Angiogenesis in the Avian Embryo Chorioallantoic Membrane: A Perspective on Research Trends and a Case Study on Toxicant Vascular Effects
Abstract
1. Introduction
“In the case of the hen, the first signs of the embryo are seen after three days and nights …the heart is no bigger than just a small blood-spot in the white …and beats and moves as though it were alive; and from it, as it grows, two vein-like vessels with blood in them lead on a twisted course to each of the two surrounding membranes. A membrane with bloody fibers already surrounds the white of the egg, at this time coming from the vessel-like channels.”Aristotle, Historia Animalium.
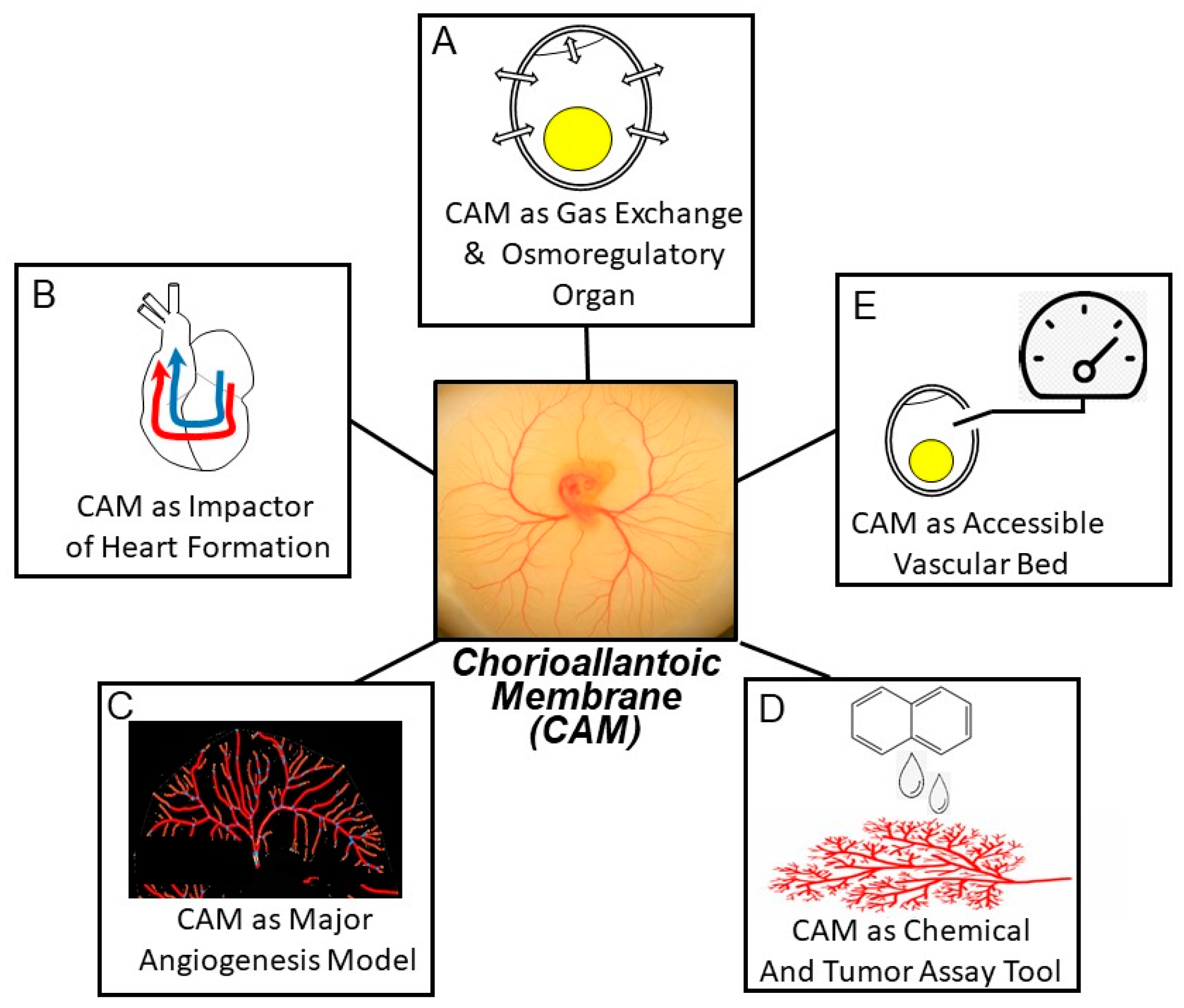
2. Contemporary Themes in Chorioallantoic Membrane Investigation
2.1. The CAM as an Intrinsically Interesting Gas Exchange and Osmoregulation Organ
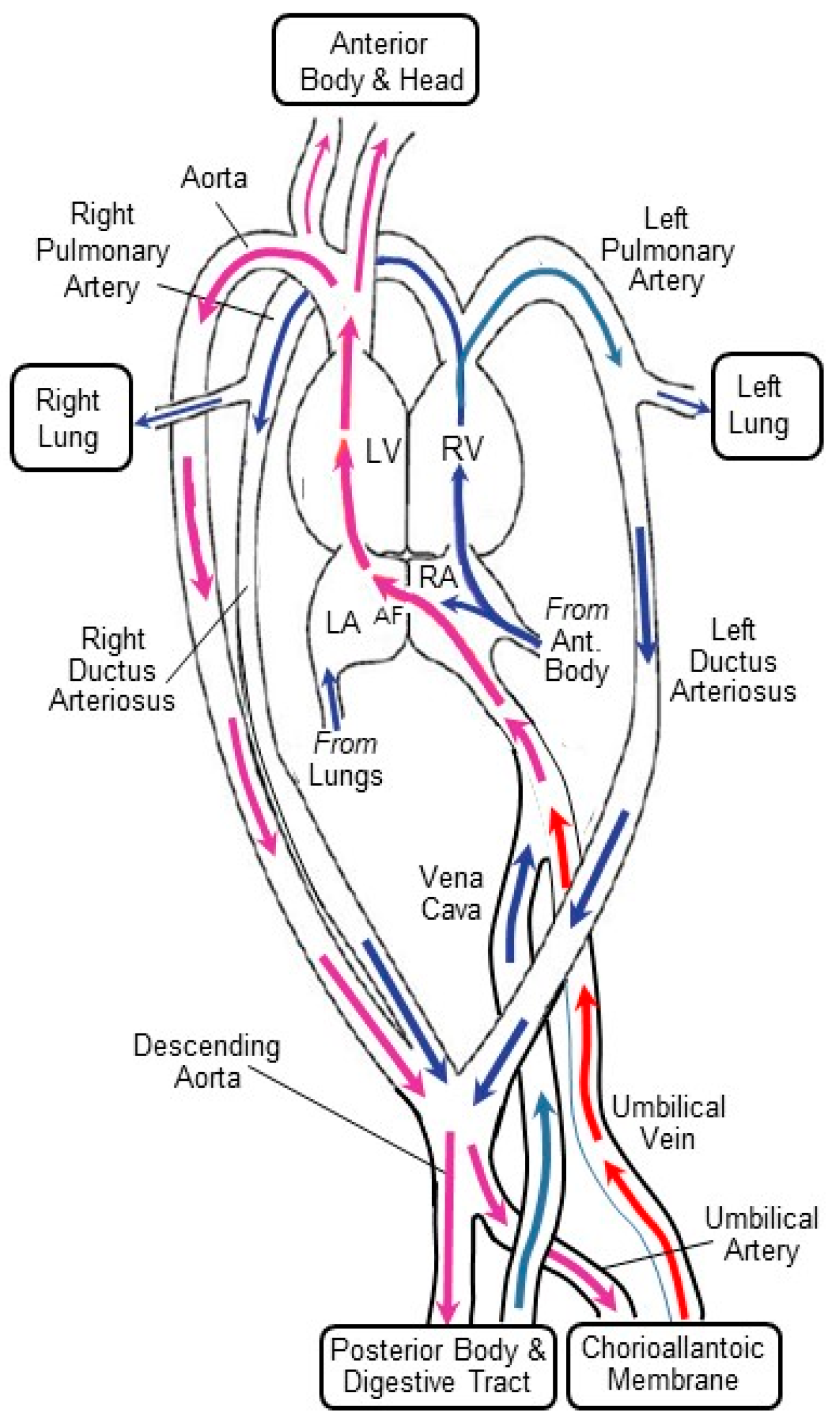
2.2. The CAM’s Hemodynamic Influence on the Developing Heart and Central Vessels
2.2.1. Preload
2.2.2. Afterload
2.2.3. Shear Stress
2.3. The CAM as Model Vascular Bed for Studying Angiogenesis
2.4. The CAM as an Assay Tool
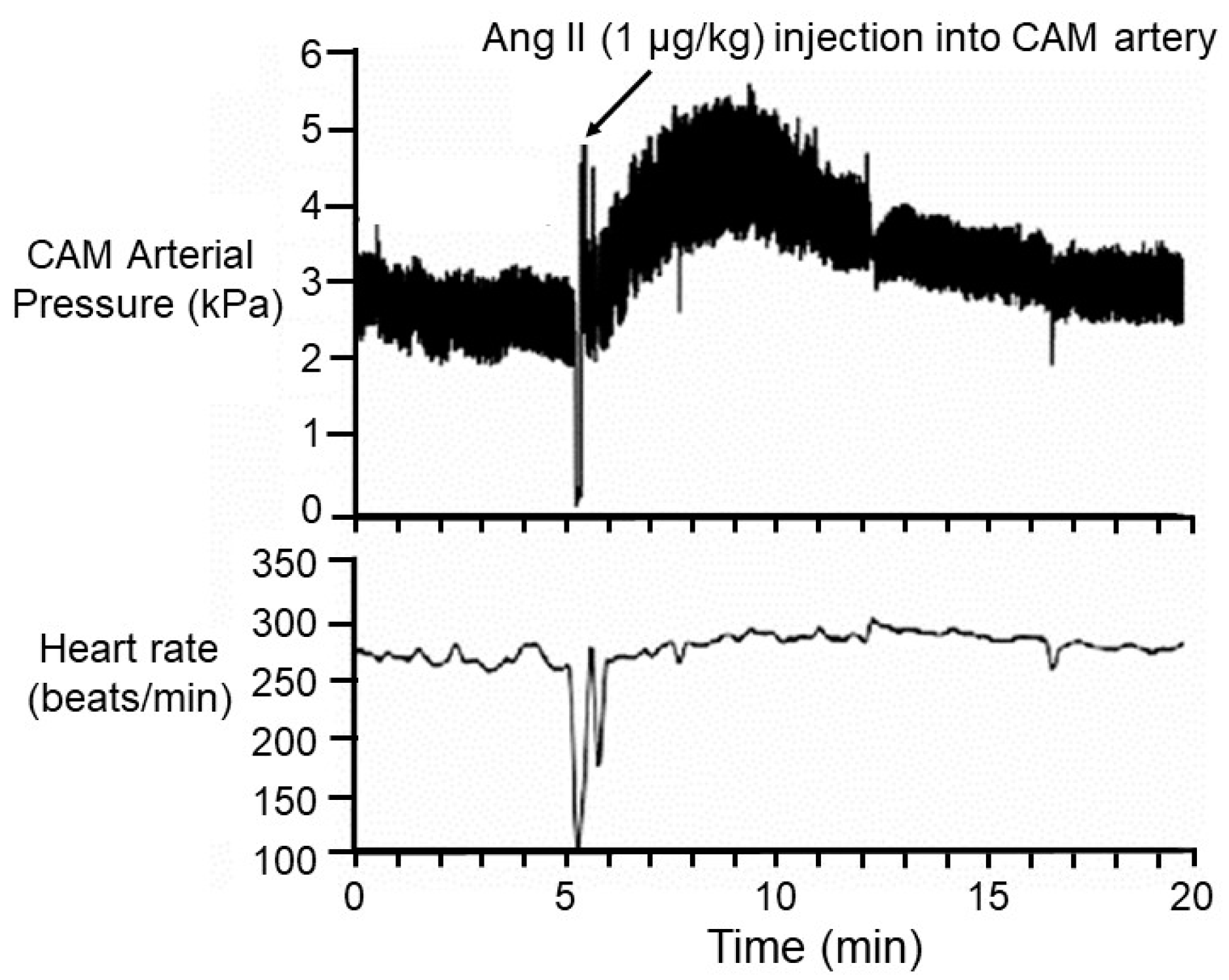
2.5. The CAM as an Accessible Vascular Bed
2.6. Untested Assumptions Regarding the CAM Vasculature
3. A Case Study in Vasculature Analysis of the Chicken CAM
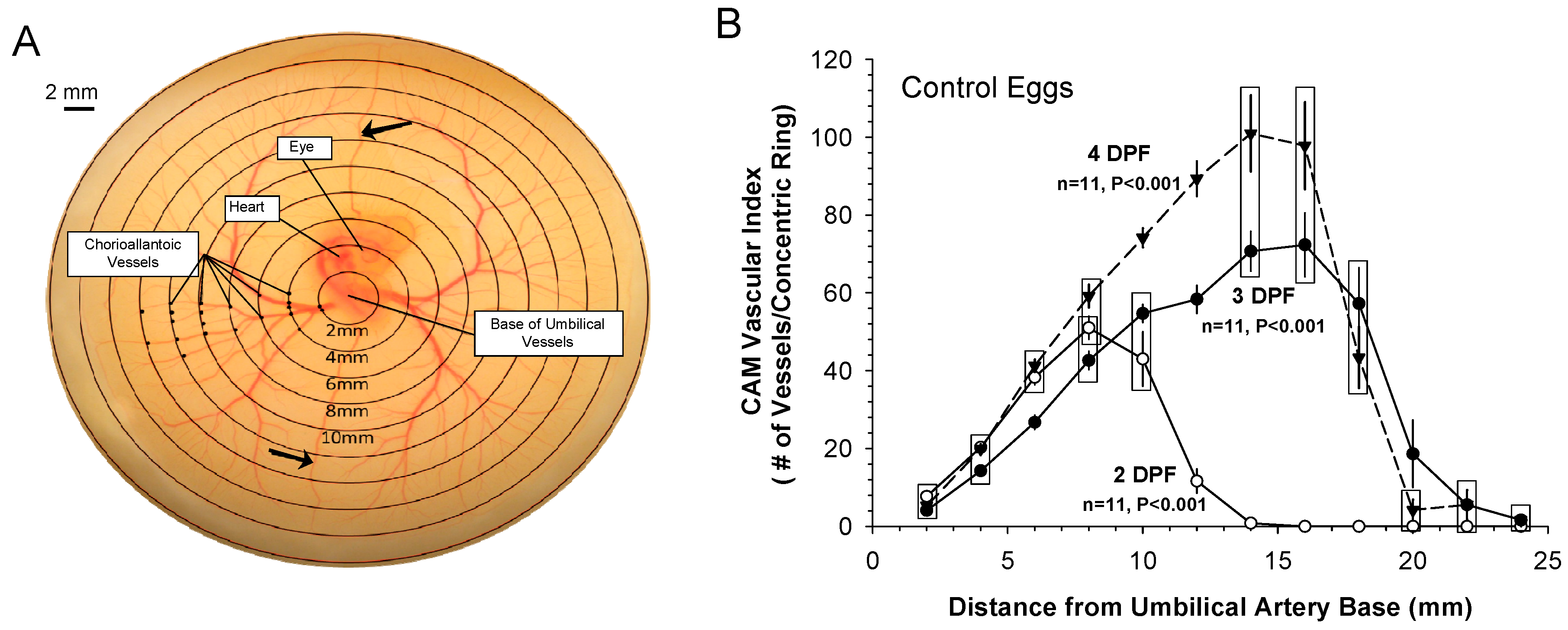
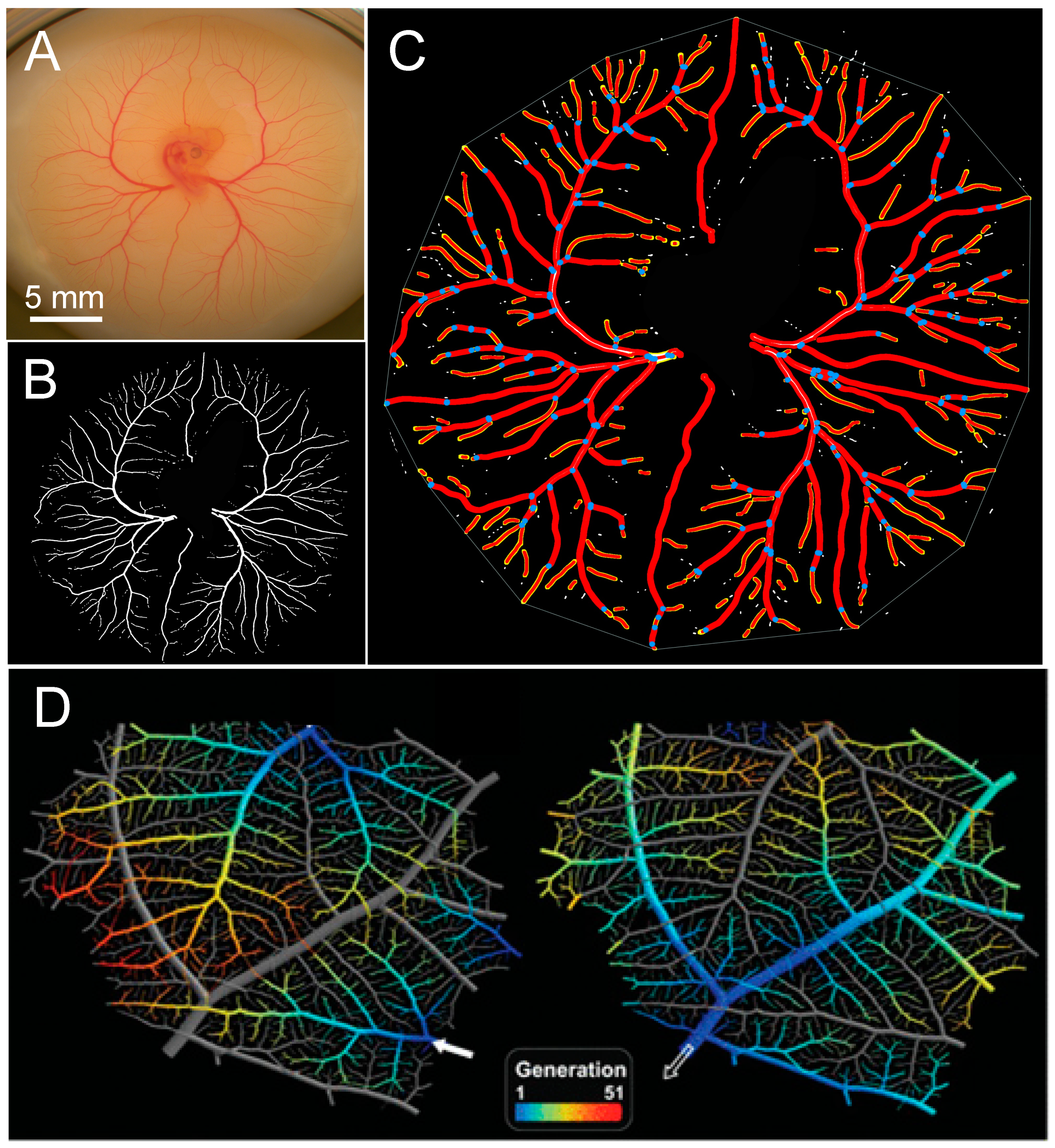
3.1. Patterns of Normal Vasculature Growth
3.2. Vascularity and Cardiac Perfusion
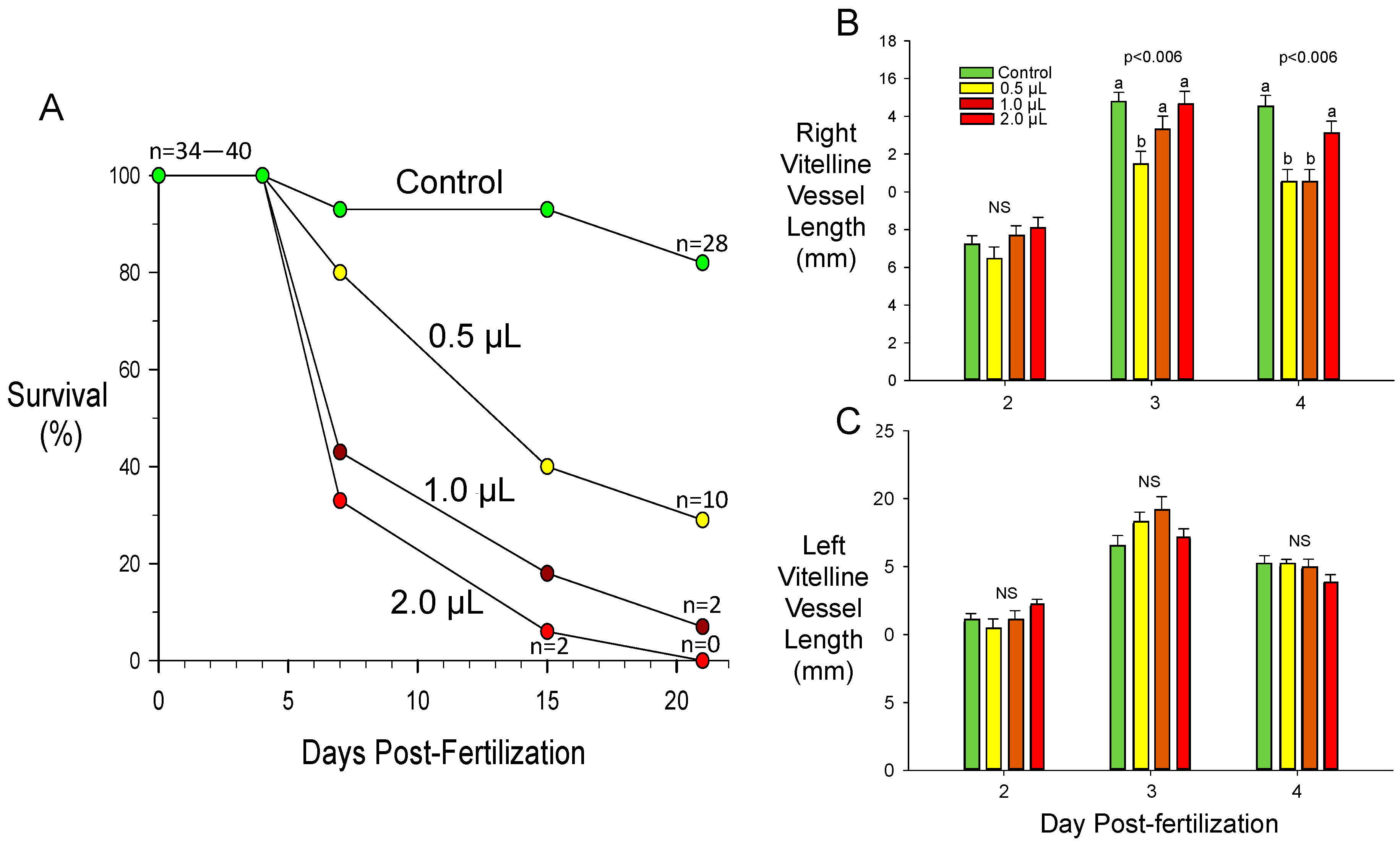
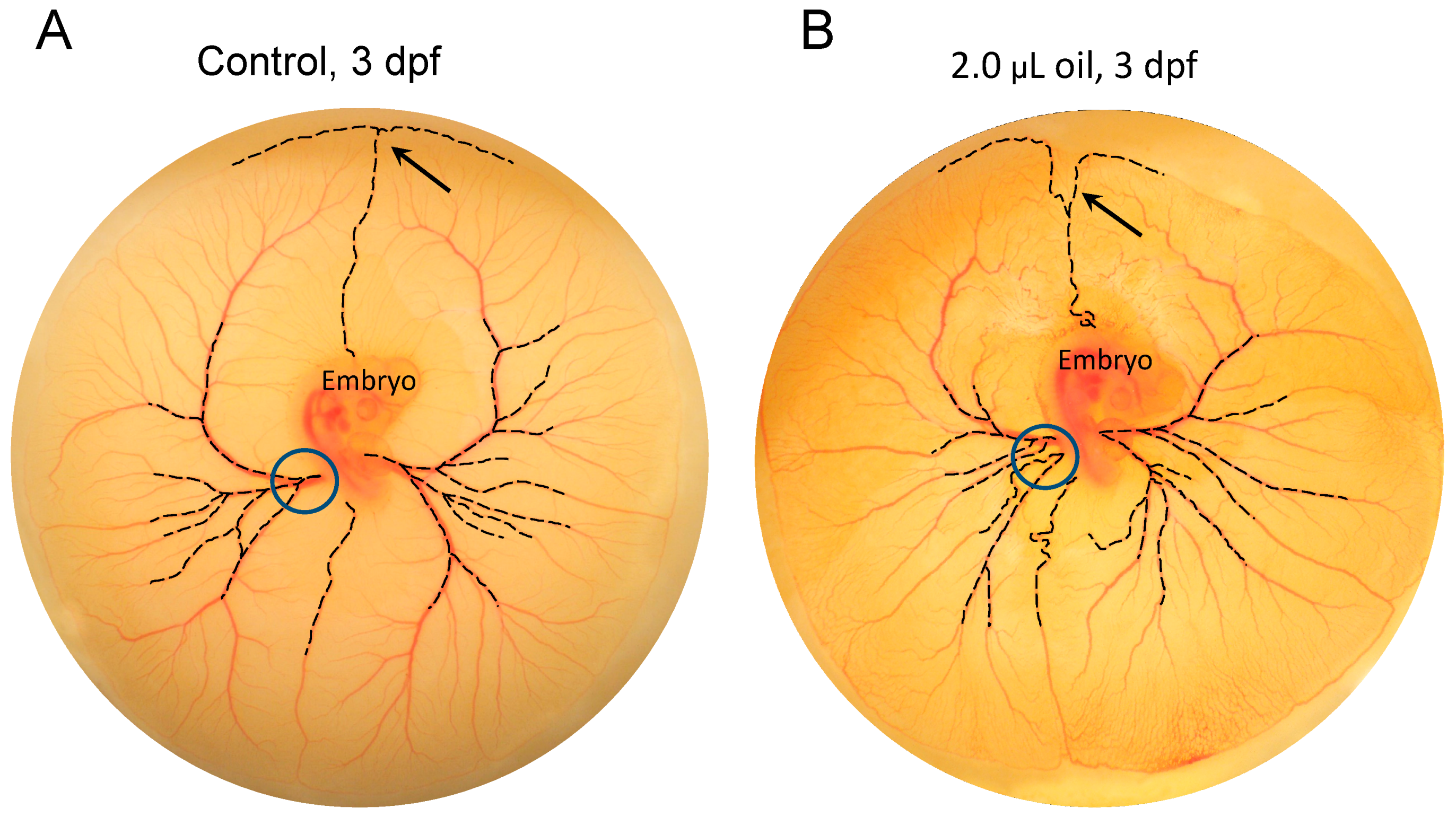
3.3. Toxicant-Induced Alterations of CAM Vascular Growth
3.4. Assessing Difference between Vascular Density vs. Vascular Pattern
4. Unanswered Questions and Future Directions
- (1)
- How can we quantify subtle differences in patterns of angiogenesis in the CAM, and do they have any biological significance? Reproducible changes in the pattern of CAM vasculature can signal changes in the local chemical environment regulating the growth and branching of CAM vasculature, and should be investigated in more detail alongside absolute levels of vascularization.
- (2)
- How does the amount of vascularization of the CAM compare to the amount of vasculature in the embryo per se (measured by length, numbers, cross-sectional area, or other metrics) as embryonic development progresses? Knowing this information will help cardiovascular physiologists better interpret changes in blood pressure measured in major CAM arteries, for example. Leading on from this question, we have the following:
- (3)
- Is the CAM a representative vasculature bed of the embryo, overall? The CAM is a venerable model for angiogenesis, but it is also a highly specialized vasculature bed. Experiments that compare the compliance, pharmacology, and other characteristics of the CAM to, for example, a typical systemic vascular bed will be important to verify our findings from the CAM, especially in physiological studies.
- (4)
- Is the chicken embryo’s CAM broadly representative of birds? Certainly, the CAM of ducks [116], geese [117], quail [118], and other birds has been investigated, often from a morphological perspective. However, to our knowledge, a comparative study of the morphology, physiology, and molecular biology of the avian CAM has not been completed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bjornstad, S.; Austdal, L.P.; Roald, B.; Glover, J.C.; Paulsen, R.E. Cracking the Egg: Potential of the Developing Chicken as a Model System for Nonclinical Safety Studies of Pharmaceuticals. J. Pharmacol. Exp. Ther. 2015, 355, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.G.; Accili, D. The chick chorioallantoic membrane: A model of molecular, structural, and functional adaptation to transepithelial ion transport and barrier function during embryonic development. J. Biomed. Technol. 2010, 2010, 940741. [Google Scholar] [CrossRef] [PubMed]
- Jedelska, J.; Strehlow, B.; Bakowsky, U.; Aigner, A.; Hobel, S.; Bette, M.; Roessler, M.; Franke, N.; Teymoortash, A.; Werner, J.A.; et al. The chorioallantoic membrane assay is a promising ex vivo model system for the study of vascular anomalies. In Vivo 2013, 27, 701–705. [Google Scholar] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Burggren, W.W.; Flores Santin, J.; Rojas, M. Cardio-respiratory development in bird embryos: New insights from a venerable animal model. Rev. Bras. Zootec. 2016, 45, 709–728. [Google Scholar] [CrossRef]
- Burggren, W.W.; Keller, B.B. Development of Cardiovascular Systems; Cambridge University Press: Cambridge, UK, 1997; p. 360. [Google Scholar]
- Mueller, C.A.; Burggren, W.W.; Tazawa, H. The Physiology of the Avian Embryo. In Sturkie’s Avian Physiology, 6th ed.; Whittow, G.C., Ed.; Elsevier: New York, NY, USA, 2015; pp. 739–766. [Google Scholar]
- Rahn, H.; Wangensteen, O.D.; Farhi, L.E. Convection and diffusion gas exchange in air or water. Respir. Physiol. 1971, 12, 1–6. [Google Scholar] [CrossRef]
- Wangensteen, O.D.; Rahn, H. Respiratory gas exchange by the avian embryo. Respir. Physiol. 1970, 11, 31–45. [Google Scholar] [CrossRef]
- Piiper, J.; Tazawa, H.; Ar, A.; Rahn, H. Analysis of chorioallantoic gas exchange in the chick embryo. Respir. Physiol. 1980, 39, 273–284. [Google Scholar] [CrossRef]
- Tazawa, H.; Visschedijk, A.H.; Wittmann, J.; Piiper, J. Gas exchange, blood gases and acid-base status in the chick before, during and after hatching. Respir. Physiol. 1983, 53, 173–185. [Google Scholar] [CrossRef]
- Tazawa, H.; Ar, A.; Rahn, H.; Piiper, J. Repetitive and simultaneous sampling from the air cell and blood vessels in the chick embryo. Respir. Physiol. 1980, 39, 265–272. [Google Scholar] [CrossRef]
- Dzialowski, E.M. Comparative physiology of the ductus arteriosus among vertebrates. Semin. Perinatol. 2018, 42, 203–211. [Google Scholar] [CrossRef]
- White, P.T. Experimental studies on the circulatory system of the late chick embryo. J. Exp. Biol. 1974, 61, 571–592. [Google Scholar] [PubMed]
- Burggren, W.W.; Filigonio, R.; Wang, T. Cardiovascular shunting in vertebrates: A practical integration of competing hypotheses. Biol. Rev. Camb. Philos. Soc. 2019, 95, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; DeFouw, D.O. Microvascular permselectivity in the chick chorioallantoic membrane during endothelial cell senescence. Int. J. Microcirc. Clin. Exp. 1997, 17, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.N.; Dimova, I.; Koller, T.; Styp-Rekowska, B.; Djonov, V. Dynamics of the Developing Chick Chorioallantoic Membrane Assessed by Stereology, Allometry, Immunohistochemistry and Molecular Analysis. PLoS ONE 2016, 11, e0152821. [Google Scholar] [CrossRef]
- Davis, T.A.; Shen, S.S.; Ackerman, R.A. Embryonic osmoregulation: Consequences of high and low water loss during incubation of the chicken egg. J. Exp. Zool. 1988, 245, 144–156. [Google Scholar] [CrossRef]
- Garrison, J.C.; Terepka, A.R. The interrelationships between sodium ion, calcium transport and oxygen utilization in the isolated chick chorioallantoic membrane. J. Membr. Biol. 1972, 7, 146–163. [Google Scholar] [CrossRef]
- Moriarty, C.M.; Hogben, C.A. Active Na plus and Cl minus transport by the isolation chick chorioallantoic membrane. Biochim. Biophys. Acta 1970, 219, 463–470. [Google Scholar] [CrossRef]
- Graves, J.S.; Dunn, B.E.; Brown, S.C. Embryonic chick allantois: Functional isolation and development of sodium transport. Am. J. Physiol. 1986, 251, C787–C794. [Google Scholar] [CrossRef]
- Bolin, G.; Burggren, W.W. Metanephric kidney development in the chicken embryo: Glomerular numbers, characteristics and perfusion. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 343–350. [Google Scholar] [CrossRef]
- Bolin, G.; Dubansky, B.; Burggren, W.W. Incubation relative humidity induces renal morphological and physiological remodeling in the embryo of the chicken (Gallus gallus domesticus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 204, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Shell, L.; Burggren, W.; Muirhead, D.; Nelson, T.C.; Dzialowski, E.M. Circulatory changes associated with the closure of the ductus arteriosus in hatching emu (Dromaius novaehollandiae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Keller, B. Embryonic cardiovascular function, coupling and maturation: A species view. In Development of Cardiovascular Systems; Burggren, W., Keller, B., Eds.; Cambridge University Press: Cambridge, UK, 1998; Volume 43–56. [Google Scholar]
- Midgett, M.; Goenezen, S.; Rugonyi, S. Blood flow dynamics reflect degree of outflow tract banding in Hamburger-Hamilton stage 18 chicken embryos. J. R. Soc. Interface 2014, 11, 20140643. [Google Scholar] [CrossRef] [PubMed]
- Midgett, M.; Rugonyi, S. Congenital heart malformations induced by hemodynamic altering surgical interventions. Front. Physiol. 2014, 5, 287. [Google Scholar] [CrossRef]
- Stovall, S.; Midgett, M.; Thornburg, K.; Rugonyi, S. Changes in dynamic embryonic heart wall motion in response to outflow tract banding measured using video densitometry. J. Biomed. Opt. 2016, 21, 116003. [Google Scholar] [CrossRef]
- Midgett, M.; López, C.S.; David, L.; Maloyan, A.; Rugonyi, S. Increased Hemodynamic Load in Early Embryonic Stages Alters Endocardial to Mesenchymal Transition. Front. Physiol. 2017, 8, 56. [Google Scholar] [CrossRef]
- Broekhuizen, M.L.; Hogers, B.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; Wladimiroff, J.W. Altered hemodynamics in chick embryos after extraembryonic venous obstruction. Ultrasound Obstet. Gynecol. 1999, 13, 437–445. [Google Scholar] [CrossRef]
- Ursem, N.T.; Stekelenburg-de Vos, S.; Wladimiroff, J.W.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; Hu, N.; Clark, E.B. Ventricular diastolic filling characteristics in stage-24 chick embryos after extra-embryonic venous obstruction. J. Exp. Biol. 2004, 207, 1487–1490. [Google Scholar] [CrossRef][Green Version]
- Stekelenburg-de Vos, S.; Ursem, N.T.; Hop, W.C.; Wladimiroff, J.W.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Acutely altered hemodynamics following venous obstruction in the early chick embryo. J. Exp. Biol. 2003, 206, 1051–1057. [Google Scholar] [CrossRef]
- Hogers, B.; DeRuiter, M.C.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ. Res. 1997, 80, 473–481. [Google Scholar] [CrossRef]
- Hogers, B.; DeRuiter, M.C.; Baasten, A.M.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Intracardiac blood flow patterns related to the yolk sac circulation of the chick embryo. Circ. Res. 1995, 76, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.B.; Kowalski, W.J.; Tinney, J.P.; Tobita, K.; Hu, N. Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2020, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Bowers, P.N.; Tinney, J.P.; Keller, B.B. Nitroprusside selectively reduces ventricular preload in the stage 21 chick embryo. Cardiovasc. Res. 1996, 31, E132–E138. [Google Scholar] [CrossRef]
- Sharma, S.K.; Lucitti, J.L.; Nordman, C.; Tinney, J.P.; Tobita, K.; Keller, B.B. Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr. Res. 2006, 59, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Druyan, S.; Levi, E. Reduced O2 concentration during CAM development—Its effect on angiogenesis and gene expression in the broiler embryo CAM. Gene Expr. Patterns 2012, 12, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Druyan, S.; Levi, E.; Shinder, D.; Stern, T. Reduced O2 concentration during CAM development--its effect on physiological parameters of broiler embryos. Poult. Sci. 2012, 91, 987–997. [Google Scholar] [CrossRef]
- Strick, D.M.; Waycaster, R.L.; Montani, J.P.; Gay, W.J.; Adair, T.H. Morphometric measurements of chorioallantoic membrane vascularity: Effects of hypoxia and hyperoxia. Am. J. Physiol. 1991, 260, H1385–H1389. [Google Scholar] [CrossRef]
- Noiman, T.; Buzhor, E.; Metsuyanim, S.; Harari-Steinberg, O.; Morgenshtern, C.; Dekel, B.; Goldstein, R.S. A rapid in vivo assay system for analyzing the organogenetic capacity of human kidney cells. Organogenesis 2011, 7, 140–144. [Google Scholar] [CrossRef]
- Burggren, W.W.; Crossley, D.A. Comparative cardiovascular development: Improving the conceptual framework. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 661–674. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Rychterova, V.; Hu, N.; Clark, E.B. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat. Rec. 1999, 254, 238–252. [Google Scholar] [CrossRef]
- Perdios, C.; Parnall, M.; Pang, K.L.; Loughna, S. Altered haemodynamics causes aberrations in the epicardium. J. Anat. 2019, 234, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Tobita, K.; Schroder, E.A.; Tinney, J.P.; Garrison, J.B.; Keller, B.B. Regional passive ventricular stress-strain relations during development of altered loads in chick embryo. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2386–H2396. [Google Scholar] [CrossRef] [PubMed]
- Buffinton, C.M.; Faas, D.; Sedmera, D. Stress and strain adaptation in load-dependent remodeling of the embryonic left ventricle. Biomech. Model. Mechanobiol. 2013, 12, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.B.; Yoshigi, M.; Tinney, J.P. Ventricular-vascular uncoupling by acute conotruncal occlusion in the stage 21 chick embryo. Am. J. Physiol. 1997, 273, H2861–H2866. [Google Scholar] [CrossRef] [PubMed]
- Tobita, K.; Keller, B.B. End-systolic myocardial stiffness is a load-independent index of contractility in stage 24 chick embryonic heart. Am. J. Physiol. 1999, 276, H2102–H2108. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.W., Jr.; Hughes, S.F.; Hu, N.; Clark, E.B. Effect of heart rate increase on dorsal aortic flow before and after volume loading in the stage 24 chick embryo. Pediatr. Res. 1989, 26, 438–441. [Google Scholar] [CrossRef]
- Wagman, A.J.; Hu, N.; Clark, E.B. Effect of changes in circulating blood volume on cardiac output and arterial and ventricular blood pressure in the stage 18, 24, and 29 chick embryo. Circ. Res. 1990, 67, 187–192. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Hierck, B.P.; Vrolijk, J.; Baiker, M.; Pourquie, M.J.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ. Res. 2005, 96, 1291–1298. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Hierck, B.P.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev. Dyn. 2004, 230, 57–68. [Google Scholar] [CrossRef]
- Kowalski, W.J.; Teslovich, N.C.; Menon, P.G.; Tinney, J.P.; Keller, B.B.; Pekkan, K. Left atrial ligation alters intracardiac flow patterns and the biomechanical landscape in the chick embryo. Dev. Dyn. 2014, 243, 652–662. [Google Scholar] [CrossRef]
- Hierck, B.P.; van der Heiden, K.; DeRuiter, M.C.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Fluid shear stress controls cardiovascular development. A functionomic approach. Wien. Klin. Wochenschr. 2007, 119, 10–13. [Google Scholar] [PubMed]
- Yalcin, H.C.; Shekhar, A.; McQuinn, T.C.; Butcher, J.T. Hemodynamic patterning of the avian atrioventricular valve. Dev. Dyn. 2011, 240, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M.G.; Taber, L.A.; Wagenseil, J.E. Reduced embryonic blood flow impacts extracellular matrix deposition in the maturing aorta. Dev. Dyn. 2018, 247, 914–923. [Google Scholar] [CrossRef]
- Celik, M.; Goktas, S.; Karakaya, C.; Cakiroglu, A.I.; Karahuseyinoglu, S.; Lashkarinia, S.S.; Ermek, E.; Pekkan, K. Microstructure of early embryonic aortic arch and its reversibility following mechanically altered hemodynamic load release. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1208–H1218. [Google Scholar] [CrossRef] [PubMed]
- Midgett, M.; Chivukula, V.K.; Dorn, C.; Wallace, S.; Rugonyi, S. Blood flow through the embryonic heart outflow tract during cardiac looping in HH13-HH18 chicken embryos. J. R. Soc. Interface 2015, 12, 20150652. [Google Scholar] [CrossRef]
- Lindsey, S.E.; Menon, P.G.; Kowalski, W.J.; Shekhar, A.; Yalcin, H.C.; Nishimura, N.; Schaffer, C.B.; Butcher, J.T.; Pekkan, K. Growth and hemodynamics after early embryonic aortic arch occlusion. Biomech. Model. Mechanobiol. 2015, 14, 735–751. [Google Scholar] [CrossRef]
- Maibier, M.; Reglin, B.; Nitzsche, B.; Xiang, W.; Rong, W.W.; Hoffmann, B.; Djonov, V.; Secomb, T.W.; Pries, A.R. Structure and hemodynamics of vascular networks in the chorioallantoic membrane of the chicken. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H913–H926. [Google Scholar] [CrossRef]
- Xiang, W.; Reglin, B.; Nitzsche, B.; Maibier, M.; Rong, W.W.; Hoffmann, B.; Ruggeri, A.; Guimarães, P.; Secomb, T.W.; Pries, A.R. Dynamic remodeling of arteriolar collaterals after acute occlusion in chick chorioallantoic membrane. Microcirculation 2017, 24. [Google Scholar] [CrossRef]
- Le Noble, F.; Moyon, D.; Pardanaud, L.; Yuan, L.; Djonov, V.; Matthijsen, R.; Bréant, C.; Fleury, V.; Eichmann, A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 2004, 131, 361–375. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane in the study of tumor angiogenesis. Rom. J. Morphol. Embryol. 2008, 49, 131–135. [Google Scholar]
- Nowak-Sliwinska, P.; van Beijnum, J.R.; van Berkel, M.; van den Bergh, H.; Griffioen, A.W. Vascular regrowth following photodynamic therapy in the chicken embryo chorioallantoic membrane. Angiogenesis 2010, 13, 281–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamanoi, F. Recent excitements in the study of the CAM assay. Enzymes 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tufan, A.C.; Satiroglu-Tufan, N.L. The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr. Cancer Drug Targets 2005, 5, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.T.; Smith, C.F.; Burger, P.C.; Klintworth, G.K. An evaluation of methods to quantitate the chick chorioallantoic membrane assay in angiogenesis. Lab. Investig. 1985, 53, 499–508. [Google Scholar]
- Liu, M.; Xie, S.; Zhou, J. Use of animal models for the imaging and quantification of angiogenesis. Exp. Anim. 2018, 67, 1–6. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Leighton, J.; Nassauer, J.; Tchao, R. The chick embryo in toxicology: An alternative to the rabbit eye. Food Chem. Toxicol. 1985, 23, 293–298. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Victorelli, F.D.; Cardoso, V.M.O.; Ferreira, N.N.; Calixto, G.M.F.; Fontana, C.R.; Baltazar, F.; Gremião, M.P.D.; Chorilli, M. Chick embryo chorioallantoic membrane as a suitable in vivo model to evaluate drug delivery systems for cancer treatment: A review. Eur. J. Pharm. Biopharm. 2020, 153, 273–284. [Google Scholar] [CrossRef]
- Barile, F.A. Validating and troubleshooting ocular in vitro toxicology tests. J. Pharmacol. Toxicol. Methods 2010, 61, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Grace Intasa-Ard, S.; Birault, A. Nanoparticles characterization using the CAM assay. Enzymes 2019, 46, 129–160. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Fernandes, A.R.; Baptista, P.V. Counteracting the effect of leukemia exosomes by antiangiogenic gold nanoparticles. Int. J. Nanomed. 2019, 14, 6843–6854. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Tekrony, A.; Yaehne, K.; Cramb, D.T. Designing a better theranostic nanocarrier for cancer applications. Nanomedicine 2014, 9, 2371–2386. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.W.; Strano, M.S. Single walled carbon nanotubes as reporters for the optical detection of glucose. J. Diabetes Sci. Technol. 2009, 3, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R. The chick embryo chorioallantoic membrane as an in vivo experimental model to study multiple myeloma. Enzymes 2019, 46, 23–35. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef]
- Altimiras, J.; Crossley, D.A. Control of blood pressure mediated by baroreflex changes of heart rate in the chicken embryo (Gallus gallus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R980–R986. [Google Scholar] [CrossRef]
- Crossley, D.A.; Bagatto, B.P.; Dzialowski, E.M.; Burggren, W.W. Maturation of cardiovascular control mechanisms in the embryonic emu (Dromiceius novaehollandiae). J. Exp. Biol. 2003, 206, 2703–2710. [Google Scholar] [CrossRef]
- Crossley, D.A.; Jonker, S.S.; Hicks, J.W.; Thornburg, K.L. Maturation of the angiotensin II cardiovascular response in the embryonic White Leghorn chicken (Gallus gallus). J. Comp. Physiol. B 2010, 180, 1057–1065. [Google Scholar] [CrossRef]
- Lindgren, I.; Crossley, D.; Villamor, E.; Altimiras, J. Hypotension in the chronically hypoxic chicken embryo is related to the beta-adrenergic response of chorioallantoic and femoral arteries and not to bradycardia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1161–R1168. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.A.; Burggren, W.W.; Crossley, D.A. ANG II and baroreflex control of heart rate in embryonic chickens (Gallus gallus domesticus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R855–R863. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.A.; Crossley, D.A.; Burggren, W.W. The actions of the renin-angiotensin system on cardiovascular and osmoregulatory function in embryonic chickens (Gallus gallus domesticus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 178, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Crossley, D.A.; Burggren, W.W.; Altimiras, J. Cardiovascular regulation during hypoxia in embryos of the domestic chicken Gallus gallus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R219–R226. [Google Scholar] [CrossRef] [PubMed]
- Crossley, D.A.I.; Altimiras, J. Ontogeny of cholinergic and adrenergic cardiovascular regulation in the domestic chicken (Gallus gallus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1091–R1098. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.E.; Wilson, D.J. Early embryonic angiogenesis in the chick area vasculosa. J. Anat. 1993, 183 Pt 3, 579–585. [Google Scholar]
- Lindgren, I.; Zoer, B.; Altimiras, J.; Villamor, E. Reactivity of chicken chorioallantoic arteries, avian homologue of human fetoplacental arteries. J. Physiol. Pharmacol. 2010, 61, 619–628. [Google Scholar]
- Lorigo, M.; Mariana, M.; Feiteiro, J.; Cairrao, E. How is the human umbilical artery regulated? J. Obstet. Gynaecol. Res. 2018, 44, 1193–1201. [Google Scholar] [CrossRef]
- Corona, T.B.; Warburton, S.J. Regional hypoxia elicits regional changes in chorioallantoic membrane vascular density in alligator but not chicken embryos. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 57–61. [Google Scholar] [CrossRef]
- Höper, J.; Jahn, H. Influence of environmental oxygen concentration on growth and vascular density of the area vasculosa in chick embryos. Int. J. Microcirc. Clin. Exp. 1995, 15, 186–192. [Google Scholar] [CrossRef]
- Branum, S.R.; Yamada-Fisher, M.; Burggren, W. Reduced heart rate and cardiac output differentially affect angiogenesis, growth, and development in early chicken embryos (Gallus domesticus). Physiol. Biochem. Zool. 2013, 86, 370–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guidolin, D.; Tamma, R.; Tortorella, C.; Annese, T.; Ruggieri, S.; Marzullo, A.; Ribatti, D. Morphometric analysis of the branching of the vascular tree in the chick embryo area vasculosa. Microvasc. Res. 2020, 128, 103935. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W.W. Developing animals flout prominent assumptions of ecological physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W.W.; Warburton, S.J.; Slivkoff, M.D. Interruption of cardiac output does not affect short-term growth and metabolic rate in day 3 and 4 chick embryos. J. Exp. Biol. 2000, 203, 3831–3838. [Google Scholar] [PubMed]
- Hughes, M.C.; Zimmer, A.M.; Perry, S.F. The role of internal convection in respiratory gas transfer and aerobic metabolism in larval zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019. [Google Scholar] [CrossRef]
- Bagatto, B.; Pelster, B.; Burggren, W.W. Growth and metabolism of larval zebrafish: Effects of swim training. J. Exp. Biol. 2001, 204, 4335–4343. [Google Scholar]
- Burggren, W.W. Cardiovascular development and angiogenesis in the early vertebrate embryo. Cardiovasc. Eng. Technol. 2013, 4, 234–245. [Google Scholar] [CrossRef]
- Clément, R.; Mauroy, B.; Cornelissen, A.J.M. Tissue growth pressure drives early blood flow in the chicken yolk sac. Dev. Dyn. 2017, 246, 573–584. [Google Scholar] [CrossRef]
- Burggren, W.W.; Dubansky, B.; Roberts, A.; Alloy, M. Deepwater Horizon Oil Spill as a Case Study for Interdisciplinary Cooperation within Developmental Biology, Environmental Sciences and Physiology. World J. Eng. Technol. 2015, 3, 7–23. [Google Scholar] [CrossRef]
- Pasparakis, C.; Grosell, M.; Esbaugh, A.; Burggren, W.W. Physiological effects of DeepWater Horizon oil on fish. Comp. Physiol. Biochem. Part C Toxicol. Pharmacol. 2019, 224, 108558. [Google Scholar] [CrossRef]
- Dubansky, B.; Verbeck, G.; Mach, P.; Burggren, W. Methodology for exposing avian embryos to quantified levels of airborne aromatic compounds associated with crude oil spills. Environ. Toxicol. Pharmacol. 2018, 58, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Franci, C.D.; Aleksieva, A.; Boulanger, E.; Brandenburg, J.; Johnston, T.; Malinova, A.; Head, J.A. Potency of polycyclic aromatic hydrocarbons in chicken and Japanese quail embryos. Environ. Toxicol. Chem. 2018, 37, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Finch, B.E.; Wooten, K.J.; Faust, D.R.; Smith, P.N. Embryotoxicity of mixtures of weathered crude oil collected from the Gulf of Mexico and Corexit 9500 in mallard ducks (Anas platyrhynchos). Sci. Total Environ. 2012, 426, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J. Embryotoxic and teratogenic effects of petroleum hydrocarbons in mallards (Anas platyrhynchos). J. Toxicol. Environ. Health 1979, 5, 835–844. [Google Scholar] [CrossRef]
- Goodchild, C.G.; Grisham, K.; Belden, J.B.; DuRant, S.E. Effects of sublethal application of Deepwater Horizon oil to bird eggs on embryonic heart and metabolic rate. Conserv. Biol. 2020, 34, 1262–1270. [Google Scholar] [CrossRef]
- Dohle, D.S.; Pasa, S.D.; Gustmann, S.; Laub, M.; Wissler, J.H.; Jennissen, H.P.; Dünker, N. Chick ex ovo culture and ex ovo CAM assay: How it really works. J. Vis. Exp. 2009. [Google Scholar] [CrossRef]
- Cloney, K.; Franz-Odendaal, T.A. Optimized ex-ovo culturing of chick embryos to advanced stages of development. J. Vis. Exp. 2015, 52129. [Google Scholar] [CrossRef]
- Farzaneh, M.; Attari, F.; Khoshnam, S.E.; Mozdziak, P.E. The method of chicken whole embryo culture using the eggshell windowing, surrogate eggshell and ex ovo culture system. Br. Poult. Sci. 2018, 59, 240–244. [Google Scholar] [CrossRef]
- Reizis, A.; Hammel, I.; Ar, A. Regional and developmental variations of blood vessel morphometry in the chick embryo chorioallantoic membrane. J. Exp. Biol. 2005, 208, 2483–2488. [Google Scholar] [CrossRef]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef]
- Bentley, K.; Chakravartula, S. The temporal basis of angiogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Paine, I.S.; Lewis, M.T. The Terminal End Bud: The Little Engine that Could. J. Mammary Gland Biol. Neoplasia 2017, 22, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Lusimbo, W.S.; Leighton, F.A.; Wobeser, G.A. Histology and ultrastructure of the chorioallantoic membrane of the mallard duck (Anas platyrhynchos). Anat. Rec. 2000, 259, 25–34. [Google Scholar] [CrossRef]
- Fehér, G. The structure of the shell membrane, the development and structural change of the amnion and chorioallantoic membrane during hatching in the goose. Anat. Histol. Embryol. 1984, 13, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, P.; Lahiani, A.; Gincberg, G.; Haham, D.; Marcinkiewicz, C.; Lelkes, P.I. Nerve Growth Factor-Induced Angiogenesis: 2. The Quail Chorioallantoic Membrane Assay. Methods Mol. Biol. 2018, 1727, 251–259. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burggren, W.; Rojas Antich, M. Angiogenesis in the Avian Embryo Chorioallantoic Membrane: A Perspective on Research Trends and a Case Study on Toxicant Vascular Effects. J. Cardiovasc. Dev. Dis. 2020, 7, 56. https://doi.org/10.3390/jcdd7040056
Burggren W, Rojas Antich M. Angiogenesis in the Avian Embryo Chorioallantoic Membrane: A Perspective on Research Trends and a Case Study on Toxicant Vascular Effects. Journal of Cardiovascular Development and Disease. 2020; 7(4):56. https://doi.org/10.3390/jcdd7040056
Chicago/Turabian StyleBurggren, Warren, and Maria Rojas Antich. 2020. "Angiogenesis in the Avian Embryo Chorioallantoic Membrane: A Perspective on Research Trends and a Case Study on Toxicant Vascular Effects" Journal of Cardiovascular Development and Disease 7, no. 4: 56. https://doi.org/10.3390/jcdd7040056
APA StyleBurggren, W., & Rojas Antich, M. (2020). Angiogenesis in the Avian Embryo Chorioallantoic Membrane: A Perspective on Research Trends and a Case Study on Toxicant Vascular Effects. Journal of Cardiovascular Development and Disease, 7(4), 56. https://doi.org/10.3390/jcdd7040056





