Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review
Abstract
1. Introduction
1.1. Occurrences of EDCs as Contaminants and Its Sources
1.2. Adverse Effects of EDCs on the Environment
2. Rejection of EDCs by Membranes
2.1. Membrane Materials Used in the Rejection of EDCs
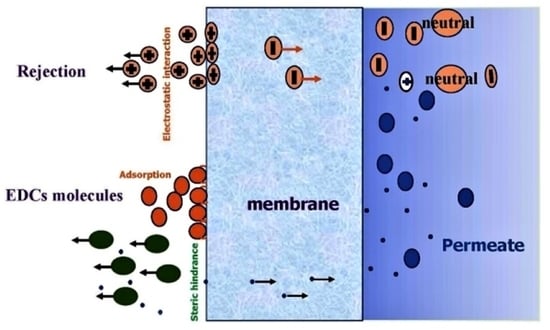
2.2. Removal Mechanisms of Endocrine-Disrupting Compounds during Membrane Processes
2.2.1. Removal Mechanisms of Endocrine-Disrupting Compounds during MF and UF Processes
2.2.2. Removal Mechanisms of Endocrine-Disrupting Compounds during NF and RO Membrane Processes
2.3. Evaluation of Membrane Processes in the Rejection of EDCs
2.3.1. Evaluation of the Performance of MF and UF Membrane Systems in the Rejection of EDCs
2.3.2. Evaluation of the Performance of NF and RO Membranes in the Rejection of EDCs
2.4. Factors Affecting the Membrane Rejection Performance of EDCs
2.5. Membrane Fouling Challenges
2.6. Mechanism of Fouling in Membrane Filtration
2.7. Nanomaterials and Membrane Technology
2.8. Removal of EDCs by Membrane Bioreactor Processes
2.8.1. Mechanisms of EDCs Removal during MBR Process
2.8.2. Performance of MBRs in the Removal of EDCs from Water
2.8.3. Optimization of MBRs Process in the Removal of EDCs from Water
| Matrices Type | Major Contaminants | Corresponding Concentrations (ng/L) | Locations | Major Effects | References |
|---|---|---|---|---|---|
| Drinking water | NP, BPA | 505; 1430 | France | Disruption of the normal hormone functions and physiological statue in human beings and animals. | [29] |
| Mangrove sediments | E2, EE2, E1 | 0.03–39.77; 0.45–129.78; 0.02–49.27 (ng/g) | Mangrove, Brazil | Feminization of fish, mimicking estrogens and disruption of homeostasis. | [322] |
| Biological wastewater treatment plants (WWTPs) | E1, E2, E2-17A, EE2 | 3050; 776; 2300; 3180 | State of Ceará, Brazil | Negative effects on the reproductive and sexual systems in humans, wildlife, and fish. | [325] |
| River water | E1, E2, E3, EE2, BPA | 26.5; 15.5; 3.6; 68.8; 37 (µg/L) | Malaysia | Diabetes, neurological challenges, cancer, tumors, obesity, damaged reproductive function, immune effects, heart disease in humans. Stimulation of breast cancer cells. | [326] |
| Surface water | E1, E2, EE2, E3 | 1.40–5.74; 1.10–5.39; 11.70–14.00; 2.15–5.20 | Watershed, Istanbul, Turkey | Hazard to public health via uninterrupted exposure and food-chain. | [327] |
| Treated sewage effluent | AMP, SFZ, CBZ, SMZ, IBF | 9299; 0.843; 125; 140; 203 | Johor, Malaysia | Alteration of the potential function, the pattern of the male reproductive system, and male behavior. | [328] |
| Yellow river | E1, E2, E3, EE2, DES, EV, 4-t-OP, 4NP, BPA | 2.98; 1.07; 4.37; 2.67; 2.52; 1.96; 89.52; 280.19; 710.65 | China | Alterations in reproductive capacity and sex in aquatic organisms. | [329] |
| Tap water | NP, BPA | 57.9 ± 18.0; 5.1 ± 8.8 | Taipei and Kaohsiung (Northern and Southern Taiwan | Biomagnification and bioaccumulation through the food supply chain. Elevated-trophic-level species in humans and the ecosystem via food intake. | [330] |
| Surface water/sediment of the Yellow River | 4-t-OP, BPA, E1, E2, TCS, 4-NP | 4.7, 46.7; 1.3; ND; 6.8; 577.9 | China | Uneven development of gonads and vitellogenin initiation in fish. | [331] |
| River water | BPA | ˂215 | Langat River Malaysia | Interrupt the release of adipokine that shield humans from a metabolic disorder. | [332] |
| Drinking water | BPA, NP, E1, E2 | 4.7−512; 8.2−918; ND-9.9; ND-3.2 | China | Obesity, persistent miscarriages, and polycystic ovarian disease in women. | [333] |
| Drinking water | Styrene | 45.11–203.48 (µg/L) | Johor Bahru Malaysia | Mimicking, blocking, development disturbances, and change function systems of hormones in humans and animals. | [334] |
| Surface sediments | BPA, 4-NP, 4t-OP | 25.15; 356.5; 176 (µg/kg) | India | Irregularities in the reproductive system of aquatic species, humans and wildlife. | [335] |
| Surface water and fish muscle tissue | 4-t-OP, NP, BPA, E1, E2, EE2, and E3 | 126.0; 634.8; 1573.1; 55.9; 23.9; 31.5; 5.2 and 26.4, 103.5, 146.9, 14.2, 9.3, 13.8, and 1.3 ng/g | Bahe River, China | Contamination in these regions triggered inhibition of gonad growth, elevated growth conditions, and repression of spermatogenesis in H. leucisculus. | [336] |
| Water, sediment, fish samples | OP, NP, BPA | ND-102; ND-127; ND ND-1.90 µg/g; ND-2.51 µg/g; ND-5.08 µg/g ND-643 ng/g; ND-476 ng/g; ND-1139 ng/g | Lagos Lagoon, Nigeria | It causes endocrine facilitated anomalies in fish, invertebrates, reptilian avian, and mammals (humans). | [337] |
| Estuarine water | DIC, E2, E1, EE2, Testosterone, Progesterone DMS, Propanolol, Caffeine, BPA | ˂0.47–79.89; ˂5.28–31.43; ˂0.56–1.92; ˂ 0.30–7.67; 0.51–2.30; ˂0.41–0.46; ˂ 1.00–1.51; ˂ 0.25–0.34; 0.13–0.33; 0.19–0.47 | Pulau kukup, Johor mariculture site, Malaysia | Influence on human development throughout the fetal period and potential cancer. | [338,339] |
| Mariculture fish | BPA, 4OP, 4NP | 0.023; 0.084; 0.078 (ng/g) | Malaysia | Biomagnification and bioaccumulation via food web or food chain in humans. | [340,341] |
| Maternal blood and amniotic fluid | BPA, OP, TCS, NP | 7.43 ng/mL and 7.75; 5.46 ng/mL and 5.72; 7.17 ng/mL and 7.04; 9.38 ng/mL and 8.44 | India | These compounds have a propensity to cross the placental barrier and may affect the fetus. | [342,343] |
| River water and sediment | BPA | 134; 275 | Indonesia | An acute contaminant with severe impact on human organs, such as reproductive system, breast, adipose, and pancreas tissue. | [344] |
| Coastal groundwater/seawater | BPA, NP, E1 | 46.3–66.5 18.9–30.9 | Coastal region of China | Disturb hormone biosynthesis and metabolism or cause a deviation from normal homeostatic control/reproduction. | [15,345] |
| Sewage water | Diethyl phthalate, estrone, nonylphenol-di-ethoxylate, | 445–4635; 11–33; 747–3945 | Hong Kong | Carcinogenicity, reproductive impairment, obesity and metabolic and disorders. | [346,347] |
| Major Membrane Materials | Fabrication Methods | Working Conditions | Pore Sizes (µm) | Nanomaterials Used | Membrane Configurations | Major Findings | References |
|---|---|---|---|---|---|---|---|
| PVDF | phase inversion | Nil | 10.59–357 | TiO2 | HF | Enriched membrane strength and better hydrophilicity. | [21] |
| PES | Phase inversion | Pressure: 300 kPa | 0.1–0.15 | SiO2 | HF | Expansion in adsorption site of the modified membrane. | [123] |
| PVDF | Irradiation graft polymerization and phase inversion | flow rates of 10 to 50 mL/min; Temp: 25 °C; Pressure: 0.02–0.2; MPa; pH: 6.5 | 0.00518 | Functionalized polypropylene (PP) non-woven fabric | FS | Excellent mechanical properties and better reusability. | [266] |
| PVDF | NIPS | TMP: 0.21 Mpa, 0.14 Mpa, and 0.07 Mpa; pH: 7 | NA | AgBiO3 | FS | improving the flux, enhanced the protein fouling resistance. Resisted a bacterial fouling attack both in the presence and absence of visible light | [270] |
| PSF | Interfacial polymerization | Pressure: 5 bar; Temp: 25 °C | 0.155 | PA/TiO2 | FS | Increased hydrophilicity. | [271] |
| PVDF | Phase inversion | Pressure: 0.1 bar; Temp: 24 °C; Filtration period: 10–50 m | 0.25 | CNT | FS | Substantially hydrophilic, enhanced wettability, additional open structured membrane developed. | [272] |
| PA | Electrospinning | Temp: 30 °C; s injection flow: 0.4 mL h−1; drum speed rate: 1000 rpm; voltage: 26 kV; working distance: 15 cm | 0.02–0.09 | Nanofibrous | HF | Considerably increased stability for the reuse application. Formation of homogenous fibers and porous structure with no significant changes during the reuse application. Excellent hydrophilic quality. | [273] |
| PVDF | Electrospinning | Temp: 27 °C; volume of permeates: 0.145–0.291; filtration period: 150 to 210 min; pressure: 1.0–25 bar; pH: 2.87 and 6.24 | 0.10501 | MnO2 | FS | Superior retention potential for the entire sampling time. Improved reusability for BPA removal. | [274] |
| CA | Diffusion induced phase separation | pH: 7; pressure: 200–1000 kPa | 0.0003–0.001 | Zeolites-zinc Oxide | FS | Introduction of Zeolite oxide build-up hydrophilicity of the membrane. | [275] |
| PSF | Phase inversion | Pressure: 200–1000 kPa | 0.002–0.004 | Polyaniline modified halloysite nanotubes (PANi-HNT) and | FS | A higher amount of PANi-HNT advances hydrophilicity and lead to a significant enhancement of the water flux | [276] |
| CA | NIPS separation (phase inversion) | T: 25 °C; contact time: 1–1500 m 250 rpm | 50–200 | Poly (4-vinylpyridine-b-ethylene oxide) (P4 VP-b PEO) | FS | Incorporation of 1% P4 VP-b-PEO to the membrane matrix enhances the adsorptive performance of the membrane. | [346] |
| PAN | Electrospinning | pH: 10.60; Time: 10 m; flow rate: 0.2 mL h−1 | NA | Mono-porphyrin (2) | HF | Electrospinning supports shows excellent promise as a real-life application for water purification. | [347] |
| Major Contaminants | Treatment Process | Treatment Factors | Brief Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|---|---|
| BPA, NP/WWTP influent | MF and RO membrane | Ph = 7.0–8.1; TOC (mg/L) = 0.8–77.4 TN (mg/L) = 0–52.5 EC (Ms/cm) = 0.1–3.1 UV (L/cm) = 0.00–1.77 | Physicochemical water characterization. Analysis of micropollutant. Identification and quantification of compounds and filtration. | 97% removal efficiency was achieved. | BPA (500 ng/L) was detected in the effluent. High energy demand. | [58] |
| BPA/biologically treated wastewater | MF and NF | Flux (MF) = 6.0–18.6 L/m2h; Flux (NF): 80 L/m2h; Temp = 21 °C TMP = 0.3 mPa (MF) 0.7 mPa (NF) | Circulation of the module with pure water. Determination of pure water infiltration. | Both techniques eliminate BPA. BPA removal efficiency: (61–75%) with NF. | Fouling. A decline in permeate flux in MF. | [74] |
| E1, E2, progesterone, testosterone/Purified water | UF membrane | MWCO: 1–100 kDa Pressure: 0.5–5 bar Pure water flux (L/m2h) 20.8–359.2 Final flux: 21.9–288.5 Time: 2–40 m Ph: 8 | Stirring feed solution at 200 rpm for 16 h. Filtering of purified membrane for 30 min. Measurement of pure water flux. Collection of permeate. | Removal via solute-solute interactions for E1 corresponds to a higher proportion of organic matter at 25–50 mg/L for 10 kDa (48–52%); 100 kDa (33–38%) membranes. | Poor removals of E1 and hormone contaminants (52% and 38%). | [115] |
| BPA/drinking water | UF-PS (PS) membrane. | Temp: 25 ± 0.5 °C. pH: 7–13 BPA concentration: 100–500 μg/L. Ph: (3.68–10) | Measurement of pure water flux. Filtration of BPA solution. | Higher removal at the initial stage of the filtration. | Lower removal efficiency (20%). Fouling. | [117] |
| BPA, CBZ, IBF, and SFZ/drinking water | UF membrane | Operating speed: 50 psi. Flow rate: 0.65 L/m per cell. | Initial partial removal of BPA. | Poor BPA removal using modified PES membranes. | [118] | |
| BPA, E2, E1, E3, EE2/synthetic wastewater | UF membrane | Working pressures (25, 30, 50, 75 kPa); temp: 20 ± 2 °C; TOC = 7 mg/L; pH: 7.6 | Soaking of fresh membrane for 24 h. Removal of impurities. Determination of flux. | EDCs removal rates of (10–76%) were achieved via a fouled membrane. | Poor removal of E3 (10–17%). | [166] |
| BPA/model solution | NF and RO membranes | Temp: 45–50 °C; Maximum pressure: 31–83 bar, pH: 2–11; permeability: 0.85–4.86 (Lm2h) Filtration period:30–360 m | ≥98% BPA rejection was achieved with polyamide-based RO membranes. | High energy demand. Too many modular units. | [167] | |
| BPA and oxybenzone/feed solution | Nanocomposite membrane | Pressure: 1 bar; Filtration time: 2 h; Temp: 20 °C | 5 mg/L BPA solution and 25 mg/L solution of oxybenzone was run via the HFM samples and the permeate was taken, and analyzed for oxybenzone/BPA using a UV–visible spectrophotometer | Higher removal of BPA (95%) and oxybenzone (98%) attained. Elevated pure water permeability (528.2 ± 44.6 mL/m2/h/mmHg) | Nil | [257] |
| BPA, DBP, DMP, DOP, NP/water | Nanocomposite membrane | Pressure: 0.02–0.5 Mpa; PEG feed concentration: 0.5 µg/L, operating pressure: 0.1 Mpa, Temp: 25 °C; pH: 6.5 | The target contaminants were dissolved in deionized water. The filtration experiment was undertaken. | ˃80% BPA removal was attained at 1.3 s contact time. | Nil | [268] |
| BPA/feed solution | UF(TFC) immobilized with TiO2 | Preparation of feed solution. Quantification of the feed and the permeate solution. | Almost 99% BPA rejection was attained. | Nil | [271] | |
| BPA/feed solution | Nanofibrous membrane | Temp: 30 °C, pH: 7; 150 rpm | Preparation of the stock solution. Batch experimentation. Sample analysis. | 98% rejection of BPA was achieved. | Nil | [273] |
| BPA/drinking water | Nanocomposite membrane | Temp: 27 °C; Pressure ranges: 0.5–2.5 bars; filtration period: 1 h | The experiments comprise of uninterrupted filtration of BPA. The feed tank was loaded with 2 L of 500 ppb BPA. Collection of permeates and the solute for analysis. | Almost complete removal of BPA was achieved. | Nil | [274] |
| BP-3/feed solution | Nanocomposite membrane | pressure range: 200–1000 kPa; feed conc: 1, 3, 6 ppm; | Preparation of BP-3 solution. Measurement of the concentration of BP-3 in feed and permeate using UV spectrophotometer. | 98% elimination of benzophenone-3 at pH (7). | Nil | [275] |
| Atrazine, oxybenzene/feed solution | Nanocomposite membrane | Pressure: 200 to 1000 kPa | The filtration unit is loaded with 3 ppm of EDCs and was run separately for each compound. | 98% oxybenzene removal was attained. | Average removal of atrazine (50%) | [276] |
| BPA/feed solution | UF membrane | pH: (3–13) MWCO: 100 Da TMP: 0.1 × 106–0.3 × 106 Pa Temp: 20 ± 2 °C BPA conc.: 5 mg/L | The UF membrane was installed, and the solution was introduced into the UF cup. Magnetic stirring. | Both salt and acidic Ph improve the transportation of BPA. | Decline BPA rejection decreased significantly when the BPA molecule was ionized. | [348] |
| DMP, DEP, DBP, DnOP, DEHP/water | NF membrane | pH: 4–9; pure water flux: 47.5 L/m2h; Temp: 25–45 °C. | Preparation of a feed solution. Measurement of concentrations of PAEs in both the feed and permeate. | Removal efficiencies of 95.4%; 95.1% and 91.5% were recorded for DEHP, DnOP, and DBP. | Lower adsorption rates. Low rejection of sulfamides. | [349] |
| Major Contaminants/Sources | Treatment Process | Treatment Factor | Brief Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|---|---|
| Steroid hormones, alkyl phenolic surfactants, pesticides, PPCPs, and industrial chemicals/synthetic wastewater | MBR treatment with UV oxidation/(NF and RO) | pH. (7.2–7.5): initial start-up and acclimatization (51 d), MBR period (5 d). | Preparation of stock solution. Introduction of stock solution to the synthetic Wastewater. | Acetaminophen removal = 87.1% carbamazepine removal ≥ 96%. | Removal efficiency by MBR and UV varied from 17 to 32%. Long term operation of MBR (196 days). | [284] |
| Steroidal hormones, xenoestrogens, pesticides, caffeine, pharmaceuticals, and personal care products (PPCPs)/wastewater | AMBR | SRT: 10–15 d; HRT; 1 d; MLSS: 7.5–8.5 g/L; aeration: 10 m cycle; pH: 7.0–7.7. | Loading of the influent into the reactor. Supply of compressed air to the aerobic tank. Sample collection and analysis | (˃90%) removals of target trace organic contaminants | (24–68%) removals for DIC, CBZ, amitriptyline, trimethoprim, diazepam, gemfibrozil, omeprazole, sulfamethoxazole and fluoxetine | [304] |
| BPA/ samples from sewage plants | secondary treatment and MBR. | HRT = 20–180 0.3–2.8, 1.1–7, 5.3–25 SRT (d) = 3.3–20 MLSS = 780–6900 (mg/L) Temp (°C): 3–25, 2–23 6–23, 7–24. | ND | Removal efficiency ranged between 1–77% | Chemically assisted primary treatment achieved very low removal. | [306] |
| BPA/Synthetic municipal wastewater | MBR, OMBR, FO membrane | HRT:4,8,12 h) MLSS: 6.5 g/L and 8.5 g/L BPA sludge loading: (0.05–0.16 mg/g/d) | ND | Overall removal of 70% was achieved. Conventional MBR: 93.9% and 98%, respectively. | The rejection of BPA by the MF membrane was very low (10.3%). Salt leakage problem. Fouling. | [307] |
| Caffeine, naproxen, and acetaminophen/wastewater | AMBR | average flow rate: 8800 m3/d; Temp: 21 °C; HRT: 11 h; SRT: 6–8 d, MLSS: 5700 mg/L; daily sludge production: 90 m3/d. | Feeding the reactor with an influent sample. Continuous aeration at 2.5 mg/L. Sample collection and analysis. | Complete removal of target compounds. | Negative removals of trimethoprim (−2%) and clarithromycin (−34%) | [308] |
| BPA, TCS, diazinon, triclocarbon, 4-n-nonylphenol, caffeine, DIC, CBZ/synthetic wastewater | Anaerobic membrane bioreactor (AnMBR) | Digester temp: (35 ± 1 °C), HRT: (4 d), permeate flux: (1.8 L/m2 h) and organic loading rate: (1.3 gCOD/L d), MLSS concentration: (10 g/L), SRT: (180 d). | Inoculation. Dilution of wastewater. Acclimatization. Analysis of sludge and permeate samples. | (˃70%) hydrophobic contaminants were removed. ˃70% of hydrophilic EDCs were removed. | Poor removal of diclofenac (2.8%), DEET (19.5%) and carbamazepine (39.2%), respectively. | [309] |
| Clozapine, butylparaben, diazinon, triclocarbon, NP, atrazine (herbicide), phenyl phenol, BPA, and TCS/municipal wastewater | Biosorption and biodegradation | HRT = 5 d; mixed liquor pH = 7 ± 0.1; temp = 35 ± 1 °C | Feeding the Bioreactor. Circulation of digested sludge. Mixing of the sludge. | Trimethoprim, carazolol, hydroxyzine, amitriptyline, and linuron removals = ˃80%. The removal of phenyl phenol was 60%. | Poor Atrazine removal 6.8%. BPA removal ranged between 40% to 20%. | [310] |
| TCT, CBZ, DIC, caffeine, theophylline, naproxen, acetaminophen, mefenamic, atenolol, furosemide, ketoprofen/wastewater | Coagulated-AMBR | HRT: 9 h; SRT 25 d; MLSS: 8 g/L; pH: 6–8 | Loading of the influent into the reactor. Supply of compressed air to the aerobic tank. Sample collection and analysis. | Acetaminophen, theophylline, caffeine and naproxen (˃90%); TCT, mefenamic (˃80%) removals, | CBZ, sulpiride and DIC | [319] |
| DIC, IBF, EI, EE2/synthetic wastewater | AASMBR | T: 8 °C and 12 °C; SRT: 30, 60, 90 d; Time points: 0.2, 0.5, 1.5, 2.3, 3, 4.5, 5, 8.5, 12, 12.5, 24 h; flow rate: 15 L d−1; operational flux: 0.14 md−1. Fine | Instantaneous spiking of EDCs stock solutions to the reactors. Constant aeration. Sample analysis (LS-MS/MS). | Complete removal of IBF and E1, EE2 (66%). | Poor removal of DIC (31%). | [320] |
| BPA, DIC, CBZ, BIS/urban wastewater | AMBR | Temp: 7–20 °C; HRT: 35 h; aeration intensity: 0.4–0.6 m−3m−2h−1; pH: 6.6–7.3; operation interval: 120 d; Flux (continuous): 7.8 Lm−2h−1. | The MBR unit was loaded with clarified wastewater. The SRT was maintained via sludge stabilization in the membrane compartment. Collection of treated permeate water and wasted sludge in the permeate and sludge tanks. | BPA (97%), Bisoprolol (65%) | Membrane fouling. Poor removal of CBZ (28%) and DIC (38%). | [350] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olmez-Hanci, T.; Dogruel, S.; Emek, A.D.A.; Yılmazer, C.E.; Çınar, S.; Kiraz, O.; Citil, E.; Orhon, A.K.; Siltu, E.; Gucver, S.M.; et al. Performance of ozone and peroxone on the removal of endocrine disrupting chemicals (EDCs) coupled with cost analysis. Water Sci. Technol. 2020, 1–11. [Google Scholar] [CrossRef]
- Omar, T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S. Occurrence, distribution, and sources of emerging organic contaminants in tropical coastal sediments of anthropogenically impacted Klang River estuary, Malaysia. Mar. Pollut. Bull. 2018, 131, 284–293. [Google Scholar] [CrossRef]
- Birnbaum, L.S. The State of the Science on Endocrine Disruptors. Environ. Health Perspect. 2014, 106, 7. [Google Scholar] [CrossRef] [PubMed]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Acharya, K. Removal of seven endocrine disrupting chemicals (EDCs) from municipal wastewater effluents by a freshwater green alga. Environ. Pollut. 2019, 247, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Attia, T.M.S.; Hu, X.-L.; Yin, D.Q. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, H.; Wen, X.; Si, X. Degradation behavior of 17α-ethinylestradiol by ozonation in the synthetic secondary effluent. J. Environ. Sci. 2012, 24, 228–233. [Google Scholar] [CrossRef]
- Aris, A.Z.; Hir, Z.A.M.; Razak, M.R. Metal-organic frameworks (MOFs) for the adsorptive removal of selected endocrine disrupting compounds (EDCs) from aqueous solution: A review. Appl. Mater. Today 2020, 21, 100796. [Google Scholar] [CrossRef]
- Kim, S.; Cho, H.; Joo, H.; Her, N.; Han, J.; Yi, K.; Kim, J.-O.; Yoon, J. Evaluation of performance with small and scale-up rotating and flat reactors; photocatalytic degradation of bisphenol A, 17β–estradiol, and 17α–ethynyl estradiol under solar irradiation. J. Hazard. Mater. 2017, 336, 21–32. [Google Scholar] [CrossRef]
- Frontistis, Z.; Fatta-Kassinos, D.; Mantzavinos, D.; Xekoukoulotakis, N.P. Photocatalytic degradation of 17α-ethynylestradiol in environmental samples by ZnO under simulated solar radiation. J. Chem. Technol. Biotechnol. 2012, 87, 1051–1058. [Google Scholar] [CrossRef]
- Lubliner, M.B.; Redding, D.R. Pharmaceuticals and Personal Care Products in Municipal Wastewater and Their Removal by Nutrient Treatment Technologies; Washington State Department of Ecology: Olympia, WA, USA, 2010.
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A. Target quantification of azole antifungals and retrospective screening of other emerging pollutants in wastewater effluent using UHPLC–QTOF-MS. Environ. Pollut. 2019, 253, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ. Int. 2019, 125, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Zbucka-Kretowska, M.; Zbucki, R.; Parfieniuk, E.; Maslyk, M.; Lazarek, U.; Miltyk, W.; Czerniecki, J.; Wolczynski, S.; Kretowski, A.; Ciborowski, M. Evaluation of Bisphenol A influence on endocannabinoid system in pregnant women. Chemosphere 2018, 203, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, J.; Stoffella, P.J.; Wilson, P.C. Isotope dilution-gas chromatography/mass spectrometry method for the analysis of alkylphenols, bisphenol A, and estrogens in food crops. J. Chromatogr. A 2012, 1258, 128–135. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Y. Removal of phenolic endocrine disrupting compounds from waste activated sludge using UV, H2O2, and UV/H2O2 oxidation processes: Effects of reaction conditions and sludge matrix. Sci. Total Environ. 2014, 493, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Deem, S.L.; Holliday, D.K.; Jandegian, C.M.; Kassotis, C.D.; Nagel, S.C.; Tillitt, D.E.; Saal, F.S.V.; Rosenfeld, C.S. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen. Comp. Endocrinol. 2015, 214, 195–219. [Google Scholar] [CrossRef]
- Cook, M.M.; Symonds, E.; Gerber, B.; Hoare, A.; Van Vleet, E.S.; Breitbart, M. Removal of Six Estrogenic Endocrine-Disrupting Compounds (EDCs) from Municipal Wastewater Using Aluminum Electrocoagulation. Water 2016, 8, 128. [Google Scholar] [CrossRef]
- Kamaludin, R. The Morphological Properties Study of Photocatalytic TiO2/Pvdf Dual Layer Hollow Fiber Membrane for Endocrine Disrupting Compounds Degradation. Malays. J. Anal. Sci. 2017, 21, 426–434. [Google Scholar] [CrossRef]
- Petrovic, M.; De Alda, M.J.L.; Díaz-Cruz, M.S.; Postigo, C.; Radjenovic, J.; Gros, M.; Barcelo, D. Fate and removal of pharmaceuticals and illicit drugs in conventional and membrane bioreactor wastewater treatment plants and by riverbank filtration. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3979–4003. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.M.; Aravind, U.K.; Aravindakumar, C.T. Emerging contaminants in Indian environmental matrices—A review. Chemosphere 2018, 190, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Pojana, G.; Gomiero, A.; Jonkers, N.; Marcomini, A. Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ. Int. 2007, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R.; Chapman, H.F.; Moore, M.R.; Ranmuthugala, G. Endocrine-disrupting compounds: A review of their challenge to sustainable and safe water supply and water reuse. Environ. Toxicol. 2006, 21, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.; Cai, W. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1–59. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Lee, S.; Liao, C.; Song, G.-J.; Ra, K.; Kannan, K.; Moon, H.-B. Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere 2015, 119, 1000–1006. [Google Scholar] [CrossRef]
- Colin, A.; Bach, C.; Rosin, C.; Munoz, J.-F.; Dauchy, X. Is Drinking Water a Major Route of Human Exposure to Alkylphenol and Bisphenol Contaminants in France? Arch. Environ. Contam. Toxicol. 2013, 66, 86–99. [Google Scholar] [CrossRef]
- Sornalingam, K.; McDonagh, A.; Zhou, J.L. Photodegradation of estrogenic endocrine disrupting steroidal hormones in aqueous systems: Progress and future challenges. Sci. Total Environ. 2016, 550, 209–224. [Google Scholar] [CrossRef]
- Ferreiro, C.; Iker, G.; Lombraña, I.; De Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of Emerging Concern Removal in an E ffl uent of Wastewater Treatment Plant under Biological and Continuous Mode Ultrafiltration Treatment. Sustainability 2020, 12, 725. [Google Scholar] [CrossRef]
- Jonkers, N.; Kohler, H.-P.E.; Dammshäuser, A.; Giger, W. Mass flows of endocrine disruptors in the Glatt River during varying weather conditions. Environ. Pollut. 2009, 157, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.-Y.; Huang, H.-J.; Lü, H.; Xiang, L.; Zhang, J.; Zeng, Q.-Y.; Tian, J.-J.; Li, Y.-W.; Wu, X.-L. Occurrence of Nonylphenol and Nonylphenol Monoethoxylate in Soil and Vegetables from Vegetable Farms in the Pearl River Delta, South China. Arch. Environ. Contam. Toxicol. 2012, 63, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Ademollo, N.; Orrù, M.A.; Silvestroni, L.; Funari, E. Alkylphenols in adipose tissues of Italian population. Chemosphere 2011, 82, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Bing, S.; Xiaoyan, W.; Xiaojie, S.; Yongning, W. A study on bisphenol A, nonylphenol, and octylphenol in human urine amples detected by SPE-UPLC-MS. Biomed. Environ. Sci. 2011, 24, 40–46. [Google Scholar] [PubMed]
- Raecker, T.; Thiele, B.; Boehme, R.M.; Günther, K. Endocrine disrupting nonyl- and octylphenol in infant food in Germany: Considerable daily intake of nonylphenol for babies. Chemosphere 2011, 82, 1533–1540. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Chen, M.-L.; Sung, F.-C.; Wang, P.S.-G.; Mao, I.-F. Daily intake of 4-nonylphenol in Taiwanese. Environ. Int. 2007, 33, 903–910. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Shen, Y.-P.; Chen, S.-C. Assessing bisphenol A (BPA) exposure risk from long-term dietary intakes in Taiwan. Sci. Total Environ. 2016, 543, 140–146. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res. 2002, 36, 5013–5022. [Google Scholar] [CrossRef]
- Gröger, T.M.; Käfer, U.; Zimmermann, R. Gas chromatography in combination with fast high-resolution time-of-flight mass spectrometry: Technical overview and perspectives for data visualization. TrAC Trends Anal. Chem. 2020, 122, 115677. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.F.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Dantas, G.; Sommer, M.O.A.; Oluwasegun, R.D.; Church, G.M. Bacteria Subsisting on Antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Bergman, Å.; Heindel, J.; Jobling, S.; Kidd, K.; Zoeller, R.T. State-of-the-science of endocrine disrupting chemicals. Toxicol. Lett. 2012, 211, S3. [Google Scholar] [CrossRef]
- Benotti, M.J.; Stanford, B.D.; Wert, E.C.; Snyder, S.A. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res. 2009, 43, 1513–1522. [Google Scholar] [CrossRef]
- Jarošová, B.; Bláha, L.; Giesy, J.P.; Hilscherová, K. What level of estrogenic activity determined by in vitro assays in municipal waste waters can be considered as safe? Environ. Int. 2014, 64, 98–109. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Křesinová, Z.; Linhartová, L.; Filipová, A.; Ezechiáš, M.; Mašín, P.; Cajthaml, T. Biodegradation of endocrine disruptors in urban wastewater using Pleurotus ostreatus bioreactor. New Biotechnol. 2018, 43, 53–61. [Google Scholar] [CrossRef]
- Zacharakis, A.; Chatzisymeon, E.; Binas, V.; Frontistis, Z.; Venieri, D.; Mantzavinos, D. Solar Photocatalytic Degradation of Bisphenol A on Immobilized ZnO or TiO2. Int. J. Photoenergy 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Komesli, O.; Muz, M.; Ak, M.; Bakirdere, S.; Gokcay, C.F. Occurrence, fate and removal of endocrine disrupting compounds (EDCs) in Turkish wastewater treatment plants. Chem. Eng. J. 2015, 277, 202–208. [Google Scholar] [CrossRef]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic transgenerational actions of endocrine disruptors. Reprod. Toxicol. 2011, 31, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Pérez, J.A.S.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Pereira, J.; Ribeiro, C.; Gombek, C.J.; Gama, F.; Gomes, A.; Patterson, D.; Lanceros-Méndez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym. Test. 2015, 44, 234–241. [Google Scholar] [CrossRef]
- Ismail, N.A.H.; Wee, S.Y.; Kamarulzaman, N.H.; Aris, A.Z. Quantification of multi-classes of endocrine-disrupting compounds in estuarine water. Environ. Pollut. 2019, 249, 1019–1028. [Google Scholar] [CrossRef]
- Al-Rifai, J.H.; Khabbaz, H.; Schaefer, A.I. Removal of pharmaceuticals and endocrine disrupting compounds in a water recycling process using reverse osmosis systems. Sep. Purif. Technol. 2011, 77, 60–67. [Google Scholar] [CrossRef]
- Silva, L.L.; Sales, J.C.S.; Campos, J.C.; Bila, D.M.; Fonseca, F.V. Advanced oxidative processes and membrane separation for micropollutant removal from biotreated domestic wastewater. Environ. Sci. Pollut. Res. 2017, 24, 6329–6338. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, X.; Si, X. Pre-ultrafiltration or pre-ozonation for EDCs removal in a combined ultrafiltration and ozonation process. J. Chem. Technol. Biotechnol. 2016, 91, 2929–2934. [Google Scholar] [CrossRef]
- Fernández, M.F.; Román, M.; Arrebola, J.P.; Olea, N. Endocrine Disruptors: Time to Act. Curr. Environ. Health Rep. 2014, 1, 325–332. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Lu, S.; Deng, S.; Wang, B.; Zhao, W.; Qiu, Z.; Yu, G. Removal of pharmaceutical and personal care products by sequential ultraviolet and ozonation process in a full-scale wastewater treatment plant. Front. Environ. Sci. Eng. 2014, 8, 62–68. [Google Scholar] [CrossRef]
- Solak, S.; Vakondios, N.; Tzatzimaki, I.; Diamadopoulos, E.; Arda, M.; Kabay, N.; Yuksel, M. A comparative study of removal of endocrine disrupting compounds (EDCs) from treated wastewater using highly crosslinked polymeric adsorbents and activated carbon. J. Chem. Technol. Biotechnol. 2014, 89, 819–824. [Google Scholar] [CrossRef]
- Jin, P.; Jin, X.; Wang, X.C.; Shi, X. An analysis of the chemical safety of secondary effluent for reuse purposes and the requirement for advanced treatment. Chemosphere 2013, 91, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Dong, H.; Zhu, B.; Qu, J.; Nie, Y. A comparison of various rural wastewater treatment processes for the removal of endocrine-disrupting chemicals (EDCs). Chemosphere 2013, 92, 986–992. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Cao, Z.; Hu, J.; Gao, W. Adsorption of ethinylestradiol (EE2) on polyamide 612: Molecular modeling and effects of water chemistry. Water Res. 2013, 47, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report of the Joint Ipcs-Japan Workshop on “Endocrine Disruptors: Research Needs and Future Directions”; WHO: Geneva, Switzerland, 2004; pp. 1–52. [Google Scholar]
- Bayen, S.; Zhang, H.; Desai, M.M.; Ooi, S.K.; Kelly, B.C. Occurrence and distribution of pharmaceutically active and endocrine disrupting compounds in Singapore’s marine environment: Influence of hydrodynamics and physical–chemical properties. Environ. Pollut. 2013, 182, 1–8. [Google Scholar] [CrossRef]
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 2014, 69, 104–119. [Google Scholar] [CrossRef]
- Mortazavi, S.; Bakhtiari, A.R.; Sari, A.E.; Bahramifar, N.; Rahbarizade, F. Phenolic endocrine disrupting chemicals (EDCs) in Anzali Wetland, Iran: Elevated concentrations of 4-nonylphenol, octhylphenol and bisphenol A. Mar. Pollut. Bull. 2012, 64, 1067–1073. [Google Scholar] [CrossRef]
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S.; Kannan, N. Occurrence of synthetic hormones in sewage effluents and Langat River and its tributaries, Malaysia. Int. J. Environ. Anal. Chem. 2013, 93, 1457–1469. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, R.; Liu, Y.; Jin, F.; An, L. Phenolic endocrine-disrupting chemicals and intersex in wild crucian carp from Hun River, China. Chemosphere 2015, 120, 743–749. [Google Scholar] [CrossRef]
- Adeogun, A.O.; Onibonoje, K.; Ibor, O.R.; Omiwole, R.A.; Chukwuka, A.V.; Ugwumba, A.O.; Ugwumba, A.A.; Arukwe, A. Endocrine-disruptor molecular responses, occurrence of intersex and gonado-histopathological changes in tilapia species from a tropical freshwater dam (Awba Dam) in Ibadan, Nigeria. Aquat. Toxicol. 2016, 174, 10–21. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K.; Cydzik-Kwiatkowska, A.; Bernat, K.; Wojnowska-Baryła, I. Removal of bisphenol A (BPA) from biologically treated wastewater by microfiltration and nanofiltration. Int. J. Environ. Sci. Technol. 2016, 13, 2239–2248. [Google Scholar] [CrossRef]
- Ratola, N.; Cincinelli, A.; Alves, A.; Katsoyiannis, A. Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. J. Hazard. Mater. 2012, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Ratajczak-Wrona, W.; Górska, M.; Jablonska, E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front. Neurosci. 2015, 9, 57. [Google Scholar] [CrossRef]
- Kay, V.R.; Chambers, C.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Giulivo, M.; De Alda, M.J.L.; Capri, E.; Barcelo, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef]
- Galus, M.; Jeyaranjaan, J.; Smith, E.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat. Toxicol. 2013, 212–222. [Google Scholar] [CrossRef]
- Galus, M.; Kirischian, N.; Higgins, S.; Purdy, J.; Chow, J.; Rangaranjan, S.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic, low concentration exposure to pharmaceuticals impacts multiple organ systems in zebrafish. Aquat. Toxicol. 2013, 200–211. [Google Scholar] [CrossRef]
- Gunnarsson, L.; Adolfsson-Erici, M.; Björlenius, B.; Rutgersson, C.; Förlin, L.; Larsson, D.G.J. Comparison of six different sewage treatment processes—Reduction of estrogenic substances and effects on gene expression in exposed male fish. Sci. Total Environ. 2009, 407, 5235–5242. [Google Scholar] [CrossRef]
- Desai, M.; Jellyman, J.K.; Ross, M.G. Epigenomics, gestational programming and risk of metabolic syndrome. Int. J. Obes. 2015, 39, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Kummerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Fernández, F.N.; Fernández-Güelfo, L.A.; García, M.P.; García-Morales, J. New approach for integral treatment of OFMSW: Comparative analysis of its methane performance versus a conventional continuously stirred tank reactor. Chem. Eng. J. 2013, 233, 282–291. [Google Scholar] [CrossRef]
- Meng, S.; Greenlee, L.F.; Shen, Y.R.; Wang, E. Basic science of water: Challenges and current status towards a molecular picture. Nano Res. 2015, 8, 3085–3110. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of Endocrine-Disruptor, Pharmaceutical, and Personal Care Product Chemicals during Simulated Drinking Water Treatment Processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Jaafar, J.; Ismail, A.; Harun, Z. Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J. Colloid Interface Sci. 2018, 532, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.E.; Courtenay, S.C.; Hewitt, L.M.; MacLatchy, D.L. Effects of 17α-ethynylestradiol on early-life development, sex differentiation and vitellogenin induction in mummichog (Fundulus heteroclitus). Mar. Environ. Res. 2010, 69, 178–186. [Google Scholar] [CrossRef]
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of Trace Organic Compounds by Forward Osmosis Membranes: A Literature Review. Environ. Sci. Technol. 2014, 48, 3612–3624. [Google Scholar] [CrossRef]
- Tang, C.Y.; Yang, Z.; Guo, H.; Wen, J.J.; Nghiem, L.D.; Cornelissen, E.R. Potable Water Reuse through Advanced Membrane Technology. Environ. Sci. Technol. 2018, 52, 10215–10223. [Google Scholar] [CrossRef]
- Nasirabadi, P.S.; Saljoughi, E.; Mousavi, S.M. Membrane processes used for removal of pharmaceuticals, hormones, endocrine disruptors and their metabolites from wastewaters: A review. Desalin. Water Treat. 2016, 57, 24146–24175. [Google Scholar] [CrossRef]
- Cui, Z.F.; Jiang, Y.; Field, R.W. Fundamentals of Pressure-Driven Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Wang, J.; Chen, X.; Reis, R.; Chen, Z.; Milne, N.A.; Winther-Jensen, B.; Kong, L.; Dumée, L.F. Plasma Modification and Synthesis of Membrane Materials—A Mechanistic Review. Membranes 2018, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Jin, L.; Ong, S.L.; Ng, H.Y. Comparison of fouling characteristics in different pore-sized submerged ceramic membrane bioreactors. Water Res. 2010, 44, 5907–5918. [Google Scholar] [CrossRef] [PubMed]
- Mutamim, N.S.A.; Noor, Z.Z.; Abu Hassan, M.A.; Yuniarto, A.; Olsson, G. Membrane bioreactor: Applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 2013, 225, 109–119. [Google Scholar] [CrossRef]
- Ghyoot, W.R.; Verstraete, W.H. Coupling Membrane Filtration to Anaerobic Primary Sludge Digestion. Environ. Technol. 1997, 18, 569–580. [Google Scholar] [CrossRef]
- Ersu, C.B.; Ong, S.K. Treatment of Wastewater Containing Phenol Using a Tubular Ceramic Membrane Bioreactor. Environ. Technol. 2008, 29, 225–234. [Google Scholar] [CrossRef]
- Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from inexpensive raw materials by extrusion method and its performance in microfiltration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Lee, S.-J.; Dilaver, M.; Park, P.-K.; Kim, J.-H. Comparative analysis of fouling characteristics of ceramic and polymeric microfiltration membranes using filtration models. J. Membr. Sci. 2013, 432, 97–105. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process. Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Ahmad, M.I. Renewable Energy and Sustainable Technologies for Building and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–252. [Google Scholar]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Licona, K.; Geaquinto, L.D.O.; Nicolini, J.; Figueiredo, N.; Chiapetta, S.; Habert, A.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process. Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Process. Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Neale, P.A.; Schäfer, A.I. Quantification of solute–solute interactions in steroidal hormone removal by ultrafiltration membranes. Sep. Purif. Technol. 2012, 90, 31–38. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Coleman, P.J. NF/RO filtration of the hydrophobic ionogenic compound triclosan: Transport mechanisms and the influence of membrane fouling. Sep. Purif. Technol. 2008, 62, 709–716. [Google Scholar] [CrossRef]
- Bing-Zhi, D.; Lin, W.; Nai-Yun, G. The removal of bisphenol A by ultrafiltration. Desalination 2008, 221, 312–317. [Google Scholar] [CrossRef]
- Su-Hua, W.; Bing-Zhi, D.; Yu, H. Adsorption of bisphenol A by polysulphone membrane. Desalination 2010, 253, 22–29. [Google Scholar] [CrossRef]
- Rana, D.; Narbaitz, R.M.; Garand-Sheridan, A.-M.; Westgate, A.; Matsuura, T.; Tabe, S.; Jasim, S.Y. Development of novel charged surface modifying macromolecule blended PES membranes to remove EDCs and PPCPs from drinking water sources. J. Mater. Chem. A 2014, 2, 10059–10072. [Google Scholar] [CrossRef]
- Chon, K.; Shon, H.K.; Cho, J. Membrane bioreactor and nanofiltration hybrid system for reclamation of municipal wastewater: Removal of nutrients, organic matter and micropollutants. Bioresour. Technol. 2012, 122, 181–188. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Cheung, W.; Szeto, Y.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Ohore, O.E.; Zhang, S. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J.; Hadibarata, T.; Yusop, Z. Removal of bisphenol A by adsorption mechanism using PES–SiO2composite membranes. Environ. Technol. 2016, 37, 1959–1969. [Google Scholar] [CrossRef]
- Xue, W.; Xiao, K.; Liang, P.; Huang, X. Roles of membrane and organic fouling layers on the removal of endocrine disrupting chemicals in microfiltration. J. Environ. Sci. 2018, 72, 176–184. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Kiso, Y. Effects of hydrophobicity and molecular size on rejection of aromatic pesticides with nanofiltration membranes. J. Membr. Sci. 2001, 192, 1–10. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Braeken, L.; Vandecasteele, C. Evaluation of parameters describing flux decline in nanofiltration of aqueous solutions containing organic compounds. Desalination 2002, 147, 281–288. [Google Scholar] [CrossRef]
- Ojajuni, O.; Saroj, D.; Cavalli, G. Removal of organic micropollutants using membrane-assisted processes: A review of recent progress. Environ. Technol. Rev. 2015, 4, 17–37. [Google Scholar] [CrossRef]
- Hu, Z.; Si, X.; Zhang, Z.; Wen, X. Enhanced EDCs removal by membrane fouling during the UF process. Desalination 2014, 336, 18–23. [Google Scholar] [CrossRef]
- Tiraferri, A.; Elimelech, M. Direct quantification of negatively charged functional groups on membrane surfaces. J. Membr. Sci. 2012, 389, 499–508. [Google Scholar] [CrossRef]
- Shao, J.; Hou, J.; Song, H. Comparison of humic acid rejection and flux decline during filtration with negatively charged and uncharged ultrafiltration membranes. Water Res. 2011, 45, 473–482. [Google Scholar] [CrossRef]
- Kimura, K.; Amy, G.L.; Drewes, J.; Watanabe, Y. Adsorption of hydrophobic compounds onto NF/RO membranes: An artifact leading to overestimation of rejection. J. Membr. Sci. 2003, 221, 89–101. [Google Scholar] [CrossRef]
- Oulton, R.L.; Kohn, T.; Cwiertny, D.M. Pharmaceuticals and personal care products in effluent matrices: A survey of transformation and removal during wastewater treatment and implications for wastewater management. J. Environ. Monit. 2010, 12, 1956–1978. [Google Scholar] [CrossRef]
- Khazaali, F.; Kargari, A.; Rokhsaran, M. Application of low-pressure reverse osmosis for effective recovery of bisphenol A from aqueous wastes. Desalin. Water Treat. 2013, 52, 7543–7551. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Yoon, J.; Snyder, S.A. Removal of 17β Estradiol and Fluoranthene by Nanofiltration and Ultrafiltration. J. Environ. Eng. 2004, 130, 1460–1467. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Yu, S.; Dong, B.; Gao, N.; Wang, X. A review of advances in EDCs and PhACs removal by nanofiltration: Mechanisms, impact factors and the influence of organic matter. Chem. Eng. J. 2021, 406, 126722. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Deng, S.; Yu, G.; Fan, Q. Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res. 2010, 44, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.L.S.; Moreira, C.G.; Curzio, B.A.; Da Fonseca, F.V. Micropollutant Removal from Water by Membrane and Advanced Oxidation Processes—A Review. J. Water Resour. Prot. 2017, 9, 411–431. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Feng, Y.; Yang, F.; Zhang, J. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Cartagena, P.; El Kaddouri, M.; Cases, V.; Trapote, A.; Prats, D. Reduction of emerging micropollutants, organic matter, nutrients and salinity from real wastewater by combined MBR–NF/RO treatment. Sep. Purif. Technol. 2013, 110, 132–143. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Verliefde, A.; Cornelissen, E.R.; Heijman, S.; Verberk, J.; Amy, G.; Van Der Bruggen, B.; Van Dijk, J. The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J. Membr. Sci. 2008, 52–66. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- D’Haese, A.; Le-Clech, P.; Van Nevel, S.; Verbeken, K.; Cornelissen, E.R.; Khan, S.J.; Verliefde, A.R. Trace organic solutes in closed-loop forward osmosis applications: Influence of membrane fouling and modeling of solute build-up. Water Res. 2013, 47, 5232–5244. [Google Scholar] [CrossRef]
- Ghiem, L.D.N.; Schäfer, A.I.; Limelech, M. Pharmaceutical Retention Mechanisms by Nanofiltration Membranes. Environ. Sci. Technol. 2005, 39, 7698–7705. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, M.; Wang, H.; Wang, X.; Wen, X. Influences of temperature on the retention of PPCPs by nanofiltration membranes: Experiments and modeling assessment. J. Membr. Sci. 2020, 599, 117817. [Google Scholar] [CrossRef]
- Braeken, L.; Van Der Bruggen, B. Feasibility of nanofiltration for the removal of endocrine disrupting compounds. Desalination 2009, 240, 127–131. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hane, Y.; Watanabe, Y.; Amy, G.; Ohkuma, N. Irreversible membrane fouling during ultrafiltration of surface water. Water Res. 2004, 38, 3431–3441. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Basile, T.; Petrella, A.; Petrella, M.; Boghetich, G.; Petruzzelli, V.; Colasuonno, S.; Petruzzelli, D. Review of Endocrine-Disrupting-Compound Removal Technologies in Water and Wastewater Treatment Plants: An EU Perspective. Ind. Eng. Chem. Res. 2011, 50, 8389–8401. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Johir, M.A.; Nguyen, T.V.; Kandasamy, J.; Vigneswaran, S. Experimental evaluation of microfiltration–granular activated carbon (MF–GAC)/nano filter hybrid system in high quality water reuse. J. Membr. Sci. 2015, 476, 1–9. [Google Scholar] [CrossRef]
- Sahar, E.; David, I.; Gelman, Y.; Chikurel, H.; Aharoni, A.; Messalem, R.; Brenner, A. The use of RO to remove emerging micropollutants following CAS/UF or MBR treatment of municipal wastewater. Desalination 2011, 273, 142–147. [Google Scholar] [CrossRef]
- Dolar, D.; Gros, M.; Rodriguez-Mozaz, S.; Moreno, J.; Comas, J.; Rodriguez-Roda, I.; Barceló, D. Removal of emerging contaminants from municipal wastewater with an integrated membrane system, MBR-RO. J. Hazard. Mater. 2012, 239–240, 64–69. [Google Scholar] [CrossRef]
- Ernst, M.; Bismarck, A.; Springer, J.; Jekel, M. Zeta-potential and rejection rates of a polyethersulfone nanofiltration membrane in single salt solutions. J. Membr. Sci. 2000, 165, 251–259. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Rana, D.; Scheier, B.; Narbaitz, R.M.; Matsuura, T.; Tabe, S.; Jasim, S.Y.; Khulbe, K.C. Comparison of cellulose acetate (CA) membrane and novel CA membranes containing surface modifying macromolecules to remove pharmaceutical and personal care product micropollutants from drinking water. J. Membr. Sci. 2012, 409, 346–354. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low Carbon Technol. 2012, 9, 157–177. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J.; Yusop, Z. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ. Sci. Pollut. Res. 2016, 23, 11549–11567. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef]
- Secondes, M.F.N.; Naddeo, V.; Belgiorno, V.; Ballesteros, F. Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J. Hazard. Mater. 2014, 264, 342–349. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Sarker, D.C.; Suja, F. Removal of emerging perfluorooctanoic acid and perfluorooctane sulfonate contaminants from lake water. Environ. Technol. 2017, 38, 1937–1942. [Google Scholar] [CrossRef]
- Bing-Zhi, D.; Hua-Qiang, C.; Lin, W.; Sheng-Ji, X.; Nai-Yun, G. The removal of bisphenol A by hollow fiber microfiltration membrane. Desalination 2010, 250, 693–697. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Wray, H.E.; Andrews, R.C.; Bérubé, P.R. Surface shear stress and retention of emerging contaminants during ultrafiltration for drinking water treatment. Sep. Purif. Technol. 2014, 122, 183–191. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Meng, S.; Gao, W. Removal of ethinylestradiol (EE2) from water via adsorption on aliphatic polyamides. Water Res. 2012, 46, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Hu, Z.; Ding, D.; Fu, X. Effects of effluent organic matters on endocrine disrupting chemical removal by ultrafiltration and ozonation in synthetic secondary effluent. J. Environ. Sci. 2019, 76, 57–64. [Google Scholar] [CrossRef]
- Schlautman, M.A.; Morgan, J.J. Sorption of Perylene on a Nonporous Inorganic Silica Surface: Effects of Aqueous Chemistry on Sorption Rates. Environ. Sci. Technol. 1994, 28, 2184–2190. [Google Scholar] [CrossRef]
- Im, J.-K.; Cho, I.-H.; Kim, S.-K.; Zoh, K.-D. Optimization of carbamazepine removal in O3/UV/H2O2 system using a response surface methodology with central composite design. Desalination 2012, 285, 306–314. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Maeng, S.K.; Fujioka, T.; Kennedy, M.D.; Amy, G.L. Proposing nanofiltration as acceptable barrier for organic contaminants in water reuse. J. Membr. Sci. 2010, 362, 334–345. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; Decarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Steinle-Darling, E.; Litwiller, E.; Reinhard, M. Effects of Sorption on the Rejection of Trace Organic Contaminants During Nanofiltration. Environ. Sci. Technol. 2010, 44, 2592–2598. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Daughton, C.G. Emerging pollutants, and communicating the science of environmental chemistry and mass spectrometry: Pharmaceuticals in the environment. J. Am. Soc. Mass Spectrom. 2001, 12, 1067–1076. [Google Scholar] [CrossRef]
- Chon, K.; Cho, J.; Shon, H.K. A pilot-scale hybrid municipal wastewater reclamation system using combined coagulation and disk filtration, ultrafiltration, and reverse osmosis: Removal of nutrients and micropollutants, and characterization of membrane foulants. Bioresour. Technol. 2013, 141, 109–116. [Google Scholar] [CrossRef]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.; Manis, A.; Soldenhoff, K.; Schäfer, A. Estrogenic hormone removal from wastewater using NF/RO membranes. J. Membr. Sci. 2004, 242, 37–45. [Google Scholar] [CrossRef]
- Vergili, I. Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources. J. Environ. Manag. 2013, 127, 177–187. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Sadmani, A.; McConville, M.; Kennedy, M.; Amy, G. Rejection of pharmaceutically active compounds and endocrine disrupting compounds by clean and fouled nanofiltration membranes. Water Res. 2009, 43, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Kosutic, K. Porosity of some commercial reverse osmosis and nanofiltration polyamide thin-film composite membranes. J. Membr. Sci. 2000, 168, 101–108. [Google Scholar] [CrossRef]
- Dolar, D.; Drašinac, N.; Kosutic, K.; Škorić, I.; Ašperger, D. Adsorption of hydrophilic and hydrophobic pharmaceuticals on RO/NF membranes: Identification of interactions using FTIR. J. Appl. Polym. Sci. 2016, 134, 17–21. [Google Scholar] [CrossRef]
- Kiso, Y.; Nishimura, Y.; Kitao, T.; Nishimura, K. Rejection properties of non-phenylic pesticides with nanofiltration membranes. J. Membr. Sci. 2000, 171, 229–237. [Google Scholar] [CrossRef]
- Berg, P.; Hagmeyer, G.; Gimbel, R. Removal of pesticides and other micropollutants by nanofiltration. Desalination 1997, 113, 205–208. [Google Scholar] [CrossRef]
- Verliefde, A.; Van Vliet, N.; Amy, G.; Van Der Bruggen, B.; Van Dijk, J. A Semi-Quantitative Method for Prediction of the Rejection of Uncharged Organic Micropollutants with Nanofiltration. Water Pract. Technol. 2006, 1, wpt2006084. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Akanyeti, I.; Semião, A.J. Micropollutant sorption to membrane polymers: A review of mechanisms for estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Determination of membrane pore size distribution using the fractional rejection of nonionic and charged macromolecules. J. Membr. Sci. 2002, 201, 191–201. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Schaep, J.; Wilms, D.; Vandecasteele, C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Role of electrostatic interactions in the retention of pharmaceutically active contaminants by a loose nanofiltration membrane. J. Membr. Sci. 2006, 286, 52–59. [Google Scholar] [CrossRef]
- Verliefde, A.; Cornelissen, E.; Amy, G.; Van Der Bruggen, B.; Van Dijk, H. Priority organic micropollutants in water sources in Flanders and the Netherlands and assessment of removal possibilities with nanofiltration. Environ. Pollut. 2007, 146, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shan, J.; Wang, C.; Wei, J.; Tang, C.Y. Rejection of pharmaceuticals by forward osmosis membranes. J. Hazard. Mater. 2012, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Acero, J.L.; Benitez, F.J.; Teva, F.; Leal, A.I. Retention of emerging micropollutants from UP water and a municipal secondary effluent by ultrafiltration and nanofiltration. Chem. Eng. J. 2010, 163, 264–272. [Google Scholar] [CrossRef]
- De Munari, A.; Semiao, A.J.C.; Antizar-Ladislao, B. Retention of pesticide Endosulfan by nanofiltration: Influence of organic matter–pesticide complexation and solute–membrane interactions. Water Res. 2013, 47, 3484–3496. [Google Scholar] [CrossRef][Green Version]
- Tanninen, J.; Nyström, M. Separation of ions in acidic conditions using NF. Desalination 2002, 147, 295–299. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Childress, A. Zeta potential of commercial RO membranes: Influence of source water type and chemistry. Desalination 2001, 140, 87–95. [Google Scholar] [CrossRef]
- Seidel, A.; Waypa, J.J.; Elimelech, M. Role of Charge (Donnan) Exclusion in Removal of Arsenic from Water by a Negatively Charged Porous Nanofiltration Membrane. Environ. Eng. Sci. 2014, 18. [Google Scholar] [CrossRef]
- Arizaa, M.J.; Ctiasa, A.; Malfeitob, J.; Benaventea, J. Effect of pH on electrokinetic and electrochemical parameters of both sub-layers of composite polyamide/polysulfone membranes. Desalination 2002, 148, 377–382. [Google Scholar] [CrossRef]
- Zhang, Y.; Van Der Bruggen, B.; Chen, G.; Braeken, L.; Vandecasteele, C. Removal of pesticides by nanofiltration: Effect of the water matrix. Sep. Purif. Technol. 2004, 38, 163–172. [Google Scholar] [CrossRef]
- Freger, V.; Bottino, A.; Capannelli, G.; Perry, M.; Gitis, V.; Belfer, S. Characterization of novel acid-stable NF membranes before and after exposure to acid using ATR-FTIR, TEM and AFM. J. Membr. Sci. 2005, 256, 134–142. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.-H. Membrane Bioreactor (MBR) Technology for Wastewater Treatment and Reclamation: Membrane Fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef]
- Ruano, P.; Delgado, L.L.; Picco, S.; Villegas, L.; Tonelli, F.; Merlo, M.; Rigau, J.; Diaz, D.; Masuelli, M. Extraction and Characterization of Pectins From Peels of Criolla Oranges (Citrus sinensis): Experimental Reviews. Intechopen 2016. [Google Scholar] [CrossRef]
- Li, H.; Yu, C.; Chen, R.; Li, J.; Li, J. Novel ionic liquid-type Gemini surfactants: Synthesis, surface property and antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 116–124. [Google Scholar] [CrossRef]
- Nackaerts, R. Are Membranes Implemented with Nanoparticles Able to Provide a Breakthrough in Water Purification? Master’s Thesis, University of Gent, Gent, Belgium, 2014. [Google Scholar]
- Goh, P.; Lau, W.; Othman, M.; Ismail, A. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Z.; Liu, J.; Zhang, X. Double-win effects of in-situ ozonation on improved filterability of mixed liquor and ceramic UF membrane fouling mitigation in wastewater treatment? J. Membr. Sci. 2017, 533, 112–120. [Google Scholar] [CrossRef]
- Wang, H.; Ding, A.; Gan, Z.; Qu, F.; Cheng, X.; Bai, L.; Guo, S.; Li, G.; Liang, H. Fluorescent natural organic matter responsible for ultrafiltration membrane fouling: Fate, contributions and fouling mechanisms. Chemosphere 2017, 182, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuhara, T.; Hanamoto, Y.; Miyoshi, T.; Kimura, K.; Watanabe, Y. Influence of membrane properties on physically reversible and irreversible fouling in membrane bioreactors. Water Sci. Technol. 2010, 61, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Lee, S.; Cho, J.; Elimelech, M. Combined influence of natural organic matter (NOM) and colloidal particles on nanofiltration membrane fouling. J. Membr. Sci. 2005, 262, 27–41. [Google Scholar] [CrossRef]
- Huang, X.; Huanga, X. Fouling characteristics and cleaning strategies in a coagulation-microfiltration combination process for water purification. Desalination 2003, 159, 1–9. [Google Scholar] [CrossRef]
- Aoustin, E. Ultrafiltration of natural organic matter. Sep. Purif. Technol. 2001, 63–78. [Google Scholar] [CrossRef]
- Kimura, K.; Toshima, S.; Amy, G.; Watanabe, Y. Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical active compounds (PhACs) by RO membranes. J. Membr. Sci. 2004, 245, 71–78. [Google Scholar] [CrossRef]
- Nilson, J.A.; DiGiano, F.A. Influence of NOM composition on nanofiltration. J. Am. Water Work. Assoc. 1996, 88, 53–66. [Google Scholar] [CrossRef]
- Carroll, T.; King, S.; Gray, S.; Bolto, B.; Booker, N. The fouling of microfiltration membranes by NOM after coagulation treatment. Water Res. 2000, 34, 2861–2868. [Google Scholar] [CrossRef]
- Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. Mass Transf. Adv. Sustain. Energy Environ. Oriented Numer. Model. 2013. [Google Scholar] [CrossRef]
- Hlaváček, M.; Bouchet, F. Constant flowrate blocking laws and an example of their application to dead-end microfiltration of protein solutions. J. Membr. Sci. 1993, 82, 285–295. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L.; Davisb, R.H.; Zydney, A.L. Behaviour of Suspentions and Macromolecular Solutions. J. Memb. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Meng, F.; Liao, B.; Liang, S.; Yang, F.; Zhang, H.; Song, L. Morphological visualization, componential characterization and microbiological identification of membrane fouling in membrane bioreactors (MBRs). J. Membr. Sci. 2010, 361, 1–14. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef]
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for membranes and membrane processes (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 1479–1489. [Google Scholar] [CrossRef]
- Tu, X.; Zhang, S.; Xu, L.; Zhang, M.; Zhu, J. Performance and fouling characteristics in a membrane sequence batch reactor (MSBR) system coupled with aerobic granular sludge. Desalination 2010, 261, 191–196. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Vargas, A.; Moreno-Andrade, I.; Buitrón, G. Controlled backwashing in a membrane sequencing batch reactor used for toxic wastewater treatment. J. Membr. Sci. 2008, 320, 185–190. [Google Scholar] [CrossRef]
- Hasnine, T.; Hanife, S.E.; Guleda, O.E. Membrane Bioreactor (MBR) Technology for Wastewater Treatment: Approaches to Membrane Fouling Control. In Proceedings of the ICOCEE—CAPPADOCIA 2017, Nevsehir, Turkey, 8–10 May 2017; pp. 313–322. [Google Scholar]
- Meng, F.; Zhang, H.; Yang, F.; Liu, L. Characterization of Cake Layer in Submerged Membrane Bioreactor. Environ. Sci. Technol. 2007, 41, 4065–4070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chua, H.; Zhou, J.; Fane, A.G. Factors affecting the membrane performance in submerged membrane bioreactors. J. Membr. Sci. 2006, 284, 54–66. [Google Scholar] [CrossRef]
- Le-Clech, P. Membrane bioreactors and their uses in wastewater treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Le Clech, P.; Chen, V.; Fane, A. Evolution of fouling during crossflow filtration of model EPS solutions. J. Membr. Sci. 2005, 264, 190–199. [Google Scholar] [CrossRef]
- Ognier, S.; Wisniewski, C.; Grasmick, A. Membrane bioreactor fouling in sub-critical filtration conditions: A local critical flux concept. J. Membr. Sci. 2004, 229, 171–177. [Google Scholar] [CrossRef]
- Hwang, B.-K.; Lee, W.-N.; Yeon, K.-M.; Park, P.-K.; Lee, C.-H.; Chang, I.-S.; Drews, A.; Kraume, M. Correlating TMP Increases with Microbial Characteristics in the Bio-Cake on the Membrane Surface in a Membrane Bioreactor. Environ. Sci. Technol. 2008, 42, 3963–3968. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Shen, X.; Liu, P.; Shu-Biao, X.; Liu, J.-J.; Wang, R.; Zhao, H.; Liu, Q.; Xu, J.; Wang, F. Anti-Fouling and Anti-Bacterial Modification of Poly(vinylidene fluoride) Membrane by Blending with the Capsaicin-Based Copolymer. Polymers 2019, 11, 323. [Google Scholar] [CrossRef]
- Dumée, L.F.; He, L.; King, P.C.; Le Moing, M.; Guller, I.; Duke, M.; Hodgson, P.D.; Gray, S.; Poole, A.; Kong, L. Towards integrated anti-microbial capabilities: Novel bio-fouling resistant membranes by high velocity embedment of silver particles. J. Membr. Sci. 2015, 475, 552–561. [Google Scholar] [CrossRef]
- Bagheri, M.; Mirbagheri, S.A. Critical review of fouling mitigation strategies in membrane bioreactors treating water and wastewater. Bioresour. Technol. 2018, 258, 318–334. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, P.; Yao, M.; Field, R.W.; Cui, Z. Effect of the bubbling regimes on the performance and energy cost of flat sheet MBRs. Desalination 2011, 283, 221–226. [Google Scholar] [CrossRef]
- Wray, H.E.; Andrews, R.C.; Bérubé, P.R. Surface shear stress and membrane fouling when considering natural water matrices. Desalination 2013, 330, 22–27. [Google Scholar] [CrossRef]
- Chan, C.; Bérubé, P.; Hall, E. Relationship between types of surface shear stress profiles and membrane fouling. Water Res. 2011, 45, 6403–6416. [Google Scholar] [CrossRef] [PubMed]
- Jankhah, S.; Bérubé, P.R. Power induced by bubbles of different sizes and frequencies on to hollow fibers in submerged membrane systems. Water Res. 2013, 47, 6516–6526. [Google Scholar] [CrossRef] [PubMed]
- Ye, D. Shear Stress And Fouling Control In Hollow Fiber Membrane Systems Under Different Gas Sparging Conditions. Ph.D. Thesis, The Faculty of Graduate Studies. The University of British Columbia, Vancouver, BC, Canada, 2012. [Google Scholar]
- Baransi-Karkaby, K.; Bass, M.; Freger, V. In Situ Modification of Reverse Osmosis Membrane Elements for Enhanced Removal of Multiple Micropollutants. Membranes 2019, 9, 28. [Google Scholar] [CrossRef]
- Woo, S.T.; Yun, T.; Kwak, S.-Y. Fouling-resistant microfiltration membrane modified with magnetite nanoparticles by reversible conjunction. Sep. Purif. Technol. 2018, 202, 299–306. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Xie, Y.-J.; Hu, M.-X.; Wang, J.-L.; Wang, S.-Y.; Xu, Z.-K. Surface modification of polypropylene microporous membrane to improve its antifouling property in MBR: CO plasma treatment. J. Membr. Sci. 2005, 254, 219–227. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of surface properties and performance of reverse osmosis membranes after surface modification: A review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Yü, Y.; Deng, B.; Li, J.; Jin, J. Sol–gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved antifouling property. J. Membr. Sci. 2013, 432, 25–32. [Google Scholar] [CrossRef]
- Li, H.S.; Zhao, Z.; Wang, N.; Liu, H.; Umar, A.; Zhang, J.; Wu, T.; Guo, Z. Enhanced Photocatalytic Activity of B, N-Codoped TiO₂ by a New Molten Nitrate Process. J. Nanosci. Nanotechnol. 2019, 19, 839–849. [Google Scholar] [CrossRef]
- Sun, W.; Shi, J.; Chen, C.; Li, N.; Xu, Z.; Li, J.; Lv, H.; Qian, X.; Zhao, L. A review on organic–inorganic hybrid nanocomposite membranes: A versatile tool to overcome the barriers of forward osmosis. RSC Adv. 2018, 8, 10040–10056. [Google Scholar] [CrossRef]
- Liang, S.; Gao, P.; Gao, X.; Xiao, K.; Huang, X. Improved blending strategy for membrane modification by virtue of surface segregation using surface-tailored amphiphilic nanoparticles. Front. Environ. Sci. Eng. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Superhydrophilic Thin-Film Composite Forward Osmosis Membranes for Organic Fouling Control: Fouling Behavior and Antifouling Mechanisms. Environ. Sci. Technol. 2012, 46, 11135–11144. [Google Scholar] [CrossRef]
- Shen, J.-N.; Ruan, H.-M.; Wu, L.; Gao, C.-J. Preparation and characterization of PES–SiO2 organic–inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Copper sulfide nanoparticles/carboxylated graphene oxide nanosheets blended polyethersulfone hollow fiber membranes: Development and characterization for efficient separation of oxybenzone and bisphenol A from water. Polymers 2019, 163, 57–67. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol–gel coated PVDF membrane. J. Membr. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Zhao, W.; Wei, Q.; Nie, S.; Sun, S.; He, M. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Membr. Sci. 2012, 228–236. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Yang, L.; Deng, C.; Tian, Q.; Yang, B. Surface modification of ultrafiltration membranes by grafting glycine-functionalized PVA based on polydopamine coatings. Appl. Surf. Sci. 2015, 345, 301–309. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Yu, S.; Zuo, X.; Bao, R.; Xu, X.; Wang, J.; Xu, J. Effect of SiO2 nanoparticle addition on the characteristics of a new organic–inorganic hybrid membrane. Polymers 2009, 50, 553–559. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, J.; Zhang, H.; Liu, K.; Kong, Z.; Dong, Y.; Jin, G.; Tian, J.; Qin, Z. Fabrication of composite membrane with adsorption property and its application to the removal of endocrine disrupting compounds during filtration process. Chem. Eng. J. 2018, 352, 53–63. [Google Scholar] [CrossRef]
- Hou, S.; Xing, J.; Dong, X.; Zheng, J.; Li, S. Integrated antimicrobial and antifouling ultrafiltration membrane by surface grafting PEO and N-chloramine functional groups. J. Colloid Interface Sci. 2017, 500, 333–340. [Google Scholar] [CrossRef]
- Mauter, M.S.; Zucker, I.; Perreault, F.; Werber, J.R.; Kim, J.-H.; Elimelech, M. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 2018, 1, 166–175. [Google Scholar] [CrossRef]
- Man, H.C.; Abdulsalam, M.; Abba, M.U.; Syahidah, R. Utilization of Nano-TiO2 as an influential additive for Complementing Separation Performance of a Hybrid PVDF-PVP Hollow Fiber: Boron removal from leachate. Polymers 2020, 12, 2511. [Google Scholar] [CrossRef]
- Boruah, B.; Samantaray, P.K.; Madras, G.; Modak, J.M.; Bose, S. Sustainable photocatalytic water remediation via dual active strongly coupled AgBiO3 on PVDF/PBSA membranes. Chem. Eng. J. 2020, 394, 124777. [Google Scholar] [CrossRef]
- Syahida, N.; Anan, M.; Jaafar, J.; Hafiz, M.; Othman, D.; Rahman, M.A.; Aziz, F.; Shahrodin, N.S. Titanium dioxide incorporated thin film composite membrane for bisphenol A removal. Malays. J. Fundam. Appl. Sci. 2019, 15, 755–760. [Google Scholar]
- Mavukkandy, M.O.; Zaib, Q.; Arafat, H.A. CNT/PVP blend PVDF membranes for the removal of organic pollutants from simulated treated wastewater effluent. J. Environ. Chem. Eng. 2018, 6, 6733–6740. [Google Scholar] [CrossRef]
- Jasni, M.J.F.; Arulkumar, M.; Sathishkumar, P.; Yusoff, A.R.M.; Buang, N.A.; Gu, F.L. Electrospun nylon 6,6 membrane as a reusable nano-adsorbent for bisphenol A removal: Adsorption performance and mechanism. J. Colloid Interface Sci. 2017, 508, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Zahari, A.M.; Shuo, C.W.; Sathishkumar, P.; Yusoff, A.R.M.; Gu, F.L.; Buang, N.A.; Lau, W.-J.; Gohari, R.J.; Yusop, Z. A reusable electrospun PVDF-PVP-MnO2 nanocomposite membrane for bisphenol A removal from drinking water. J. Environ. Chem. Eng. 2018, 6, 5801–5811. [Google Scholar] [CrossRef]
- Rajesha, B.; Vishaka, V.H.; Balakrishna, G.R.; Padaki, M.; Nazri, N. Effective composite membranes of cellulose acetate for removal of benzophenone-3. J. Water Process. Eng. 2019, 30, 100419. [Google Scholar] [CrossRef]
- Babu, V.; Padaki, M.; D’Souza, L.P.; Déon, S.; Balakrishna, R.G.; Ismail, A. Effect of hydraulic coefficient on membrane performance for rejection of emerging contaminants. Chem. Eng. J. 2018, 334, 2392–2400. [Google Scholar] [CrossRef]
- Alvarez, P.J.; Chan, C.K.; Elimelech, M.; Halas, N.J.; Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 2018, 13, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef]
- Kamaz, M.; Wickramasinghe, S.R.; Eswaranandam, S.; Zhang, W.; Jones, S.M.; Watts, M.J.; Qian, X. Investigation into Micropollutant Removal from Wastewaters by a Membrane Bioreactor. Int. J. Environ. Res. Public Health 2019, 16, 1363. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Manikandan, N.A.; Dolman, B.; Pakshirajan, K.; Pugazhenthi, G. Biological treatment of wastewater containing a mixture of polycyclic aromatic hydrocarbons using the oleaginous bacterium Rhodococcus opacus. J. Clean. Prod. 2018, 196, 1282–1291. [Google Scholar] [CrossRef]
- Visvanathan, C.; Ben Aim, R.; Parameshwaran, K. Membrane Separation Bioreactors for Wastewater Treatment. Crit. Rev. Environ. Sci. Technol. 2000, 30, 1–48. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Removal of emerging trace organic contaminants by MBR-based hybrid treatment processes. Int. Biodeterior. Biodegrad. 2013, 85, 474–482. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, D.G. Removal of Contaminants of Emerging Concern (CECs) Using a Membrane Bioreactor (MBR): A short review. Glob. NEST J. 2019, 21, 337–346. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, J.-T.; Wang, J.; Xing, L.-L.; Jiang, N.; Gou, M.; Uddin, M.S. Population dynamics in bioaugmented membrane bioreactor for treatment of bromoamine acid wastewater. Bioresour. Technol. 2009, 100, 244–248. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; McArdell, C.S. Hospital Wastewater Treatment by Membrane Bioreactor: Performance and Efficiency for Organic Micropollutant Elimination. Environ. Sci. Technol. 2012, 46, 1536–1545. [Google Scholar] [CrossRef]
- Ma, J.; Dai, R.; Chen, M.; Khan, S.J.; Wang, Z. Applications of membrane bioreactors for water reclamation: Micropollutant removal, mechanisms and perspectives. Bioresour. Technol. 2018, 269, 532–543. [Google Scholar] [CrossRef]
- Suárez, S.; Carballa, M.; Omil, F.; Lema, J.M. How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters? Rev. Environ. Sci. Bio Technol. 2008, 7, 125–138. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. The fate of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters and pesticides during MBR treatment. Bioresour. Technol. 2013, 144, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—A review. Environ. Sci. Pollut. Res. 2014, 21, 11708–11728. [Google Scholar] [CrossRef]
- Boonnorat, J.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, K. Microbial adaptation to biodegrade toxic organic micro-pollutants in membrane bioreactor using different sludge sources. Bioresour. Technol. 2014, 165, 50–59. [Google Scholar] [CrossRef]
- Besha, A.T.; Gebreyohannes, A.Y.; Tufa, R.A.; Bekele, D.N.; Curcio, E.; Giorno, L. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: A review. J. Environ. Chem. Eng. 2017, 5, 2395–2414. [Google Scholar] [CrossRef]
- Mei, X.; Wang, Z.; Zheng, X.; Huang, F.; Ma, J.; Tang, J.; Wu, Z. Soluble microbial products in membrane bioreactors in the presence of ZnO nanoparticles. J. Membr. Sci. 2014, 451, 169–176. [Google Scholar] [CrossRef]
- Wick, A.; Marincas, O.; Moldovan, Z.; Ternes, T.A. Sorption of biocides, triazine and phenylurea herbicides, and UV-filters onto secondary sludge. Water Res. 2011, 45, 3638–3652. [Google Scholar] [CrossRef] [PubMed]
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the sorption and biotransformation of organic micropollutants in innovative biological wastewater treatment technologies. Sci. Total Environ. 2018, 615, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Cirja, M.; Ivashechkin, P.; Schäffer, A.; Corvini, P.F. Factors affecting the removal of organic micropollutants from wastewater in conventional treatment plants (CTP) and membrane bioreactors (MBR). Rev. Environ. Sci. Bio Technol. 2007, 7, 61–78. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M. Removal of cosmetic ingredients and pharmaceuticals in sewage primary treatment. Water Res. 2005, 39, 4790–4796. [Google Scholar] [CrossRef]
- Kumar, R.V.; Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Dairy wastewater treatment using a novel low cost tubular ceramic membrane and membrane fouling mechanism using pore blocking models. J. Water Process. Eng. 2016, 13, 168–175. [Google Scholar] [CrossRef]
- Li, X.; Hai, F.I.; Nghiem, L.D. Simultaneous activated carbon adsorption within a membrane bioreactor for an enhanced micropollutant removal. Bioresour. Technol. 2011, 102, 5319–5324. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-J.; Kim, S.-G.; Kim, S.-H. Removal of antibiotics by coagulation and granular activated carbon filtration. J. Hazard. Mater. 2008, 151, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Tadkaew, N.; Sivakumar, M.; Khan, S.J.; McDonald, J.A.; Nghiem, L.D. Effect of mixed liquor pH on the removal of trace organic contaminants in a membrane bioreactor. Bioresour. Technol. 2010, 101, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Gruchlik, Y.; Linge, K.; Joll, C.A. Removal of organic micropollutants in waste stabilisation ponds: A review. J. Environ. Manag. 2018, 206, 202–214. [Google Scholar] [CrossRef]
- Vyrides, I.; Santos, H.; Mingote, A.; Ray, M.J.; Stuckey, D.C. Are Compatible Solutes Compatible with Biological Treatment of Saline Wastewater? Batch and Continuous Studies Using Submerged Anaerobic Membrane Bioreactors (SAMBRs). Environ. Sci. Technol. 2010, 44, 7437–7442. [Google Scholar] [CrossRef]
- Trinh, T.; Akker, B.V.D.; Stuetz, R.M.; Coleman, H.M.; Le-Clech, P.; Khan, S.J. Removal of trace organic chemical contaminants by a membrane bioreactor. Water Sci. Technol. 2012, 66, 1856–1863. [Google Scholar] [CrossRef]
- Jiang, Q.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Price, W.E.; Zhang, J.; Liang, S.; Deng, L.; Guo, W. Effect of hydraulic retention time on the performance of a hybrid moving bed biofilm reactor-membrane bioreactor system for micropollutants removal from municipal wastewater. Bioresour. Technol. 2018, 247, 1228–1232. [Google Scholar] [CrossRef]
- Guerra, P.; Kim, M.; Teslic, S.; Alaee, M.; Smyth, S. Bisphenol-A removal in various wastewater treatment processes: Operational conditions, mass balance, and optimization. J. Environ. Manag. 2015, 152, 192–200. [Google Scholar] [CrossRef]
- Zhu, H.; Li, W. Bisphenol A removal from synthetic municipal wastewater by a bioreactor coupled with either a forward osmotic membrane or a microfiltration membrane unit. Front. Environ. Sci. Eng. 2013, 7, 294–300. [Google Scholar] [CrossRef]
- Kim, M.; Guerra, P.; Shah, A.; Parsa, M.; Alaee, M.; Smyth, S. Removal of pharmaceuticals and personal care products in a membrane bioreactor wastewater treatment plant. Water Sci. Technol. 2014, 69, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, K.C.; McDonald, J.A.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Development of a predictive framework to assess the removal of trace organic chemicals by anaerobic membrane bioreactor. Bioresour. Technol. 2015, 189, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; McDonald, J.; Price, W.E.; Khan, S.J.; Hai, F.I.; Ngo, H.H.; Guo, W.; Nghiem, L.D. Effects of salinity build-up on the performance of an anaerobic membrane bioreactor regarding basic water quality parameters and removal of trace organic contaminants. Bioresour. Technol. 2016, 216, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ma, J.; Wang, Z.; Xu, S.; Waite, T.D.; Wu, Z. Contaminant Removal from Source Waters Using Cathodic Electrochemical Membrane Filtration: Mechanisms and Implications. Environ. Sci. Technol. 2017, 51, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zhang, J.; Waite, T.D.; Wu, Z. Cost-effective Chlorella biomass production from dilute wastewater using a novel photosynthetic microbial fuel cell (PMFC). Water Res. 2017, 108, 356–364. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Love, N.; Skerlos, S.J.; Raskin, L. Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: A critical review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef]
- Suneethi, S.; Joseph, K. ANAMMOX process start up and stabilization with an anaerobic seed in Anaerobic Membrane Bioreactor (AnMBR). Bioresour. Technol. 2011, 102, 8860–8867. [Google Scholar] [CrossRef]
- Kujawa-Roeleveld, K.; Wu, E.S. Biodegradability and fate of pharmaceutical impact compounds in different treatment processes. Sustain. Water Manag. 2008, 64, 54–58. [Google Scholar]
- Saravanane, R.; Sundararaman, S. Effect of loading rate and HRT on the removal of cephalosporin and their intermediates during the operation of a membrane bioreactor treating pharmaceutical wastewater. Environ. Technol. 2009, 30, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yamashita, N.; Tanaka, H. Membrane fouling control and enhanced removal of pharmaceuticals and personal care products by coagulation-MBR. Chemosphere 2018, 197, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Kruglova, A.; Kråkström, M.; Riska, M.; Mikola, A.; Rantanen, P.; Vahala, R.; Kronberg, L. Comparative study of emerging micropollutants removal by aerobic activated sludge of large laboratory-scale membrane bioreactors and sequencing batch reactors under low-temperature conditions. Bioresour. Technol. 2016, 214, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Ran, Y.; Chen, D.-Y.; Yang, Y. Occurrence of endocrine-disrupting chemicals in riverine sediments from the Pearl River Delta, China. Mar. Pollut. Bull. 2011, 63, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, G.P.; De Souza, N.C.; Vidal, C.B.; Alves, J.A.; Firmino, P.I.M.; Nascimento, R.F.; Dos Santos, A.B. Occurrence and removal of estrogens in Brazilian wastewater treatment plants. Sci. Total Environ. 2014, 490, 288–295. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Wu, S.; Fan, Z.; Hu, J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 732–740. [Google Scholar] [CrossRef]
- Xu, W.; Yan, W.; Huang, W.; Miao, L.; Zhong, L. Endocrine-disrupting chemicals in the Pearl River Delta and coastal environment: Sources, transfer, and implications. Environ. Geochem. Health 2014, 36, 1095–1104. [Google Scholar] [CrossRef]
- Froehner, S.; Machado, K.S.; Stefan, E.; Bleninger, T.; Da Rosa, E.C.; Martins, C.C. Occurrence of selected estrogens in mangrove sediments. Mar. Pollut. Bull. 2012, 64, 75–79. [Google Scholar] [CrossRef]
- Nazifa, T.H.; Kristanti, R.A.; Ike, M.; Kuroda, M.; Hadibarata, T. Occurrence and distribution of estrogenic chemicals in river waters of Malaysia. Toxicol. Environ. Health Sci. 2020, 12, 65–74. [Google Scholar] [CrossRef]
- Aydin, E.; Talınlı, I.; Aydın, E. Analysis, occurrence and fate of commonly used pharmaceuticals and hormones in the Buyukcekmece Watershed, Turkey. Chemosphere 2013, 90, 2004–2012. [Google Scholar] [CrossRef]
- Yacob, H.; Ling, Y.E.; Hee-Young, K.; Zainura, O.; Noor, Z.; Fadhil, M.; Din, M.; Mat, S.; Hun, L.T. Identification of pharmaceutical residues in treated sewage effluents in Johor, Malaysia. Malays. J. Civ. Eng. 2017, 29, 165–173. [Google Scholar]
- Wang, L.; Ying, G.-G.; Chen, F.; Zhang, L.-J.; Zhao, J.-L.; Lai, H.-J.; Chen, Z.-F.; Tao, R. Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools. Environ. Pollut. 2012, 165, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Chen, H.-W.; Chen, W.-L.; Chen, C.-Y.; Wang, G.-S. Occurrence of nonylphenol and bisphenol A in household water pipes made of different materials. Environ. Monit. Assess. 2016, 188, 188. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Hu, J.; An, W.; Yang, M. Detection and Occurrence of Chlorinated Byproducts of Bisphenol A, Nonylphenol, and Estrogens in Drinking Water of China: Comparison to the Parent Compounds. Environ. Sci. Technol. 2013, 47, 10841–10850. [Google Scholar] [CrossRef]
- Santhi, V.; Sakai, N.; Ahmad, E.; Mustafa, A. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci. Total Environ. 2012, 332–338. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, Y.; Ma, M.; Wang, N.; Luo, Q.; Wang, Z.; Satyanarayanan, S.K. Assessment of source water contamination by estrogenic disrupting compounds in China. J. Environ. Sci. 2012, 24, 320–328. [Google Scholar] [CrossRef]
- Talib, J.; Aris, A.; Jaafar, J.; Majid, Z.A.; Syarmimi, A. Occurrence of emerging pollutants in skudai river in johor bahru region of malaysia. Indian J. Forensic Med. Toxicol. 2020, 14, 1069–1074. [Google Scholar]
- Tiwari, M.; Sahu, S.K.; Pandit, G. Distribution and estrogenic potential of endocrine disrupting chemicals (EDCs) in estuarine sediments from Mumbai, India. Environ. Sci. Pollut. Res. 2016, 23, 18789–18799. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; He, J.; Yue, X.; Pan, J.; Wang, Z. Steroidal and phenolic endocrine disrupting chemicals (EDCs) in surface water of Bahe River, China: Distribution, bioaccumulation, risk assessment and estrogenic effect on Hemiculter leucisculus. Environ. Pollut. 2018, 243, 103–114. [Google Scholar] [CrossRef]
- Adeyi, A.A. Distribution and bioaccumulation of Endocrine Disrupting Chemicals (EDCS) in Lagos Lagoon water, sediment and fish. Ife J. Sci. 2020, 24, 57–74. [Google Scholar] [CrossRef]
- Ismail, N.A.H.; Wee, S.Y.; Aris, A.Z. Bisphenol A and alkylphenols concentrations in selected mariculture fish species from Pulau Kukup, Johor, Malaysia. Mar. Pollut. Bull. 2018, 127, 536–540. [Google Scholar] [CrossRef]
- Ismail, N.A.H.; Wee, S.Y.; Aris, A.Z. Multi-class of endocrine disrupting compounds in aquaculture ecosystems and health impacts in exposed biota. Chemosphere 2017, 188, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Kristanti, R.A.; Mahmoud, A.H. Occurrence of endocrine-disrupting chemicals (EDCs) in river water and sediment of the Mahakam River. J. Water Health 2019, 18, 38–47. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Zhang, C.; Zhang, Y. Possible effect of submarine groundwater discharge on the pollution of coastal water: Occurrence, source, and risks of endocrine disrupting chemicals in coastal groundwater and adjacent seawater influenced by reclaimed water irrigation. Chemosphere 2020, 250, 126323. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lam, J.C.-W.; Kwok, K.Y.; Tsui, M.M.; Lam, P.K. Occurrence and fate of endogenous steroid hormones, alkylphenol ethoxylates, bisphenol A and phthalates in municipal sewage treatment systems. J. Environ. Sci. 2017, 61, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Pak, V.M.; McCauley, L.A.; Pinto-Martin, J. Phthalate Exposures and Human Health Concerns. AAOHN J. 2011, 59, 228–235. [Google Scholar] [CrossRef]
- Penabad-Peña, L.; Herrera-Morales, J.; Betancourt, M.; Nicolau, E. Cellulose Acetate/P4VP-b-PEO Membranes for the Adsorption of Electron-Deficient Pharmaceutical Compounds. ACS Omega 2019, 4, 22456–22463. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Mafukidze, D.; Nyokong, T. Fabrication of electrospun fibers from a porphyrin linked to polyacrylonitrile polymer for photocatalytic transformation of phenols. J. Mol. Struct. 2020, 1213, 128191. [Google Scholar] [CrossRef]
- Zhao, F.-B.; Tang, C.-C.; Liu, X.-Y.; Shi, F.-J.; Song, X.-R.; Tian, Y.; Li, Z.-S. Transportation characteristics of bisphenol A on ultrafiltration membrane with low molecule weight cut-off. Desalination 2015, 362, 18–25. [Google Scholar] [CrossRef]
- Wei, X.; Shi, Y.; Fei, Y.; Chen, J.; Lv, B.; Chen, Y.; Zheng, H.; Shen, J.; Zhu, L. Removal of trace phthalate esters from water by thin-film composite nanofiltration hollow fiber membranes. Chem. Eng. J. 2016, 292, 382–388. [Google Scholar] [CrossRef]
- Gurung, K.; Ncibi, M.C.; Sillanpää, M. Assessing membrane fouling and the performance of pilot-scale membrane bioreactor (MBR) to treat real municipal wastewater during winter season in Nordic regions. Sci. Total Environ. 2017, 579, 1289–1297. [Google Scholar] [CrossRef] [PubMed]


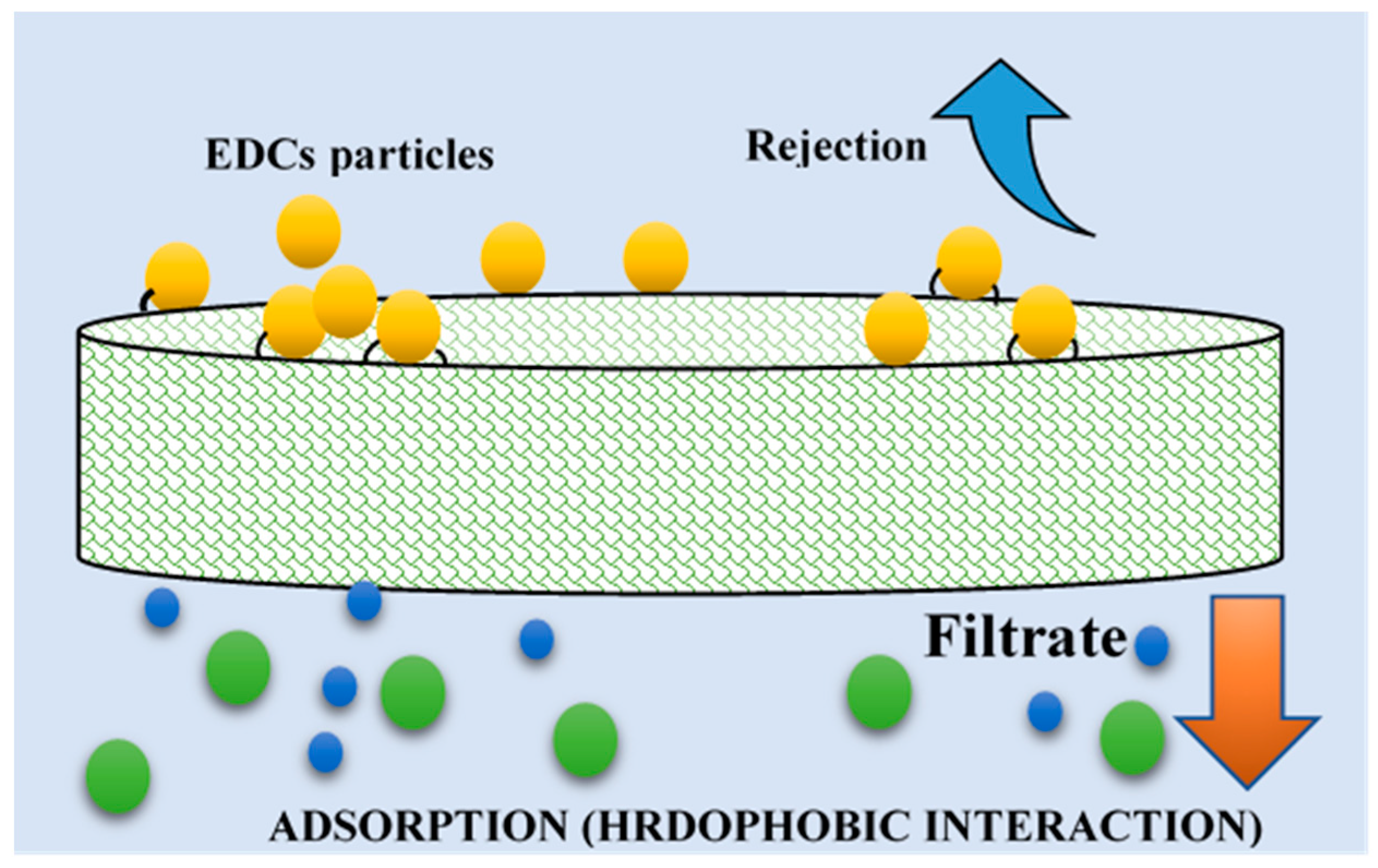
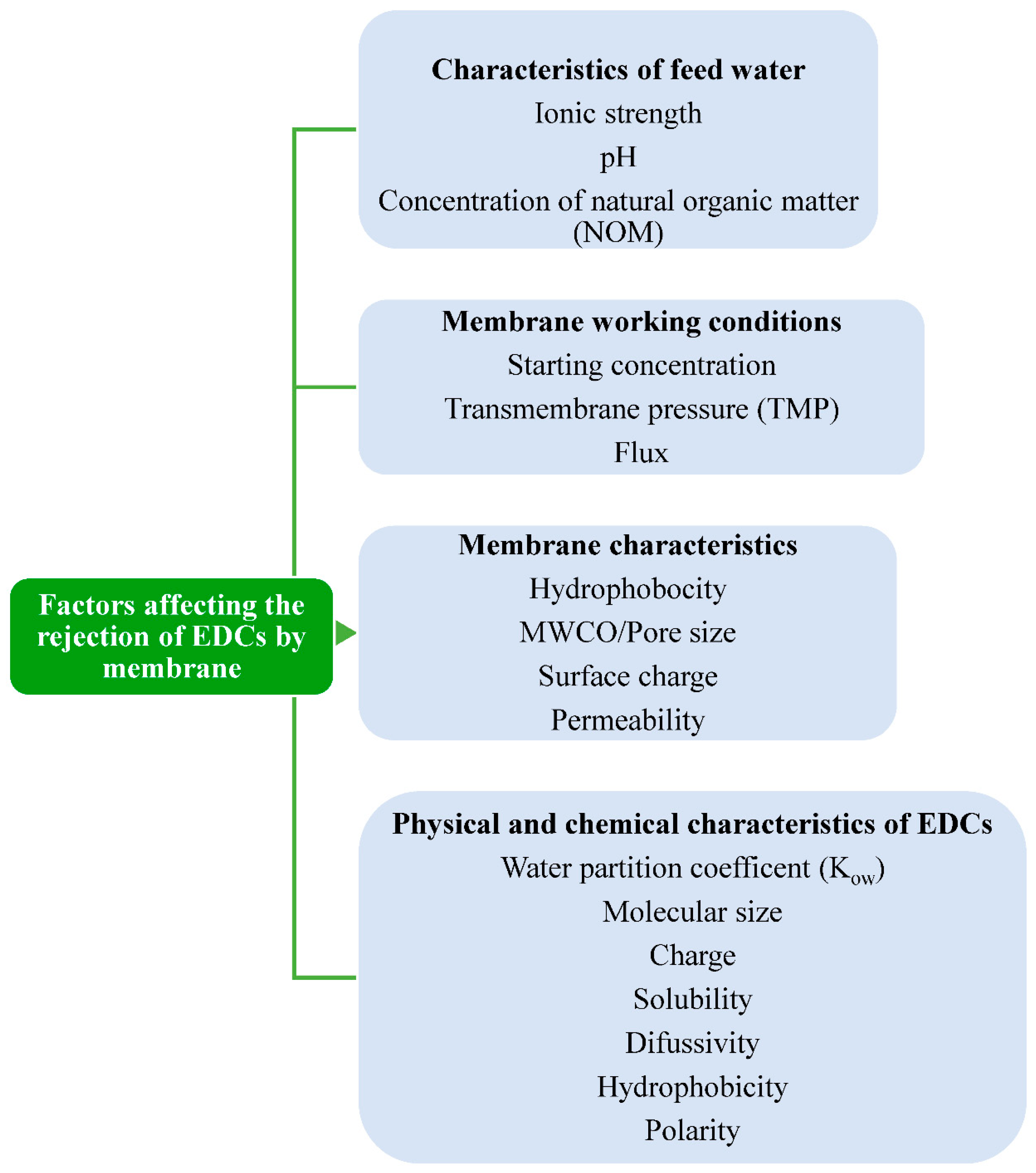

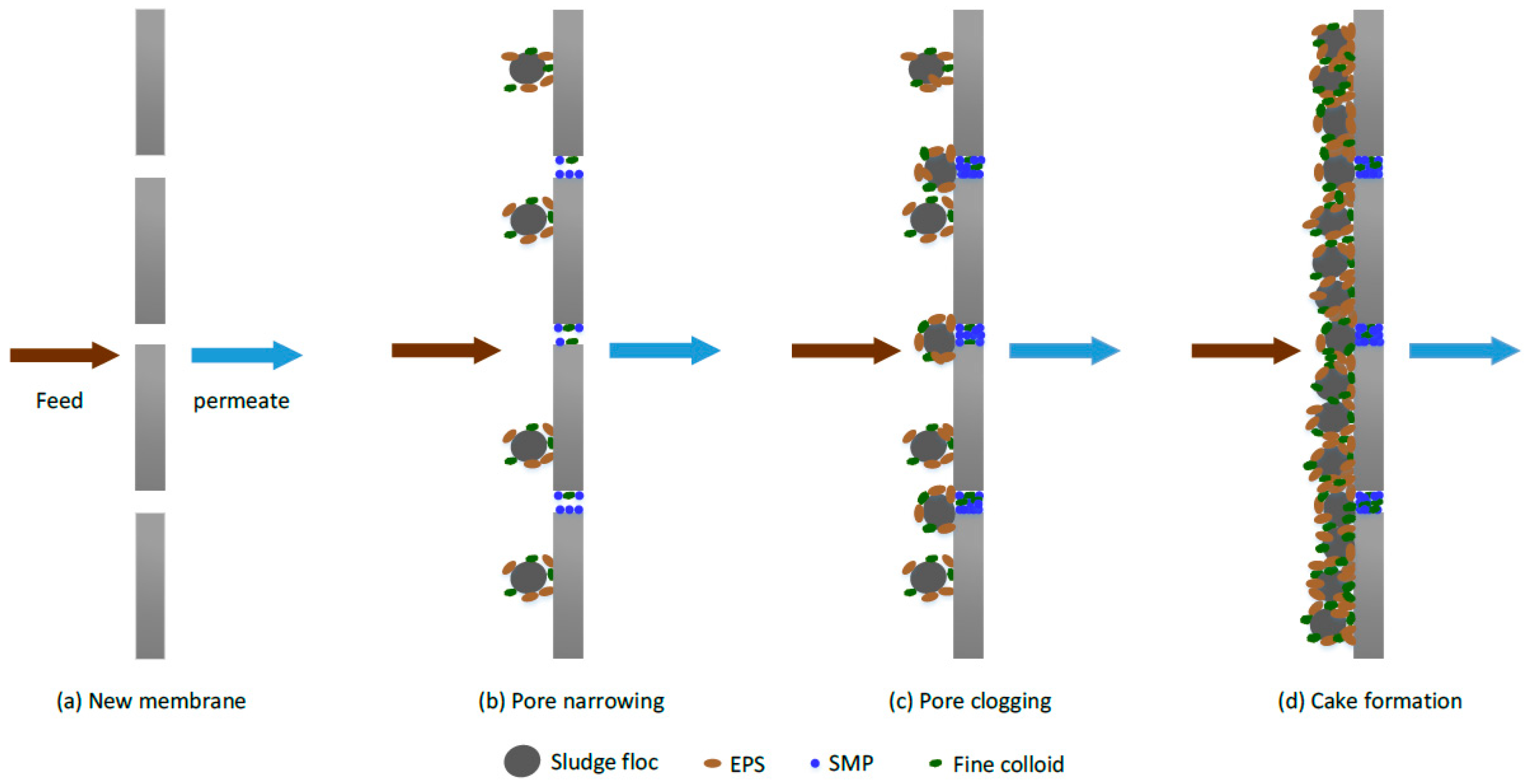

| Membranes | MF Symmetric/Asymmetric | UF Asymmetric | NF Asymmetric | RO Asymmetric |
|---|---|---|---|---|
| Pore size | 0.025–5 (µm) | 1–100 nm | 0.5–10 nm | ˂1 nm |
| Thickness (µm) | 10–150 | 150–250 | 150 | 150 |
| Operating pressure (bar) | 0.1–10 | 0.1–10 | 10–50 | 35–170 |
| Flux range (Lm−2h−1bar−1) | ˃50 | 10–50 | 1.4–12 | 0.05–1.4 |
| Separation mechanism | Sieving | Sieving | Sieving and electrostatic | Solution diffusion |
| Applications | Clarification Pre-treatment Removal of bacteria | Removal of macromolecules, bacteria, viruses. | Removal of (multivalent) ions and relatively small organics. | Ultra-pure water. Desalination. |
| Rejection: | ||||
| Monovalent ions | - | - | - | + |
| Multivalent ions | - | −/+ | + | + |
| Small organic compounds | - | - | −/+ | + |
| macromolecules | - | + | + | + |
| Particles | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katibi, K.K.; Yunos, K.F.; Che Man, H.; Aris, A.Z.; bin Mohd Nor, M.Z.; binti Azis, R.S. Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review. Polymers 2021, 13, 392. https://doi.org/10.3390/polym13030392
Katibi KK, Yunos KF, Che Man H, Aris AZ, bin Mohd Nor MZ, binti Azis RS. Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review. Polymers. 2021; 13(3):392. https://doi.org/10.3390/polym13030392
Chicago/Turabian StyleKatibi, Kamil Kayode, Khairul Faezah Yunos, Hasfalina Che Man, Ahmad Zaharin Aris, Mohd Zuhair bin Mohd Nor, and Rabaah Syahidah binti Azis. 2021. "Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review" Polymers 13, no. 3: 392. https://doi.org/10.3390/polym13030392
APA StyleKatibi, K. K., Yunos, K. F., Che Man, H., Aris, A. Z., bin Mohd Nor, M. Z., & binti Azis, R. S. (2021). Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review. Polymers, 13(3), 392. https://doi.org/10.3390/polym13030392