Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the NR Compounds
2.3. Curing the NR Compounds
2.4. Treatment of the NR Samples for Crosslink Analysis
2.5. Analysis of the Crosslink Density and Structure
2.6. Instrumentation and Equipment
3. Results and Discussion
3.1. Curing Characteristics of NR Compounds
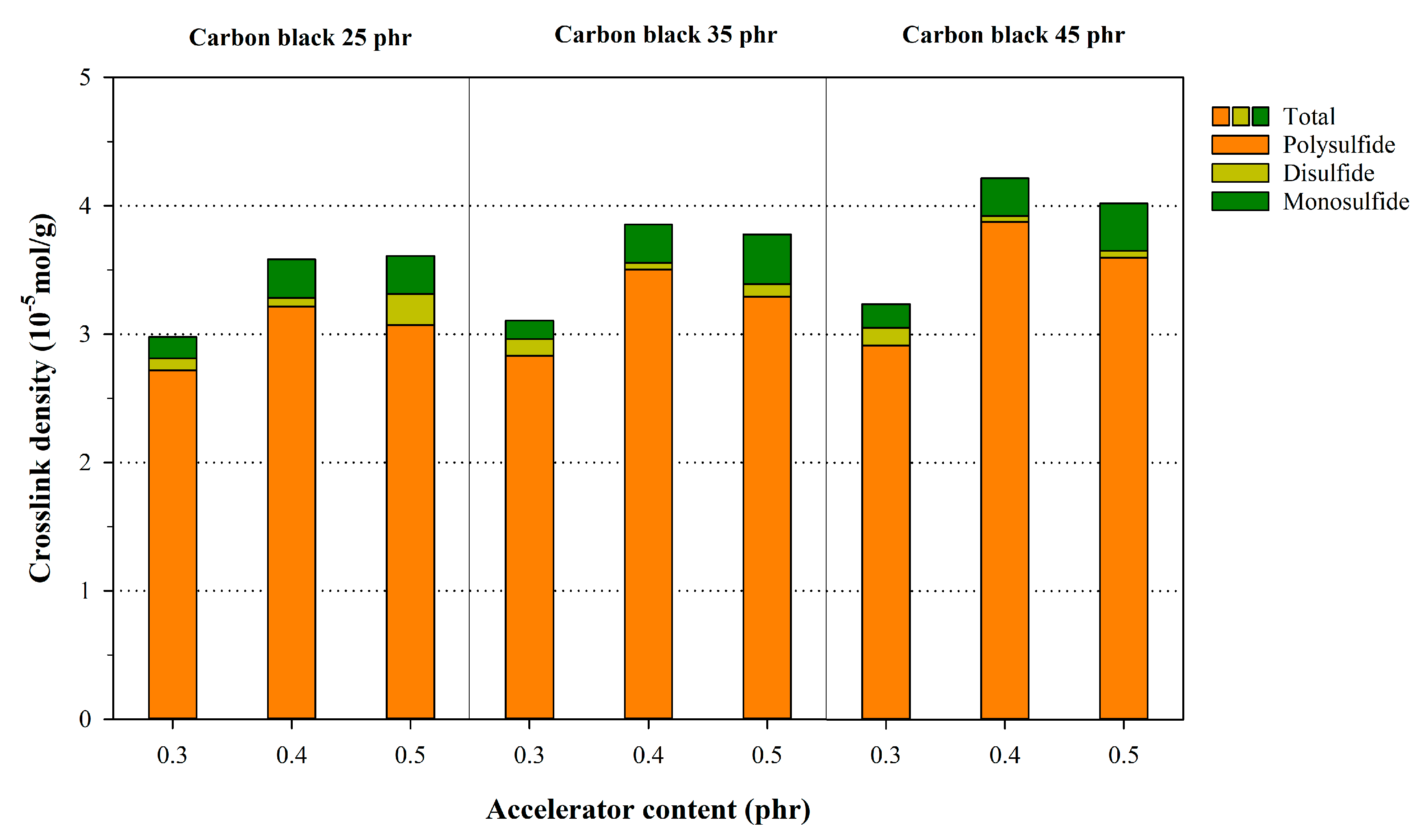
3.2. Crosslink Characteristics of NR Compounds Calculated Using the Flory–Rehner Equation
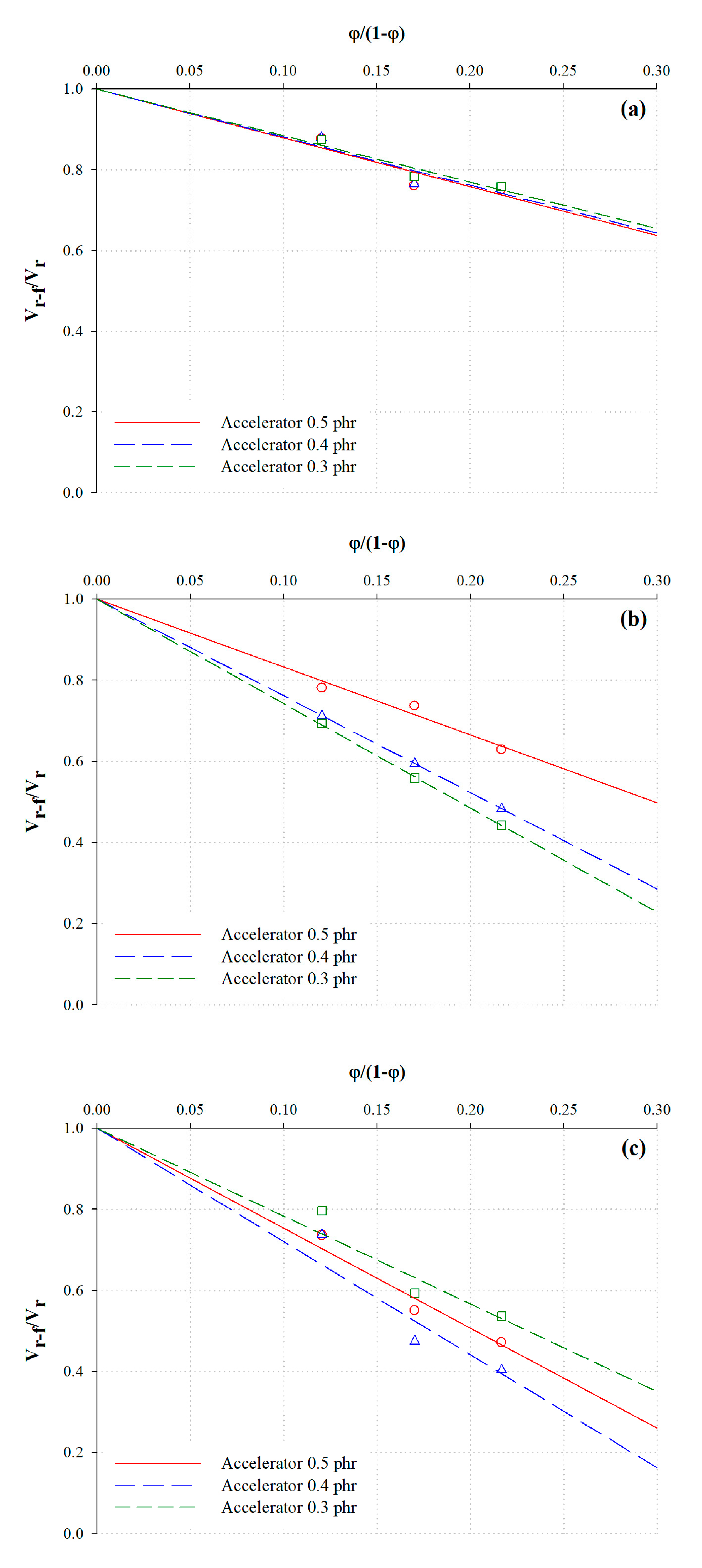
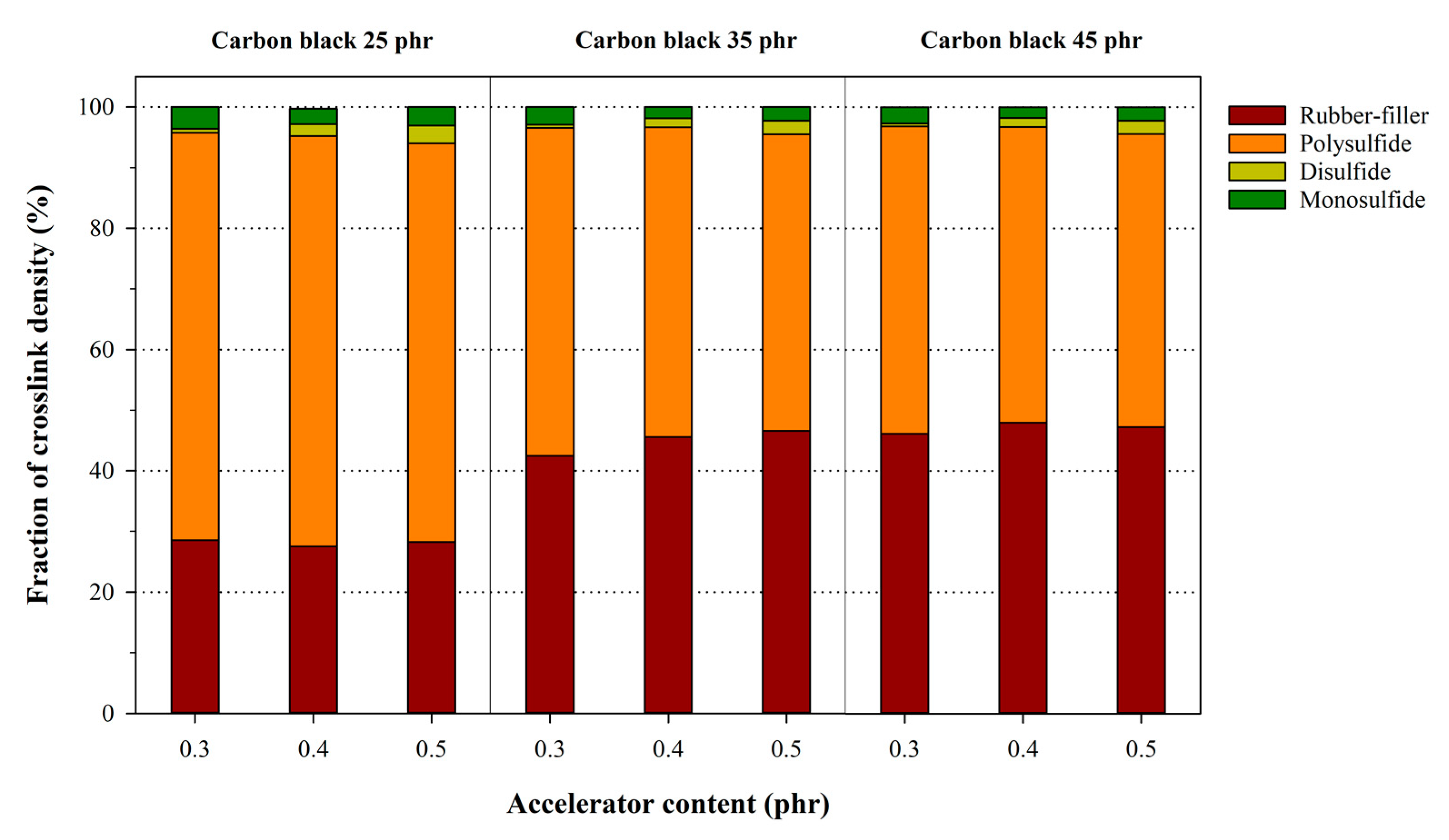
3.3. Analysis of the Chemical Crosslink Structure and Rubber–Filler Interaction Using the Kraus Equation
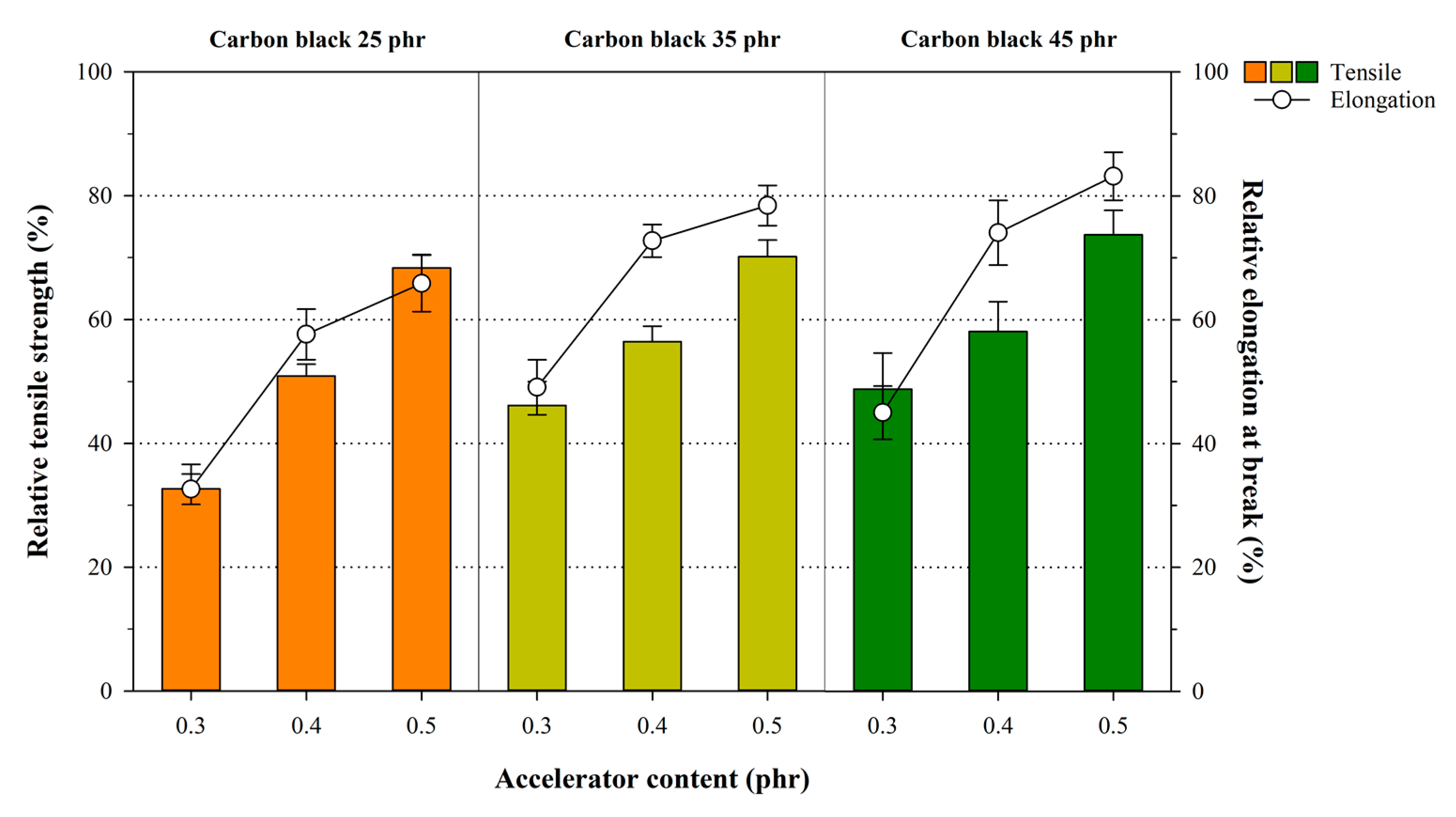
3.4. Influence of Crosslink Structures of NR Compounds on Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brydson, J.A. The Historical Development of Rubber Chemistry; Springer Science and Business Media LLC: Berlin, Germany, 1978; pp. 1–10. [Google Scholar]
- Hofmann, W. Vulcanization and Vulcanizing Agents; Maclaren: Northampton, UK, 1967. [Google Scholar]
- Aprem, A.S.; Joseph, K.; Thomas, S. Recent Developments in Crosslinking of Elastomers. Rubber Chem. Technol. 2005, 78, 458–488. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tarachiwin, L. Recent Advances in Structural Characterization of Natural Rubber. Rubber Chem. Technol. 2009, 82, 283–314. [Google Scholar] [CrossRef]
- Sperling, L. Introduction to Physical Polymer Science; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Thomas, S.; Chan, C.H.; Pothen, L.A.; Rajisha, K.; Maria, H. Natural Rubber Materials; Volume 1: Blends and IPNs; Royal Society of Chemistry: London, UK, 2013; Volume 7. [Google Scholar]
- Saville, B.; Watson, A.A. Structural Characterization of Sulfur-Vulcanized Rubber Networks. Rubber Chem. Technol. 1967, 40, 100–148. [Google Scholar] [CrossRef]
- Choi, S.-S.; Nah, C.; Jo, B.-W. Properties of natural rubber composites reinforced with silica or carbon black: Influence of cure accelerator content and filler dispersion. Polym. Int. 2003, 52, 1382–1389. [Google Scholar] [CrossRef]
- Jincheng, W.; Yuehui, C.; Jihu, W. Novel Reinforcing Filler: Application to Natural Rubber (NR) System. J. Elastomers Plast. 2005, 37, 169–180. [Google Scholar] [CrossRef]
- Krebs, H. The Mechanism of Accelerator Action. Rubber Chem. Technol. 1957, 30, 962–971. [Google Scholar] [CrossRef]
- Susamma, A.; Kurien, M.; Kuriakose, A. New binary accelerator systems for sulphur vulcanisation of styrene butadiene rubber. Plast. Rubber Compos. 2004, 33, 63–70. [Google Scholar] [CrossRef]
- Ahsan, Q.; Mohamad, N.; Soh, T. Effects of accelerators on the cure characteristics and mechanical properties of natural rubber compounds. Int. J. Automot. Mech. Eng. 2015, 12, 2954–2966. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512. [Google Scholar] [CrossRef]
- Mooney, M. A Theory of Large Elastic Deformation. J. Appl. Phys. 1940, 11, 582. [Google Scholar] [CrossRef]
- Rivlin, R.S. Large elastic deformations of isotropic materials IV. further developments of the general theory. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1948, 241, 379–397. [Google Scholar] [CrossRef]
- Ferry, J.D. Applications of a two-network model for crosslinks and trapped entanglements. Polymer 1979, 20, 1343–1348. [Google Scholar] [CrossRef]
- Kumar, N.; Rao, V.V. Hyperelastic Mooney-Rivlin model: Determination and physical interpretation of material constants. Parameters 2016, 2, 1. [Google Scholar]
- Chae, Y.K.; Kang, W.Y.; Jang, J.-H.; Choi, S.-S. A simple NMR method to measure crosslink density of natural rubber composite. Polym. Test. 2010, 29, 953–957. [Google Scholar] [CrossRef]
- Saalwächter, K. Microstructure and Molecular Dynamics of Elastomers as Studied by Advanced Low-Resolution Nuclear Magnetic Resonance Methods. Rubber Chem. Technol. 2012, 85, 350–386. [Google Scholar] [CrossRef]
- Campbell, D.S. Structural characterization of vulcanizates part X. Thiol-disulfide interchange for cleaving disulfide crosslinks in natural rubber vulcanizates. J. Appl. Polym. Sci. 1969, 13, 1201–1214. [Google Scholar] [CrossRef]
- Marzocca, A.J. Evaluation of the polymer–solvent interaction parameter χ for the system cured styrene butadiene rubber and toluene. Eur. Polym. J. 2007, 43, 2682–2689. [Google Scholar] [CrossRef]
- Marzocca, A.J.; Garraza, A.R.; Mansilla, M. Evaluation of the polymer–solvent interaction parameter χ for the system cured polybutadiene rubber and toluene. Polym. Test. 2010, 29, 119–126. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kraus, G. Degree of Cure in Filler-Reinforced Vulcanizates by the Swelling Method. Rubber Chem. Technol. 1957, 30, 928–951. [Google Scholar] [CrossRef]
- Kraus, G. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Dick, J.S. Rubber Technology: Compounding and Testing for Performance; Carl Hanser Verlag GmbH Co. KG: Munich, Germany, 2014. [Google Scholar]
- Mark, J.E.; Erman, B.; Roland, C.M. The Science and Technology of Rubber; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Morton-Jones, D.H. Rubber Technology; Springer Science and Business Media LLC: Berlin, Germany, 1989; pp. 191–219. [Google Scholar]
- Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.M.; Venkatasubramanian, V.; Walker, K.A. Sulfur Vulcanization of Natural Rubber for Benzothiazole Accelerated Formulations: From Reaction Mechanisms to a Rational Kinetic Model. Rubber Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.A. Hawley’s Condensed Chemical Dictionary; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ismail, H.; Tan, S.; Poh, B.T. Curing and Mechanical Properties of Nitrile and Natural Rubber Blends. J. Elastomers Plast. 2001, 33, 251–262. [Google Scholar] [CrossRef]
- El-Sabbagh, S.A.; Yehia, A. Detection of crosslink density by different methods for natural rubber blended with SBR and NBR. Egypt. J. Solids 2007, 30, 157–173. [Google Scholar]
- Rabiei, S.; Shojaei, A. Vulcanization kinetics and reversion behavior of natural rubber/styrene-butadiene rubber blend filled with nanodiamond–The role of sulfur curing system. Eur. Polym. J. 2016, 81, 98–113. [Google Scholar] [CrossRef]
- Bueche, F. Mullins effect and rubber–filler interaction. J. Appl. Polym. Sci. 1961, 5, 271–281. [Google Scholar] [CrossRef]
- Choi, S.-S.; Nah, C.; Lee, S.G.; Joo, C.W. Effect of filler-filler interaction on rheological behaviour of natural rubber compounds filled with both carbon black and silica. Polym. Int. 2003, 52, 23–28. [Google Scholar] [CrossRef]
- Litvinov, V.M.; Orza, R.A.; Klüppel, M.; Van Duin, M.; Magusin, P.C. Rubber–Filler Interactions and Network Structure in Relation to Stress–Strain Behavior of Vulcanized, Carbon Black Filled EPDM. Macromolecules 2011, 44, 4887–4900. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Crop Residues as an Alternative to Vulcanizates with Common Fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef] [Green Version]
- Noordermeer, J.W. Vulcanization. In Encyclopedia of Polymeric Nanomaterials; Springer Science and Business Media LLC: Berlin, Germany, 2014; pp. 1–16. [Google Scholar]
- Sae-Oui, P.; Sirisinha, C.; Thepsuwan, U.; Thapthong, P. Influence of accelerator type on properties of NR/EPDM blends. Polym. Test. 2007, 26, 1062–1067. [Google Scholar] [CrossRef]
- Coran, A.Y. Chapter 7—Vulcanization. In The Science and Technology of Rubber, Fourth ed.; Mark, J.E., Erman, B., Roland, C.M., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 337–381. [Google Scholar] [CrossRef]
- Layer, R.W. Recuring Vulcanizates. I. A Novel Way to Study the Mechanism of Vulcanization. Rubber Chem. Technol. 1992, 65, 211–222. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, J.-C. Lifetime prediction and thermal aging behaviors of SBR and NBR composites using crosslink density changes. J. Ind. Eng. Chem. 2012, 18, 1166–1170. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.H.; Nam, G.M.; Kang, D.G.; Seo, K.H. Oil resistance and low-temperature characteristics of plasticized nitrile butadiene rubber compounds. J. Appl. Polym. Sci. 2019, 136, 47851. [Google Scholar] [CrossRef]
- Rodgers, B. Rubber Compounding: Chemistry and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]


| Description | NR (wt%) | Carbon Black (phr) | MBTS (phr) | DPG (phr) | Sulfur (phr) | Lubricant (phr) | Antioxidant 1 (phr) | Antioxidant 2 (phr) |
|---|---|---|---|---|---|---|---|---|
| NR25-0.3 | 100 | 25 | 0.1 | 0.2 | 1.5 | 1.5 | 1 | 2 |
| NR25-0.4 | 100 | 25 | 0.1 | 0.3 | 1.5 | 1.5 | 1 | 2 |
| NR25-0.5 | 100 | 25 | 0.2 | 0.3 | 1.5 | 1.5 | 1 | 2 |
| NR35-0.3 | 100 | 35 | 0.1 | 0.2 | 1.5 | 1.5 | 1 | 2 |
| NR35-0.4 | 100 | 35 | 0.1 | 0.3 | 1.5 | 1.5 | 1 | 2 |
| NR35-0.5 | 100 | 35 | 0.2 | 0.3 | 1.5 | 1.5 | 1 | 2 |
| NR45-0.3 | 100 | 45 | 0.1 | 0.2 | 1.5 | 1.5 | 1 | 2 |
| NR45-0.4 | 100 | 45 | 0.1 | 0.3 | 1.5 | 1.5 | 1 | 2 |
| NR45-0.5 | 100 | 45 | 0.2 | 0.3 | 1.5 | 1.5 | 1 | 2 |
| Mixing Process | Temperature (°C) | Time (min) | Reagents | |
|---|---|---|---|---|
| Kneader | Step 1 | - | 1 | NR |
| Step 2 | - | 3 | Carbon black | |
| Step 3 | - | 3 | Lubricant | |
| Step 4 | 90 | - | Sulfur | |
| Open-roll | Step 5 | 30 | 4 | Accelerator |
| Description | ts2 (s) | t90 (s) | MH (dN·m) | ML (dN·m) | MH-ML (dN·m) |
|---|---|---|---|---|---|
| NR25-0.3 | 90 | 198 | 20.7 | 4.7 | 15.9 |
| NR25-0.4 | 55 | 156 | 22.8 | 5.5 | 17.3 |
| NR25-0.5 | 52 | 136 | 22.6 | 3.1 | 19.5 |
| NR35-0.3 | 77 | 184 | 22.9 | 4.6 | 18.3 |
| NR35-0.4 | 53 | 154 | 24.9 | 5.1 | 19.8 |
| NR35-0.5 | 47 | 131 | 25.1 | 3.7 | 21.4 |
| NR45-0.3 | 71 | 173 | 25.5 | 5.5 | 20.0 |
| NR45-0.4 | 49 | 149 | 26.4 | 5.2 | 21.2 |
| NR45-0.5 | 45 | 128 | 28.4 | 5.0 | 23.4 |
| Description | φ/(1 − φ) | Poly + Di + Mono | Di + Mono | Mono | ||||||
| NR25-0.3 | 0.1202 | 0.1655 | 0.1452 | 1.2081 | 0.0540 | 0.0422 | 1.6722 | 0.0395 | 0.0291 | 2.4648 |
| NR35-0.3 | 0.1699 | 0.1907 | 0.0572 | 0.0528 | ||||||
| NR45-0.3 | 0.2166 | 0.1932 | 0.0670 | 0.0616 | ||||||
| NR25-0.4 | 0.1203 | 0.1624 | 0.1429 | 1.1873 | 0.0496 | 0.0353 | 2.3837 | 0.0347 | 0.0256 | 2.7934 |
| NR35-0.4 | 0.1700 | 0.1867 | 0.0594 | 0.0539 | ||||||
| NR45-0.4 | 0.2168 | 0.1890 | 0.0731 | 0.0635 | ||||||
| NR25-0.5 | 0.1204 | 0.1596 | 0.1396 | 1.1492 | 0.0494 | 0.0335 | 2.5756 | 0.0383 | 0.0305 | 2.1666 |
| NR35-0.5 | 0.1702 | 0.1690 | 0.0570 | 0.0514 | ||||||
| NR45-0.5 | 0.2169 | 0.1884 | 0.0775 | 0.0568 | ||||||
| Description | Crosslink Density (10−5 mol/g) | Fraction (%) | |||
|---|---|---|---|---|---|
| Interaction | Chemical | Total | Interaction | Cehmical | |
| NR25-0.3 | 0.9757 | 2.4417 | 3.4174 | 71.45 | 28.55 |
| NR35-0.3 | 1.8023 | 4.2440 | 57.53 | 42.47 | |
| NR45-0.3 | 2.0933 | 4.5350 | 53.84 | 46.16 | |
| NR35-0.4 | 0.9927 | 2.5705 | 3.5632 | 72.14 | 27.86 |
| NR45-0.4 | 2.1555 | 4.7260 | 54.39 | 45.61 | |
| NR45-0.4 | 2.3713 | 4.9418 | 52.02 | 47.98 | |
| NR25-0.5 | 1.0491 | 2.6626 | 3.7117 | 71.74 | 28.26 |
| NR35-0.5 | 2.3245 | 4.9871 | 53.39 | 46.61 | |
| NR45-0.5 | 2.3852 | 5.0478 | 52.75 | 47.25 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers 2020, 12, 2020. https://doi.org/10.3390/polym12092020
Kim DY, Park JW, Lee DY, Seo KH. Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers. 2020; 12(9):2020. https://doi.org/10.3390/polym12092020
Chicago/Turabian StyleKim, Do Young, Jae Woo Park, Dong Yun Lee, and Kwan Ho Seo. 2020. "Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement" Polymers 12, no. 9: 2020. https://doi.org/10.3390/polym12092020






