TRIB | 0.71 ± 0.0379 a | 0.1673 ± 0.0287 b | 76.393 |
| FL91 | 0.78 ± 0.0245 a | 0.1948 ± 0.0458 b | 74.910 |
| HEAT | 0.69 ± 0.0492 a | 0.1756 ± 0.0565 b | 74.654 |
| LaF6 | 0.70 ± 0.0437 a | 0.1782 ± 0.0443 b | 74.599 |
| ARKA | 0.63 ± 0.0708 a | 0.1820 ± 0.0670 b | 70.984 |
| HT1 | 0.67 ± 0.0434 a | 0.1991 ± 0.1023 b | 70.175 |
| HOME | 0.72 ± 0.0261 a | 0.2338 ± 0.0507 b | 67.725 |
| TL | 0.73 ± 0.0357 a | 0.2343 ± 0.0782 b | 67.719 |
| NEWG | 0.80 ± 0.0507 a | 0.2588 ± 0.0657 b | 67.582 |
| PH | 0.69 ± 0.0351 a | 0.2478 ± 0.0611 b | 63.842 |
| BR | 0.60 ± 0.0322 a | 0.2216 ± 0.0712 b | 62.894 |
| BHN5 | 0.64 ± 0.1465 a | 0.2458 ± 0.0665 b | 61.858 |
| HT2 | 0.74 ± 0.0342 a | 0.2979 ± 0.0716 b | 59.619 |
| AMEL | 0.79 ± 0.0513 a | 0.3262 ± 0.1036 b | 58.436 |
| SQ | 0.71 ± 0.0455 a | 0.3249 ± 0.0895 b | 54.073 |
| HM | 0.72 ± 0.0329 a | 0.3497 ± 0.1195 b | 51.246 |
| PICU | 0.71 ± 0.0370 a | 0.3678 ± 0.0333 b | 48.307 |
| PP | 0.54 ± 0.0437 a | 0.3768 ± 0.0554 b | 30.103 |
| Intercellular CO2 Concentration (Ci, µmol CO2 mol−1) | |||
|---|---|---|---|
| Variety | Stage 1 | Stage 2 | Decrease (%) |
| HOME | 320.9 ± 3.80 a | 36.30 ± 167.4 a | |
| HT1 | 320.9 ± 3.80 a | 36.30 ± 167.4 a | |
| ARKA | 318.9 ± 17.1 a | 75.00 ± 121.9 a | |
| LaF4 | 323.1 ± 7.40 a | 82.60 ± 69.80 b | 74.4 |
| LaF6 | 333.2 ± 9.70 a | 93.10 ± 123.7 a | |
| CELE | 330.3 ± 12.3 a | 105.0 ± 93.40 a | |
| ESTI | 322.0 ± 5.90 a | 109.4 ± 83.40 b | 66.0 |
| VG | 316.0 ± 8.50 a | 109.9 ± 90.10 a | |
| RALY | 331.7 ± 7.50 a | 124.1 ± 63.80 b | 62.5 |
| TL | 328.5 ± 13.2 a | 131.1 ± 102.8 a | |
| HEAT | 322.3 ± 7.75 a | 132.5 ± 83.80 a | |
| BR | 322.6 ± 10.6 a | 146.6 ± 92.40 a | |
| TRIB | 316.5 ± 5.91 a | 145.4 ± 84.10 a | |
| FL91 | 322.5 ± 10.0 a | 161.1 ± 67.70 a | |
| BHN5 | 330.3 ± 9.81 a | 169.4 ± 80.10 a | |
| PH | 318.9 ± 12.5 a | 173.5 ± 50.20 b | 45.6 |
| SQ | 330.7 ± 9.45 a | 200.6 ± 80.70 a | |
| NEWG | 340.6 ± 10.6 a | 213.4 ± 22.30 b | 37.3 |
| PICU | 316.7 ± 7.00 a | 202.7 ± 43.30 b | 35.9 |
| HM | 324.7 ± 7.70 a | 208.9 ± 74.30 a | |
| HT2 | 332.9 ± 6.00 a | 217.1 ± 18.30 b | 34.7 |
| BHN1 | 328.7 ± 8.20 a | 221.1 ± 32.30 b | 32.7 |
| AMEL | 336.5 ± 7.30 a | 232.0 ± 29.80 b | 31.1 |
| PP | 325.2 ± 10.8 a | 236.4 ± 40.20 a | |
| Leaf Temperature (°C) | |||
|---|---|---|---|
| Variety | Stage 1 | Stage 2 | Increase (%) |
| LaF4 | 29.1 ± 0.7 b | 40.2 ± 1.8 a | 38.1 |
| BR | 28.8 ± 1.2 b | 39.6 ± 1.6 a | 37.5 |
| CELE | 28.8 ± 1.2 b | 39.6 ± 1.6 a | 37.5 |
| BHN1 | 28.4 ± 0.6 b | 39.0 ± 1.8 a | 37.5 |
| HEAT | 28.6 ± 0.9 b | 39.2 ± 2.1 a | 36.9 |
| RALY | 29.1 ± 0.8 b | 39.6 ± 2.1 a | 36.0 |
| FL91 | 29.2 ± 0.5 b | 39.3 ± 2.2 a | 34.3 |
| ESTI | 29.6 ± 0.2 b | 39.7 ± 2.2 a | 33.9 |
| VG | 29.1 ± 1.0 b | 39.1 ± 2.2 a | 33.9 |
| HT1 | 29.5 ± 0.4 b | 39.4 ± 1.8 a | 33.2 |
| NEWG | 28.6 ± 0.8 b | 38.0 ± 2.1 a | 32.9 |
| LaF6 | 29.1 ± 0.8 b | 38.6 ± 2.3 a | 32.5 |
| TRIB | 29.4 ± 0.6 b | 39.0 ± 2.4 a | 32.5 |
| ARKA | 29.8 ± 0.9 b | 39.3 ± 2.6 a | 31.6 |
| HT2 | 29.0 ± 0.9 b | 38.1 ± 1.7 a | 31.4 |
| BHN5 | 29.4 ± 1.1 b | 38.2 ± 1.5 a | 29.7 |
| TL | 30.0 ± 0.4 b | 38.9 ± 2.4 a | 29.3 |
| PH | 29.8 ± 0.9 b | 38.3 ± 1.9 a | 28.5 |
| AMEL | 29.1 ± 0.8 b | 37.3 ± 2.5 a | 28.3 |
| HM | 29.1 ± 0.9 b | 37.2 ± 2.8 a | 27.9 |
| HOME | 30.3 ± 0.5 b | 38.6 ± 2.2 a | 27.1 |
| PICU | 29.6 ± 0.2 b | 37.5 ± 1.5 a | 26.5 |
| SQ | 29.5 ± 0.1 b | 36.9 ± 2.4 a | 25.3 |
| PP | 30.1 ± 0.7 b | 36.8 ± 1.9 a | 22.1 |
| Intrinsic Water Use Efficiency (WUEinst, µmol CO2 mmol H2O−1) | |||
|---|---|---|---|
| Variety | Stage 1 | Stage 2 | Increase (%) |
| LaF6 | 27.0 ± 4.6 a | 192.2 ± 98.50 a | |
| HT1 | 33.2 ± 1.8 a | 225.1 ± 113.9 a | |
| ARKA | 34.8 ± 8.9 b | 231.2 ± 123.2 a | 564.6 |
| CELE | 28.2 ± 5.7 a | 163.8 ± 59.01 a | |
| RALY | 27.2 ± 4.0 b | 150.5 ± 39.29 a | 453.1 |
| LaF4 | 32.3 ± 4.2 b | 176.6 ± 43.62 a | 446.8 |
| TL | 28.3 ± 6.7 a | 146.0 ± 65.66 a | |
| ESTI | 32.5 ± 2.8 a | 160.3 ± 52.74 a | |
| VG | 35.3 ± 4.0 a | 159.7 ± 55.97 a | |
| HEAT | 32.8 ± 3.9 a | 145.9 ± 51.74 a | |
| HOME | 32.7 ± 5.3 a | 145.1 ± 57.50 a | |
| BHN5 | 28.1 ± 4.5 a | 122.9 ± 50.79 a | |
| BR | 32.8 ± 5.3 a | 137.2 ± 59.11 a | |
| NEWG | 22.9 ± 5.1 b | 93.00 ± 13.21 a | 305.1 |
| FL91 | 31.4 ± 4.6 a | 126.2 ± 41.32 a | |
| TRIB | 34.8 ± 3.0 a | 137.7 ± 51.55 a | |
| SQ | 28.4 ± 4.9 a | 104.7 ± 48.72 a | |
| PH | 34.0 ± 6.0 b | 118.0 ± 31.30 a | 246.8 |
| AMEL | 24.5 ± 3.4 b | 82.50 ± 16.93 a | 235.9 |
| HT2 | 26.8 ± 2.7 b | 89.60 ± 10.99 a | 233.8 |
| HM | 30.9 ± 3.7 a | 100.2 ± 46.00 a | |
| BHN1 | 28.5 ± 3.7 b | 90.60 ± 19.56 a | 217.4 |
| PICU | 34.7 ± 3.8 b | 97.70 ± 25.20 a | 181.0 |
| PP | 31.9 ± 5.6 a | 79.10 ± 24.01 a | |
References
- Rick, C.M. Potential genetic resources in tomato species: Clues from observations in native habitats. In Genes, Enzymes, and Populations; Springer: Boston, MA, USA, 1973; pp. 255–269. [Google Scholar] [CrossRef]
- FAOSTAT. 2017: Agriculture Organization of the United Nations Database. The Agricultural Production. Available online: http://www.apps.fao.org (accessed on 30 June 2020).
- Heuvelink, E.; Okello, R.C.; Peet, M.; Giovannoni, J.J.; Dorais, M. Tomato. In The Physiology of Vegetable Crops, 2nd ed.; Wien, H.C., Stützel, H., Eds.; CABI: Boston, MA, USA, 2020; pp. 138–178. [Google Scholar] [CrossRef]
- USDA ERS. Vegetable and Pulses Outlook. 2019. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/cj82k729x/t435gs076/6d570986z/VGS363.pdf (accessed on 12 July 2019).
- USDA ERS. Vegetable and Pulses Outlook. 2020. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/cj82k729x/9p290w93h/0z709g24p/VGS364.pdf (accessed on 18 September 2020).
- Kandel, D.R.; Bedre, R.H.; Mandadi, K.K.; Crosby, K.; Avila, C.A. Genetic Diversity and Population Structure of Tomato (Solanum lycopersicum) Germplasm Developed by Texas A&M Breeding Programs. Am. J. Plant Sci. 2019, 10, 1154–1180. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Poudyal, D.; Rosenqvist, E.; Ottosen, C.O. Phenotyping from lab to field–tomato lines screened for heat stress using Fv/Fm maintain high fruit yield during thermal stress in the field. Funct. Plant Biol. 2019, 46, 44–55. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.O.; Wu, Z. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Wu, Z.; Rosenqvist, E.; Wang, Y.; Zhao, L.; Zhao, T.; Ottosen, C.O. Physiological response of tomatoes at drought, heat and their combination followed by recovery. Physiol. Plant. 2019, 165, 144–154. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Nankishore, A.; Farrell, A.D. The response of contrasting tomato genotypes to combined heat and drought stress. J. Plant Physiol. 2016, 202, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Čajánek, M.; Štroch, M.; Lachetová, I.; Kalina, J.; Spunda, V. Characterization of the photosystem II inactivation of heat-stressed barley leaves as monitored by the various parameters of chlorophyll a fluorescence and delayed fluorescence. J. Photoch. Photobio. B 1998, 47, 39–45. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Müller, F.; Rieu, I. Acclimation to high temperature during pollen development. Plant Reprod. 2016, 29, 107–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stainforth, D.A.; Aina, T.; Christensen, C.; Collins, M.; Faull, N.; Frame, D.J.; Kettleborough, J.A.; Knight, S.; Martin, A.; Murphy, J.M.; et al. Uncertainty in predictions of the climate response to rising levels of greenhouse gases. Nature 2005, 433, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Asner, G.P. Climate and management contributions to recent trends in U. S. agricultural yields. Science 2003, 299, 1032. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havaux, M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993, 16, 461–467. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q.; Rajora, O.P.; Li, X.Q. Physiological and growth responses of potato varieties to heat stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Marenco, R.A.; Antezana-Vera, S.A.; Nascimento, H.C.S. Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Martínez, D.E.; Guiamet, J.J. Distortion of the SPAD 502 chlorophyll meter readings by changes in irradiance and leaf water status. Agronomie 2004, 24, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Schlemmer, M.R.; Francis, D.D.; Shanahan, J.F.; Schepers, J.S. Remotely Measuring Chlorophyll Content in Corn Leaves with Differing Nitrogen Levels and Relative Water Content. Agron. J. 2005, 97, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, M.R.; Ayyub, C.M.; Amjad, M.; Waraich, E.A. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions. J. Sci. Food Agric. 2016, 96, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Heuvelink, E. Influence of day and night temperature on the growth of young tomato plants. Sci. Hortic. 1989, 38, 11–22. [Google Scholar] [CrossRef]
- Klepper, B.; Browning, V.D.; Taylor, H.M. Stem diameter in relation to plant water status. Plant Physiol. 1971, 48, 683–685. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.; Wen, J.; Jensen, N.B.; dos Santos, T.M.; Wu, Z.; Rosenqvist, E.; Ottosen, C.O. Interactive effects of elevated CO 2 concentration and combined heat and drought stress on tomato photosynthesis. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wu, J.; Huang, G.; Zhou, S.; Liu, W.; Liao, Y.; Yang, X.; Xiao, Z.; Fan, H. Individual and interactive effects of drought and heat on leaf physiology of seedlings in an economically important crop. AoB Plants 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, M.; Keitel, C.; Ahmad, N.; Mahmood, T.; Trethowan, R. Screening of tomatoes germplasm for heat stress tolerance under controlled conditions. Procedia Environ. Sci. 2015, 29, 173–174. [Google Scholar] [CrossRef] [Green Version]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1716. [Google Scholar] [CrossRef] [Green Version]
- USDA. Grading Standards. Available online: https://www.ams.usda.gov/grades-standards/tomato-grades-and-standards (accessed on 5 July 2019).
- Soil Survey Staff, U.S.D.A. Web Soil Survey; USDA Natural Resources Conservation Service: Washington, DC, USA, 2020. [Google Scholar]
- Hong, B.; Ma, C.; Yang, Y.; Wang, T.; Yamaguchi-Shinozaki, K.; Gao, J. Over-expression of AtDREB1A in chrysanthemum enhances tolerance to heat stress. Plant Mol. Biol 2009, 70, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Y.; Chen, G.; Yang, H.; Du, T. Water use efficiency was improved at leaf and yield levels of tomato plants by continuous irrigation using semipermeable membrane. Agric. Water Manag. 2018, 203, 430–437. [Google Scholar] [CrossRef]
- Shinohara, T.; Leskovar, D.I. Effects of ABA, antitranspirants, heat and drought stress on plant growth, physiology and water status of artichoke transplants. Sci. Hortic. 2014, 165, 225–234. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, S.; Harvey, J.T.; Djidonou, D.; Leskovar, D.I. Exploring Morpho-Physiological Variation for Heat Stress Tolerance in Tomato. Plants 2021, 10, 347. https://doi.org/10.3390/plants10020347
Bhattarai S, Harvey JT, Djidonou D, Leskovar DI. Exploring Morpho-Physiological Variation for Heat Stress Tolerance in Tomato. Plants. 2021; 10(2):347. https://doi.org/10.3390/plants10020347
Chicago/Turabian StyleBhattarai, Samikshya, Joshua T. Harvey, Desire Djidonou, and Daniel I. Leskovar. 2021. "Exploring Morpho-Physiological Variation for Heat Stress Tolerance in Tomato" Plants 10, no. 2: 347. https://doi.org/10.3390/plants10020347







