Abstract
Some types of cancer are resistant to conventional treatments, such as triple-negative (TN) breast cancer (BC), which, due to its molecular characteristics, does not respond effectively. In recent years, there has been an increase in the use of medicinal plants for prevention and as an alternative in adjuvant therapy for cancer. Hibiscus sabdariffa L. (H.S.) is a plant which has anti-inflammatory and antioxidants properties. The objective of this study is to evaluate the cytotoxicity effect of the lyophilized extract of H.S. in the TN BC cell line (HCC-70) exposed to different concentrations (0.10, 0.25, 0.5 and 1.0 mg/mL). The results were evaluated by ANOVA, finding that H.S. reduces the proliferation on TN BC cell line HCC-70 exposed at 1.0 mg/mL by 62%. The use of natural extracts as coadjuvant therapy for cancer is an alternative with great potential for future studies and provides a precedent in the area of natural medicine.
1. Introduction
Cancer is a disease characterized by a high rate of cell proliferation and loss of the function of proteins that regulate the cell cycle, thus preventing cells from performing their programmed cell death cycle, resulting in the formation of neoplasms [1]. It should be noted that only 1–2% of neoplasms are the result of genetic predisposition, and 98–99% are the result of epigenetic factors [2].
According to the World Health Organization (WHO), cancer is one of the main causes of death worldwide, and it was estimated that in 2015 it caused 8.8 million deaths [3]. By 2018, the incidence of cancer rose to 18 million and 9.6 million deaths, thus placing cancer among the leading causes of death worldwide [4].
The types of cancers that are most aggressive and have the highest mortality rates are lung, liver, colorectal, gastric and breast cancer, which is the most common in the female population and represents 16%worldwide. [5]
According to gene expression, BC is classified molecularly; Luminal A: It is the most common and least aggressive positive for progesterone (RP+) and estrogen receptor (RE+); Luminal B: It has a higher proliferation index and a least response to conventional treatment (RP+, RE+ and Her2/neu); HER2/neu: It is the least common subtype and highly aggressive due to increased expression of genes related with cellular proliferation (Her2/neu positive), by last the basal subtype (TN): does not express any of the receptors named above due to this subtype not being susceptible to conventional treatment, which defines it as a cancer with heterogeneous biology and clinical behavior, making it difficult to identify and treat properly by developing early resistance to chemotherapy; no response to endocrine therapy and no effective target therapy is available [6,7].
In recent years, there has been an increase in interest in the use of medicinal plants. Therefore, the study of different natural extracts, their bioactive compounds, uses and limitations, allows us to know the effect of these extracts on health. Since 2004, the WHO has approved the use of medicinal plants and alternative therapies; additionally, H.S. is classified as a medicinal plant because of its great quantity of bioactive compounds and its relationship with health [8].
Antioxidants play an important role in cellular homeostasis, where one of their main functions is to maintain host integrity. The balance between prooxidant and antioxidant levels defines the cellular fate by maintaining the redox state of the cell preventing the development of chronic pathologies related to oxidative stress including cancer [9]. There is a wide range of antioxidants, some are synthesized by the body (endogenous) and others provided by the diet (exogenous) that are found mainly in fruits and vegetables [10].
H.S., also known as hibiscus flowers, belongs to the Malvaceae family and its origin is attributed to Sudan and tropical Asia; it was later introduced to the rest of the world. This flower has been widely studied for its therapeutic effects such as: antitumor, antihypertensive, anti-inflammatory, hepatoprotective and antimicrobial related to its bioactive compounds (flavonoids, organic acids, and antiocianins) present in the flower's calyxes. The main antioxidants present in S.H. are polyphenolic compounds called anthocyanins, which in various fruits give a red–orange to blue–violet pigmentation [11].
Anthocyanins represent the glycosylated form of anthocyanidins; that is, the combination of an anthocyanidin (aglycone) with a carbohydrate (glucose or xylose) results in anthocyanins, a stable and functional structure. Among the anthocyanins with the highest biological activity are delphinidin and pelargonidin.
Several studies have documented the anti-tumor effect of S.H. in different types of cancer. In 2018, Ríaz evaluated the effect of S.H. on carcinoma and myeloma cells and observed that, after stimulation of both cell lines with the S.H. extract, the S.H. was able to produce a positive result. the viability, proliferation and capacity of cellular transmigration were reduced by more than 50% [12]. On the other hand, it has been observed that the H.S. extract induces apoptosis in malignant cells [13], as well as a decrease in cell proliferation and viability. Similarly, in studies on BC Cheng et al. evaluated the effect of the hydroalcoholic extract of H.S. on BC cells (MCF-7) where it was reported that of the total anthocyanins of the compound, 69% were represented by delphinidin, followed by cyanidin in 27% and other anthocyanins in 4%. Likewise, they showed that the antitumor effect of H.S. was attributed to its capacity to participate as a chemoprotective agent, inducer of autophagy and necrosis in breast tumor cells [14].
The use of H.S. extract in BC RE+, RP+ and HER2/neu cell lines has been reported; however, in cell lines corresponding to TN, there is not much information about it, so the aim of this study is to evaluate HCC-70 cell line exposed to lyophilized H.S. extract.
2. Material and Methods
2.1. Chemical
Roswell Park Memorial Institute (RPMI-1640), Fetal Bovine Serum (FBS), Extraction Buffer (EB), Lyophilizede H.S. extract (HSE)), and Antibiotic-Antimycotic (AA) were purchased from Thermo Fischer ScientificTM (Walthman MA, USA). Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Phosphate Buffered Saline (PBS) Tripan Blue (TB) All solvents and chemicals were of an analytical grade by SigmaTM (St Luis MO, USA).
2.2. Plant Material
The lyophilized extract of H.S. was obtained by Dr. Gómez Leyva from the Technological Institute of Tlajomulco.
2.3. Preparation of the HSE Solution
- -
- Preparation of DMSO 0.5% in culture medium as a vehicle.
- -
- Preparation of Hibiscus Sabdariffa stock solution (1 mg/mL).
- -
- HSE 1.7 mg + Medium + DMSO 0.5%: 1700 mg.
From the stock solution, 5 mL aliquots were prepared according to the concentrations at use, being as follows: 0.10 mg/mL, 0.25 mg/mL, 0.5 mg/mL, and 1.0 mg/mL.
2.4. Cell Lines and Cell Culture
The cells lines were purchased from ATCC. Human keratinocyte transformed and immortalized HaCaT cells were maintained in RPMI supplemented with 10% of SFB and 1% of AA. This cell line was used as control for this study. Human TN BC cells HCC-70 were maintained in RPMI-1640 supplemented with 10% of SFB and 1% of AA. The cell cultures were kept in an incubator under physiological conditions (37oC, 95% humidity and 5% CO2 saturation). All cell were cultures and received the necessary culture media changes until reach a confluence of 100%.
Then cells were recovered by trypsinization technique (1–2 mL of trypsin in the culture flask without culture medium and incubate for 5 min), trypsin was inactivated with culture medium and the cells were centrifuged (1500 RPM for 5 min) to obtain a pellet. This was resuspended with 1 mL of culture medium.
2.4.1. Cell Viability Assay and Cell Count
The cell viability was evaluated through TB assay, and the cell count was performed with a Neubauer chamber. TB assay is based on the principle that live cells possess intact cell membranes that exclude certain dyes, such as trypan blue. 1 μL of cells was mixed with 9 μL of TB to create a 1:10 dilution, and this was placed in the Neubauer chamber. Viable cells were counted when the chamber was analyzed in the microscope [15].
2.4.2. Cytotoxicity Test (MTT Test)
The MTT assay is based on the capability of viable cells to convert this yellow water-soluble tetrazolium salt into insoluble purple formazan crystals by cleavage of the tetrazolium ring by dehydrogenase enzymes. This water insoluble product can be solubilized using organic solvents and the resulting colored solutions are spectrophotometrically measured, getting an absorbance directly proportional to the percentage of viable cells. Cells were seeded in triplicate in 96-well plates at a density of 1 × 104 cells/well. After 24 h of culture, cells were treated with or without increasing concentrations of HSE (culture medium was used for untreated groups) and incubated for 24 h. Then MTT (5 mg/1 mL PBS) was added to the cells, incubating for 18–24 h. The formazan crystals were dissolved with EB [16]. The absorbance was measured at a wavelength of 570 nm.
2.5. Statistical Analysis
Results were expressed as means ± SD. An ANOVA was performed using IBM SPSS Statistics 24, and a p value of <0.05 was considered statistically significant.
3. Results and Discussion
3.1. Cell Viability Assay
Figure 1A,B show the stock of HSE and the concentration the (0.10 mg/mL, 0.25 mg/mL, 0.5 mg/mL, and 1.0 mg/mL) used to measure the viability.
Figure 1.
(A) Photographs of stock solution of HSE and (B) dilution of the HSE stock solution in culture medium to obtain the desired concentrations respectively (0.10 mg/mL, 0.25 mg/mL, 0.5 mg/mL, and 1.0 mg/mL).
A total of 90% of cell confluence was required to perform the trypsinization process (Figure 2A) and then make cell count with the trypane blue method (Figure 2B).
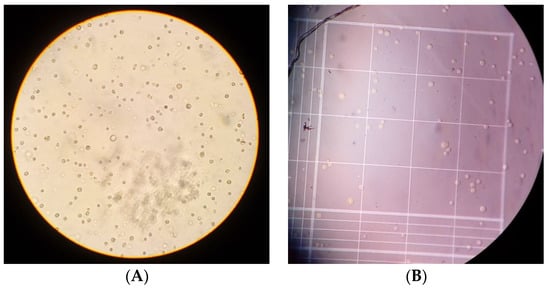
Figure 2.
Cell line BC HCC-70. (A) Cell culture after the recovery process by trypsinization, a more rounded shape is observed in cultured cells after the adherent cell recovery process; these were observed through an inverted microscope. (B) Viable cells appear white and those that are not are stained blue, practically no non-viable cells can be seen; the photograph of Neubauer’s chamber was taken by observing it under a microscope.
3.2. Cytotoxicity
The cytotoxicity of H.S on BC HCC70 cell line was determined through the MTT assay, using different concentrations of the lyophilized extract (0.10 µg/mL, 0.25 µg/mL, 0.5 µg/mL, and 1.0 µg/mL) during 24 h. After exposure to the extract, cell viability was reduced only by 1.0 µg/mL (Figure 1); however, there was no significant difference p > 0.001 (Figure 3).
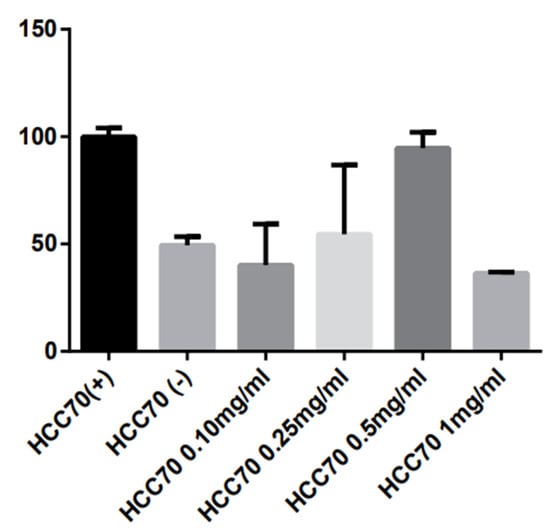
Figure 3.
Cells of the HCC-70 cell line corresponding to TN BC exposed to different concentrations of H.S. (HCC70 0.10 mg/mL, HCC70 0.25 mg/mL, HCC70 0.5 mg/mL and HCC70 1.0 mg/mL. As negative control (HCC70−), we used etoposide and in the positive control (HCC70+) no H.S. was added.
Similar studies related to the cytotoxicity of H.S. against BC have been reported, one of them was for Shahnaz Khaghani et al. in 2011, where they evaluated a water-soluble extract of HS in the BC cell line (MCF-7) at the concentration of 0.5 mg/mL. This study suggests that H.S. inhibits cell proliferation of MCF-7 line selectively in a concentration and time dependent, observing that after exposure for 72 h. cell viability was reduced by 50% [17]. Moreover, a recent study carried out by Ahirwar Bharti et al. in 2020 reported that the aqueous extract of HS reduced the growth of tumor cells from the MCF-7 breast cancer cell line by 60% at a dose of 80 mg/mL. It also significantly reduced angiogenesis (15 ± 0.7%) (p < 0.05) and inhibited metastasis compared to the control group [18].
There is a wide variety of studies related to HS and its cytotoxic activity in different types of cancers with significant results, Chung-Tang et al. 2005, observed a significant relationship between dose increase and decrease in viability with the lyophilized HS extract on human prostate cancer cells; in which an inhibitory concentration (IC50) of 3 mg/mL was reported [19]. Another study on human cervical carcinoma cells (HeLa line) reported a 14% decrease in cell proliferation rate when exposed to a 5 mg/mL concentration of lyophilized H.S. extract [20].
From this, we can emphasize that the H.S. has a great number of therapeutic characteristics, among which we can highlight the antitumor and cytotoxic effects. In our study, the H.S. extract showed greater cytotoxic effect only at the highest concentration (1.0 mg/mL); from this, it can be argued that, perhaps at lower concentrations, carbohydrates present in the extract serve as energy for tumor growth; in contrast, the higher concentration of H.S. extract higher number of anthocyanins, which allows for a desired cytotoxic effect. It is in our interest to test higher doses on the BC cell line HCC-70., as more research is needed to determine a concentration at which a reduction in cell viability can be seen with greater impact.
Acknowledgments
The authors thank Universidad de Guadalajara for providing the facilities to carry out this study.
References
- Meza-Junco, J.; Montaño-Loza, A.; Aguayo-González, Á. Bases moleculares del cáncer. Rev. Investig. Clin. 2006, 58, 56–70. [Google Scholar]
- Vallejo-Zamudio, E.; Rojas-Velázquez, A.; Torres-Bugarín, O.; Torres Bugarín, O.; Una Poderosa Herramienta en la Medicina Preventiva del Cáncer: Los Antioxidantes. El Resid. 2017, p. 104. Available online: www.medigraphic.org.mx (accessed on 10 October 2020).
- Aldaco-Sarvide, F.; Pérez-Pérez, P.; Cervantes-Sánchez, G.; Torrecillas-Torres, L.; Erazo-Valle-Solís, A.A.; Cabrera-Galeana, P.; Motola-Kuba, D.; Anaya, P.; Rivera-Rivera, S.; Cárdenas-Cárdenas, E.; et al. Mortality from cancer in Mexico: 2015 update. Gac. Mex. Oncol. 2018, 17, 28–34. [Google Scholar] [CrossRef]
- IARC. New Global Cancer Data: GLOBOCAN 2018|UICC. International Agency for Research on Cancer. 2018. Available online: https://www.uicc.org/new-global-cancer-data-globocan-2018 (accessed on 2 October 2020).
- OPS/OMS|Cáncer de Mama. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=5041:2011-breast-cancer&Itemid=3639&lang=es (accessed on 7 October 2020).
- Perou, C.M. Molecular Stratification of Triple-Negative Breast Cancers. Available online: www.TheOncologist.com (accessed on 8 October 2020).
- Zaharia, M.; Gómez, H. Cáncer de mama triple negativo: Una enfermedad de difícil diagnóstico y tratamiento. Rev. Peru. Med. Exp. Salud Publica 2014, 30, 2–8. [Google Scholar] [CrossRef]
- OMS. Nuevas Directrices de la OMS para Fomentar el uso Adecuado de las Medicinas Tradicionales; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- García, B.; Alberto Saldaña, L.S.; El Estrés Oxidativo y los Antioxidantes en la Prevención del Cáncer. Revista Habanera de Cienicas Médicas. 2013. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1729-519X2013000200005 (accessed on 7 October 2020).
- Tsuda, H.; Ohshima, Y.; Nomoto, H.; Fujita, K.; Matsuda, E.; Iigo, M.; Takasuka, N.; Malcolm, A. Cancer Prevention by Natural Compounds. Drug Metab. Pharmacokinet. 2004, 19, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, J.; Wang, C. Chemopreventive Properties and Molecular Mechanisms of the Bioactive Compounds in Hibiscus sabdariffa Linne. Curr. Med. Chem. 2011, 18, 1245–1254. [Google Scholar] [CrossRef]
- Chiu, C.T.; Hsuan, S.W.; Lin, H.H.; Hsu, C.C.; Chou, F.P.; Chen, J.H. Hibiscus sabdariffa Leaf polyphenolic extract induces human melanoma cell death, apoptosis, and autophagy. J. Food Sci. 2015, 80, H649–H658. [Google Scholar] [CrossRef]
- Lin, H.H.; Chan, K.C.; Sheu, J.Y.; Hsuan, S.W.; Wang, C.J.; Chen, J.H. Hibiscus sabdariffa leaf induces apoptosis of human prostate cancer cells in vitro and in vivo. Food Chem. 2012, 132, 880–891. [Google Scholar] [CrossRef]
- Wu, C.; Huang, C.; Hung, C.; Yao, F.; Wang, C.; Chang, Y. Delphinidin-rich extracts of Hibiscus sabdariffa L. trigger mitochondria-derived autophagy and necrosis through reactive oxygen species in human breast cancer cells. J. Funct. Foods 2016, 25, 279–290. [Google Scholar] [CrossRef]
- Cell Viability and Proliferation Assays|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biofiles/cell-viability-and-proliferation.html (accessed on 13 October 2020).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Khaghani, S.; Razi, F.; Yajloo, M.M.; Paknejad, M.; Shariftabrizi, A.; Pasalar, P. Selective Cytotoxicity and Apoptogenic Activity of Hibiscus sabdariffa Aqueous Extract Against MCF-7 Human Breast Cancer Cell Line. J. Cancer Ther. 2011, 2, 394–400. [Google Scholar] [CrossRef]
- Ahirwar, B.; Ahirwar, D. In vivo and in vitro investigation of cytotoxic and antitumor activities of polyphenolic leaf extract of Hibiscus sabdariffa against breast cancer cell lines. Res. J. Pharm. Technol. 2020, 13, 615–620. [Google Scholar] [CrossRef]
- Chiu, C.T.; Chen, J.H.; Chou, F.P.; Lin, H.H. Hibiscus sabdariffa leaf extract inhibits human prostate cancer cell invasion via down-regulation of Akt/NF-κB/MMP-9 pathway. Nutrients 2015, 7, 5065–5087. [Google Scholar] [CrossRef] [PubMed]
- Olvera-García, V.; Castaño-Tostado, E.; Rezendiz-Lopez, R.I.; Reynoso-Camacho, R.; González de Mejía, E.; Elizondo, G.; Loarca-Piña, G. Hibiscus sabdariffa L. extracts inhibit the mutagenicity in microsuspension assay and the proliferation of HeLa cells. J. Food Sci. 2008, 73, T75–T81. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).