Ethanol-Induced Oxidative Stress Modifies Inflammation and Angiogenesis Biomarkers in Retinal Pigment Epithelial Cells (ARPE-19): Role of CYP2E1 and its Inhibition by Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Determination of ROS Levels
2.3. Cell Viability
2.4. Proteome Profiling
2.5. Matrix Metalloproteinases ELISA
2.6. Western Blotting
2.7. Quantitative Real Time PCR (RT-qPCR)
2.8. Statistical Analysis
3. Results
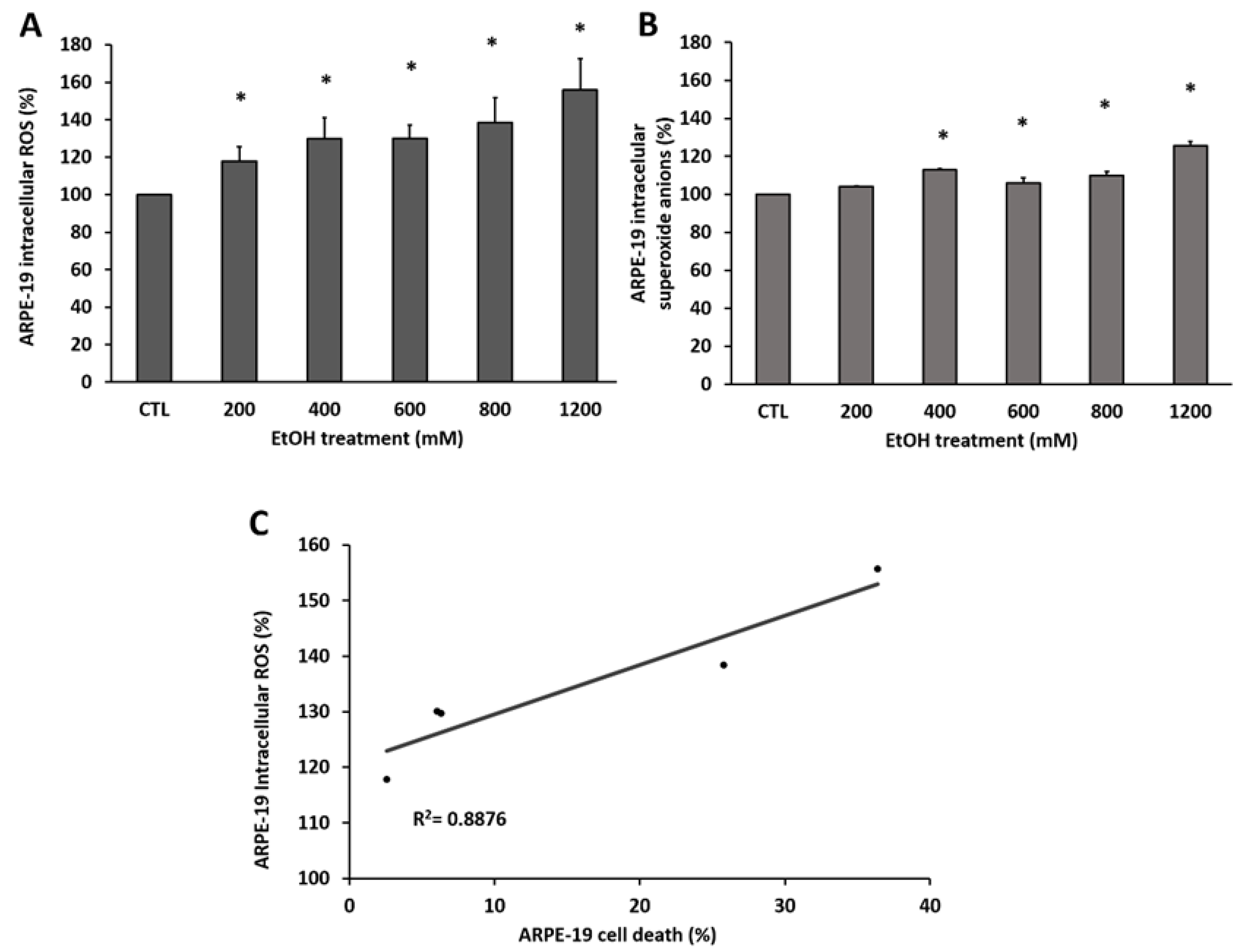
3.1. EtOH Induces ROS Accumulation in RPE Cells Promoting Death
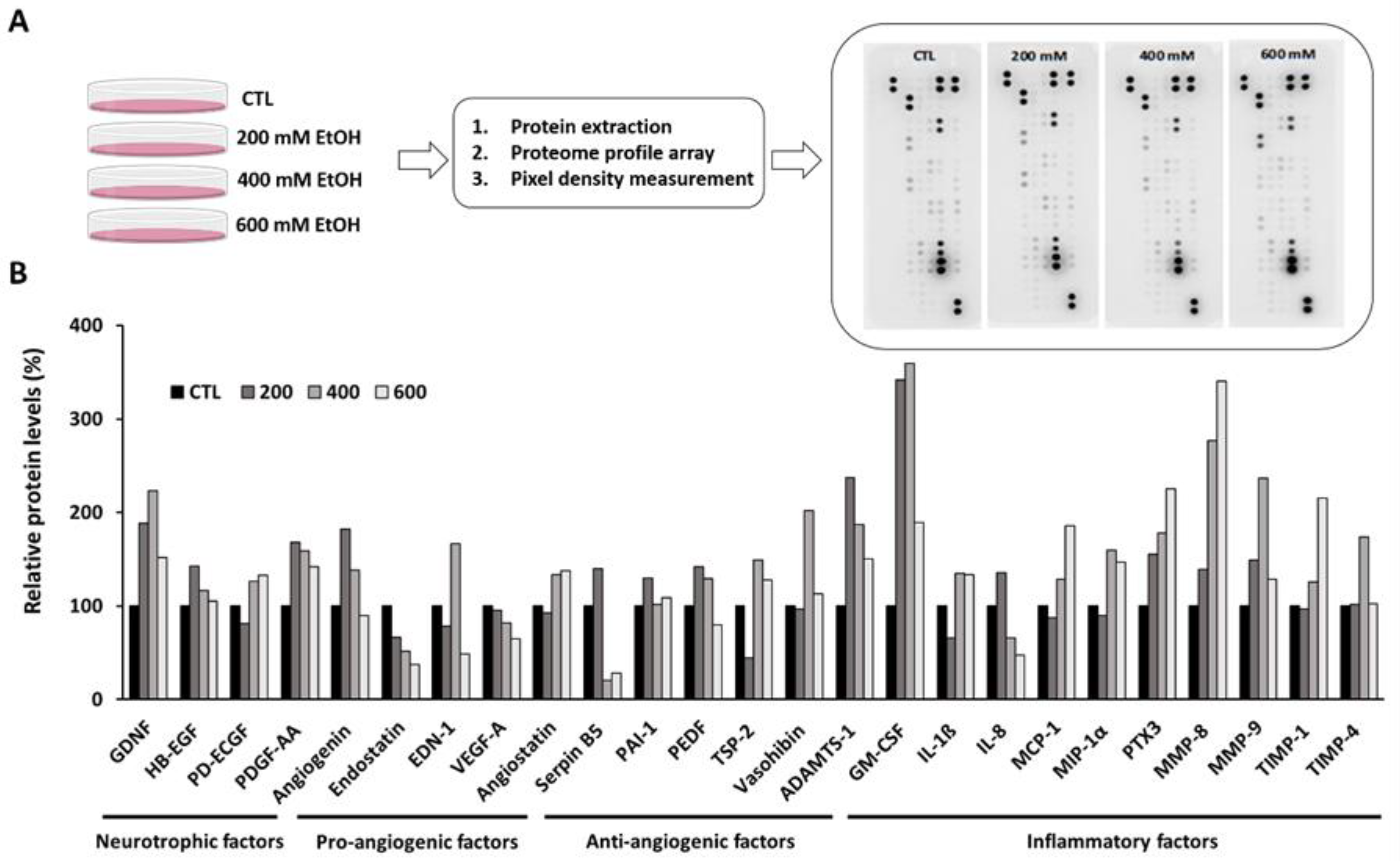
3.2. Ethanol Altered the Inflammation and Angiogenesis-Related Proteome Profile in Human RPE Cells
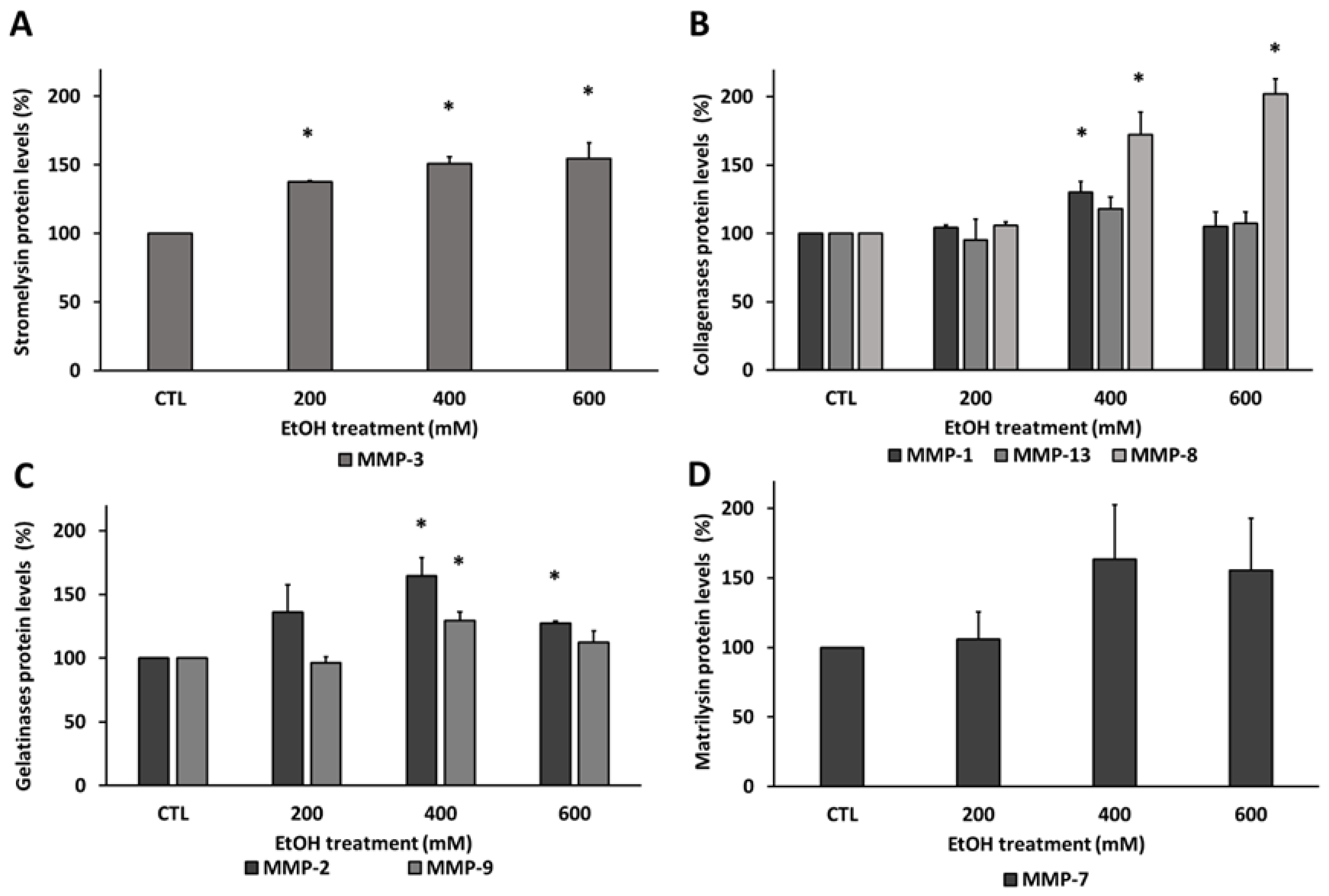
3.3. Ethanol Modified Matrix Metalloproteinases Levels in Human RPE Cells
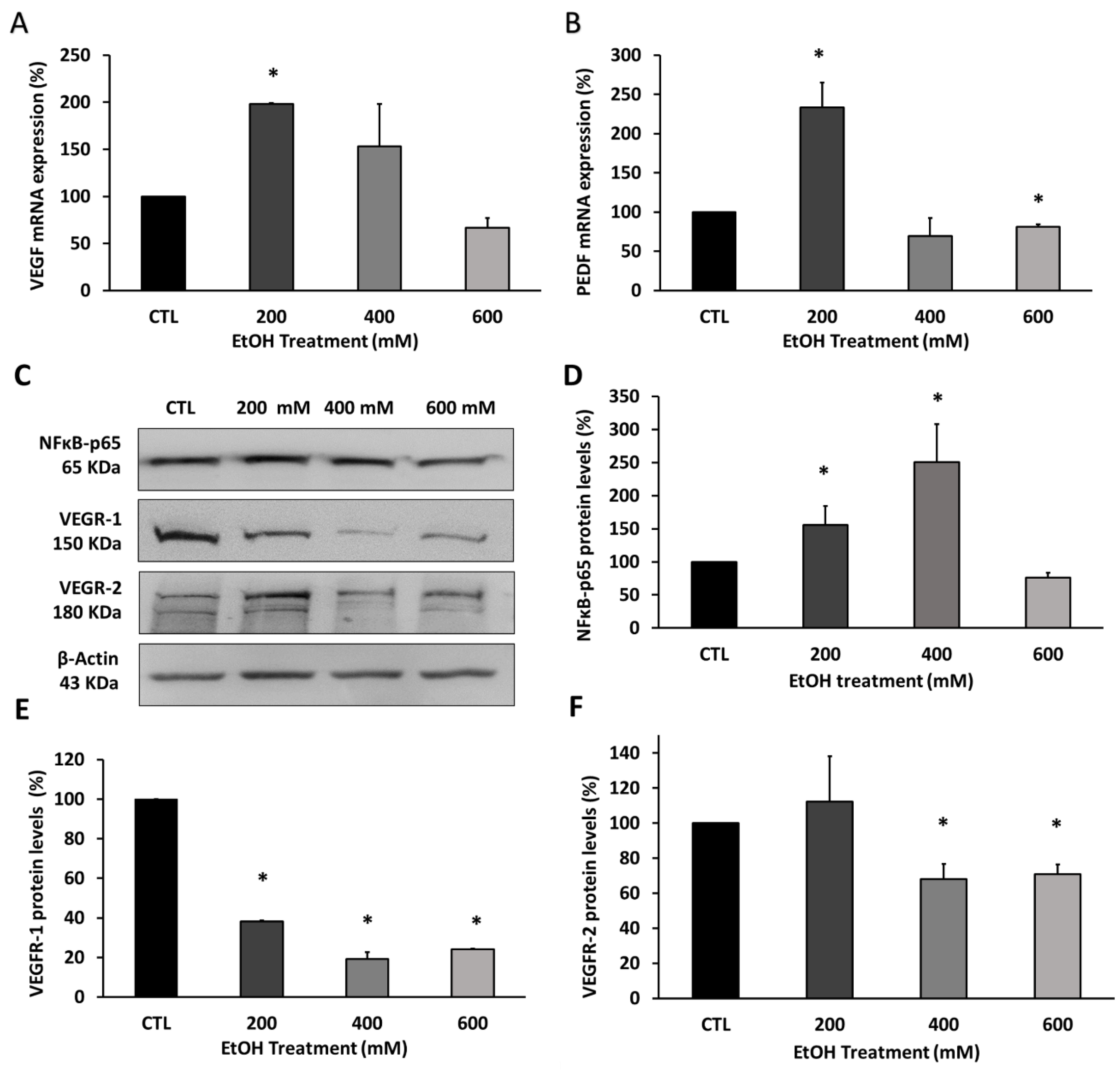
3.4. Ethanol Modified the Upstream and Downstream Angiogenesis Regulators in RPE Cells
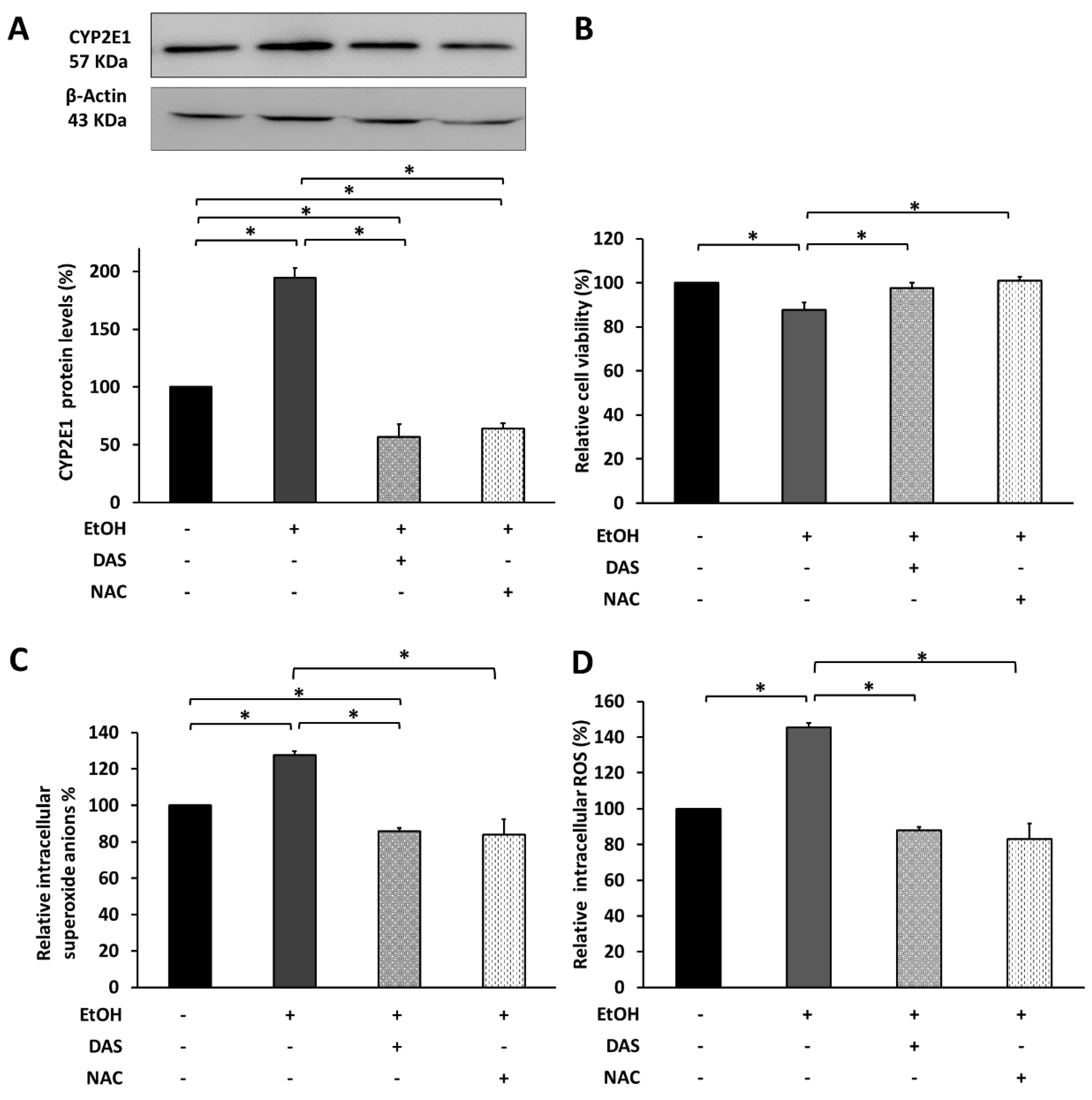
3.5. CYP2E1 Upregulation Promotes Cell Death via Oxidative Stress Induction
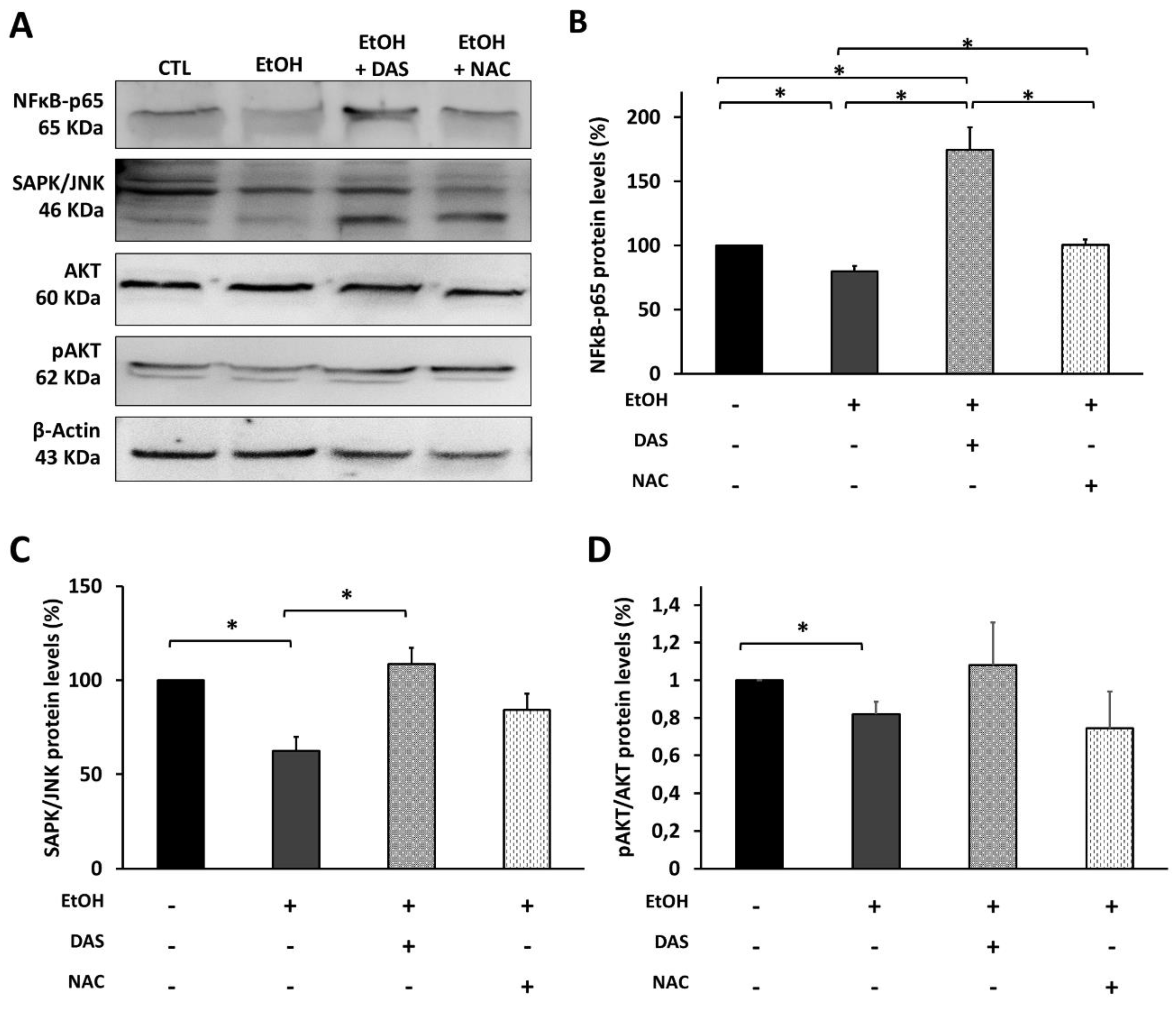
3.6. CYP2E1 Upregulation Modulates the Upstream Angiogenesis and Inflammation Regulators in RPE Cells
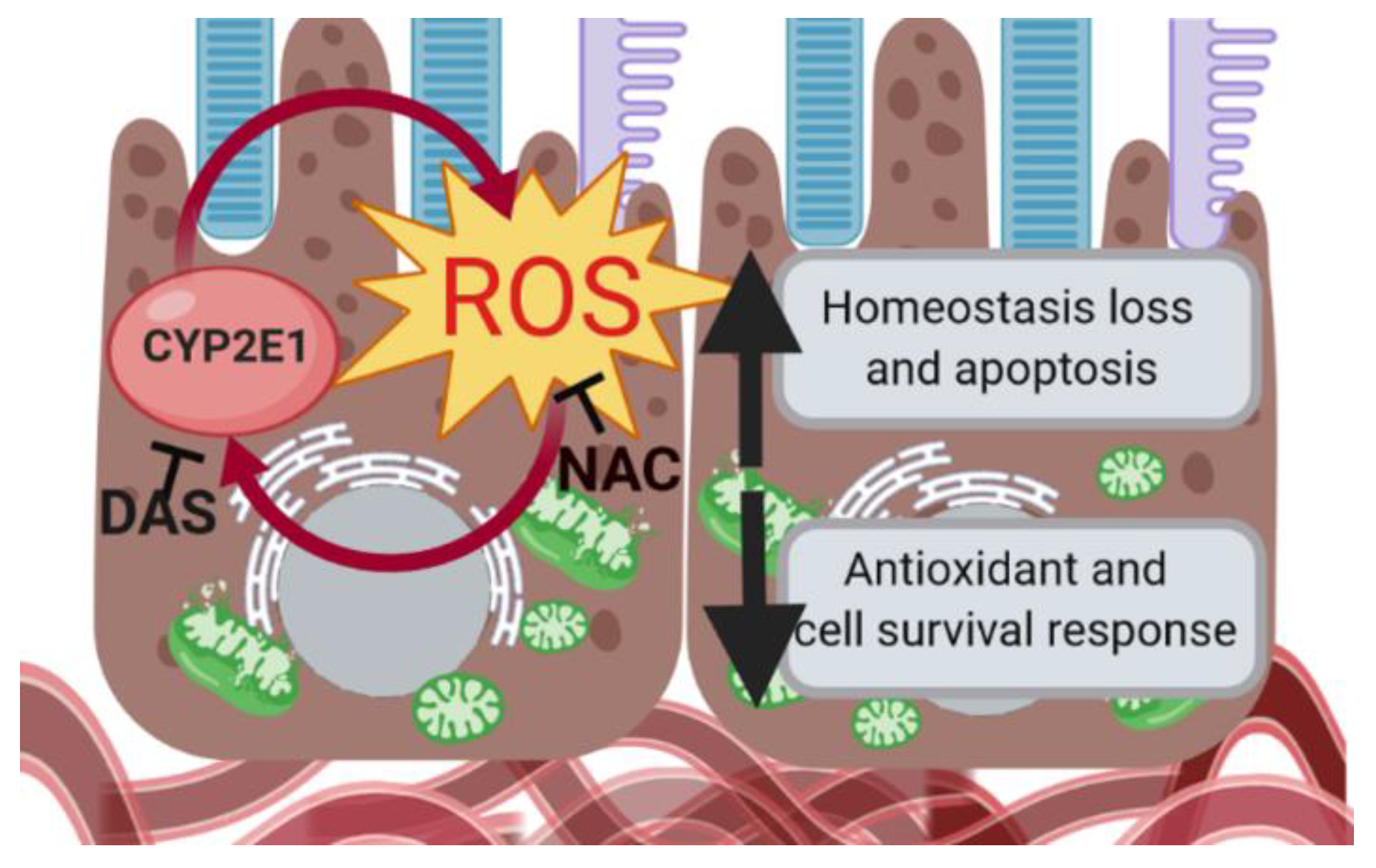
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Hamann, S. Molecular mechanisms of water transport in the eye. Int. Rev. Cytol. 2002, 215, 395–431. [Google Scholar] [PubMed]
- Kay, P.; Yang, Y.C.; Paraoan, L. Directional protein secretion by the retinal pigment epithelium: Roles in retinal health and the development of age-related macular degeneration. J. Cell. Mol. Med. 2013, 17, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21, S3–S9. [Google Scholar] [CrossRef]
- Ponnalagu, M.; Subramani, M.; Jayadev, C.; Shetty, R.; Das, D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine 2017, 95, 126–135. [Google Scholar] [CrossRef]
- Nguyen-Legros, J.; Hicks, D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int. Rev. Cytol. 2000, 196, 245–313. [Google Scholar]
- Sethna, S.; Chamakkala, T.; Gu, X.; Thompson, T.C.; Cao, G.; Elliott, M.H.; Finnemann, S.C. Regulation of Phagolysosomal Digestion by Caveolin-1 of the Retinal Pigment Epithelium Is Essential for Vision. J. Biol. Chem. 2016, 291, 6494–6506. [Google Scholar] [CrossRef]
- Thompson, D.A.; Gal, A. Vitamin A metabolism in the retinal pigment epithelium: Genes, mutations, and diseases. Prog. Retin. Eye Res. 2003, 22, 683–703. [Google Scholar] [CrossRef]
- Bavik, C.; Henry, S.H.; Zhang, Y.; Mitts, K.; McGinn, T.; Budzynski, E.; Pashko, A.; Lieu, K.L.; Zhong, S.; Blumberg, B.; et al. Visual Cycle Modulation as an Approach toward Preservation of Retinal Integrity. PLoS ONE 2015, 10, e0124940. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Handa, J.T.; Rickman, C.B.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef] [PubMed]
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y. Oxidative Stress in the RPE and Its Contribution to AMD Pathogenesis: Implication of Light Exposure. In Neuroprotection and Neuroregeneration for Retinal Diseases; Nakazawa, T.K.Y., Harada, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 239–253. [Google Scholar]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef] [PubMed]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tao, L.; Lei, C.; Wang, J.; Yang, P.; Li, Q.; Lei, B. Downregulating p22phox ameliorates inflammatory response in Angiotensin II-induced oxidative stress by regulating MAPK and NF-kappaB pathways in ARPE-19 cells. Sci. Rep. 2015, 5, 14362. [Google Scholar] [CrossRef] [PubMed]
- Klettner, A.; Westhues, D.; Lassen, J.; Bartsch, S.; Roider, J. Regulation of constitutive vascular endothelial growth factor secretion in retinal pigment epithelium/choroid organ cultures: p38, nuclear factor kappaB, and the vascular endothelial growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol. Vis. 2013, 19, 281–291. [Google Scholar]
- Faghiri, Z.; Bazan, N.G. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp. Eye Res. 2010, 90, 718–725. [Google Scholar] [CrossRef]
- Piippo, N.; Korhonen, E.; Hytti, M.; Kinnunen, K.; Kaarniranta, K.; Kauppinen, A. Oxidative Stress is the Principal Contributor to Inflammasome Activation in Retinal Pigment Epithelium Cells with Defunct Proteasomes and Autophagy. Cell. Physiol. Biochem. 2018, 49, 359–367. [Google Scholar] [CrossRef]
- Eichler, W.; Friedrichs, U.; Thies, A.; Tratz, C.; Wiedemann, P. Modulation of matrix metalloproteinase and TIMP-1 expression by cytokines in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2767–2773. [Google Scholar]
- Miranda Sanz, M.; Johnson, L.E.; Ahuja, S.; Ekstrom, P.A.R.; Romero, F.J.; Van Veen, T. Significant photreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 2007, 145, 1120–1129. [Google Scholar] [CrossRef]
- Miranda, M.; Arnal, E.; Ahuja, S.; Alvarez-Nölting, R.; Lopez-Pedrajas, R.; Ekstrom, P.; Bosch-Morell, F.; van Veen, T.; Romero, F.J. Antioxidants rescue photoreceptors in rd1 mice: Relationships with thiol metabolism. Free Radic. Biol. Med. 2010, 48, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Malo, D.; Villaron-Casares, C.A.; Alarcon-Jimenez, J.; Miranda, M.; Diaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a therapeutic option in retinal diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxid. Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef] [PubMed]
- Piano, I.; D’Antongiovanni, V.; Testai, L.; Calderone, V.; Gargini, C. A Nutraceutical Strategy to Slowing Down the Progression of Cone Death in an Animal Model of Retinitis Pigmentosa. Front. Neurosci. 2019, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Fernández-Sánchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Ret. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell. Longev. 2017, 2017, 9208489. [Google Scholar] [CrossRef]
- Blasiak, J.; Petrovski, G. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed. Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: Implications for retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Wittchen, E.S.; Hartnett, M.E. TNF-alpha mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent beta-catenin activation. Mol. Vis. 2016, 22, 116–128. [Google Scholar]
- Aouiss, A.; Anka Idrissi, D.; Kabine, M.; Zaid, Y. Update of inflammatory proliferative retinopathy: Ischemia, hypoxia and angiogenesis. Curr. Res. Transl. Med. 2019, 67, 62–71. [Google Scholar] [CrossRef]
- Papaconstantinou, J. The Role of Signaling Pathways of Inflammation and Oxidative Stress in Development of Senescence and Aging Phenotypes in Cardiovascular Disease. Cells 2019, 8, 1383. [Google Scholar] [CrossRef] [PubMed]
- Pilat, A.; Herrnreiter, A.M.; Skumatz, C.M.; Sarna, T.; Burke, J.M. Oxidative stress increases HO-1 expression in ARPE-19 cells, but melanosomes suppress the increase when light is the stressor. Investig. Ophthalmol. Vis. Sci. 2013, 54, 47–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyamura, N.; Ogawa, T.; Boylan, S.; Morse, L.S.; Handa, J.T.; Hjelmeland, L.M. Topographic and age-dependent expression of heme oxygenase-1 and catalase in the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1562–1565. [Google Scholar] [CrossRef]
- Singhal, S.S.; Godley, B.F.; Chandra, A.; Pandya, U.; Jin, G.F.; Saini, M.K.; Awasthi, S.; Awasthi, Y.C. Induction of glutathione S-transferase hGST 5.8 is an early response to oxidative stress in RPE cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2652–2659. [Google Scholar]
- Frank, R.N.; Amin, R.H.; Puklin, J.E. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am. J. Ophthalmol. 1999, 127, 694–709. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar]
- Roth, S.; Shaikh, A.R.; Hennelly, M.M.; Li, Q.; Bindokas, V.; Graham, C.E. Mitogen-activated protein kinases and retinal ischemia. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5383–5395. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Yang, L.; Wu, D.; Cederbaum, A.I. CYP2E1, oxidative stress and MAPK signaling pathways in alcohol-induced hepatotoxicity. J. Biochem. Pharmacol. Res. 2014, 2, 16. [Google Scholar]
- Abdelmegeed, M.A.; Choi, Y.; Ha, S.K.; Song, B.J. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic. Biol. Med. 2016, 91, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; Rajendran, R.; Singh, S.; Garva, R.; Krstic-Demonacos, M.; Demonacos, C. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res. 2013, 15, R107. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.M.; Russo, A.; Proença, M.A.; Gazola, N.F.; Rodrigues, G.H.; Biselli-Chicote, P.M.; Silva, A.E.; Netinho, J.G.; Pavarino, É.C.; Goloni-Bertollo, E.M. CYP1A1, CYP2E1 and EPHX1 polymorphisms in sporadic colorectal neoplasms. World J. Gastroenterol. 2016, 22, 9974–9983. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Pan, X.; Yu, J.; Chen, Z.; Xu, H.; El-Rifai, W.; Zhang, G.; Xu, Z. Functional PstI/RsaI polymorphism in CYP2E1 is associated with the development, progression and poor outcome of gastric cancer. PLoS ONE 2012, 7, e44478. [Google Scholar] [CrossRef] [PubMed]
- García-Suástegui, W.A.; Ramos-Chávez, L.A.; Rubio-Osornio, M.; Calvillo-Velasco, M.; Atzin-Méndez, J.A.; Guevara, J.; Silva-Adaya, D. The Role of CYP2E1 in the Drug Metabolism or Bioactivation in the Brain. Oxid. Med. Cell. Longev. 2017, 2017, 4680732. [Google Scholar] [CrossRef]
- Martinez-Gil, N.; Flores-Bellver, M.; Atienzar-Aroca, S.; Lopez-Malo, D.; Urdaneta, A.C.; Sancho-Pelluz, J.; Peris-Martínez, C.; Bonet-Ponce, L.; Romero, F.J.; Barcia, J.M. CYP2E1 in the Human Retinal Pigment Epithelium: Expression, Activity, and Induction by Ethanol. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6855–6863. [Google Scholar] [CrossRef]
- Flores-Bellver, M.; Bonet-Ponce, L.; Barcia, J.M.; Garcia-Verdugo, J.M.; Martinez-Gil, N.; Saez-Atienzar, S.; Sancho-Pelluz, J.; Jordan, J.; Galindo, M.F.; Romero, F.J. Autophagy and mitochondrial alterations in human retinal pigment epithelial cells induced by ethanol: Implications of 4-hydroxy-nonenal. Cell Death Dis. 2014, 5, e1328. [Google Scholar] [CrossRef]
- Jin, M.; Ande, A.; Kumar, A.; Kumar, S. Regulation of cytochrome P450 2e1 expression by ethanol: Role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013, 4, e554. [Google Scholar] [CrossRef]
- Maisto, R.; Oltra, M.; Vidal-Gil, L.; Martínez-Gil, N.; Sancho-Pellúz, J.; Filippo, C.D.; Rossi, S.; Amico, M.D.; Barcia, J.M.; Romeroc, F.J. ARPE-19-derived VEGF-containing exosomes promote neovascularization in HUVEC: The role of the melanocortin receptor 5. Cell Cycle 2019, 18, 413–424. [Google Scholar] [CrossRef]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J.M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef]
- Goldman, C.K.; Kendall, R.L.; Cabrera, G.; Soroceanu, L.; Heike, Y.; Gillespie, G.Y.; Siegal, G.P.; Mao, X.; Bett, A.J.; Huckle, W.R.; et al. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc. Natl. Acad. Sci. USA 1998, 95, 8795–8800. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cheng, R.; Benyajati, S.; Ma, J.X. PEDF and its roles in physiological and pathological conditions: Implication in diabetic and hypoxia-induced angiogenic diseases. Clin. Sci. 2015, 128, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Wang, J.J.; Gao, G.; Shao, C.; Mott, R.; Ma, J.X. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006, 20, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, M.; Rohrer, B. Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.Y.; Sivaprasad, S.; Patel, N.; Donaldson, T.A.; Luthert, P.J.; Chong, N.V. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye 2007, 21, 1511–1515. [Google Scholar] [CrossRef]
- Kernt, M.; Hirneiss, C.; Wolf, A.; Liegl, R.; Rueping, J.; Neubauer, A.; Alge, C.; Ulbig, M.; Gandorfer, A.; Kampik, A. Indocyanine green increases light-induced oxidative stress, senescence, and matrix metalloproteinases 1 and 3 in human RPE cells. Acta Ophthalmol. 2012, 90, 571–579. [Google Scholar] [CrossRef]
- Demeestere, D.; Dejonckheere, E.; Steeland, S.; Hulpiau, P.; Haustraete, J.; Devoogdt, N.; Wichert, R.; Becker-Pauly, C.; van Wonterghem, E.; Dewaele, S.; et al. Development and validation of a small single-domain antibody that effectively inhibits matrix metalloproteinase 8. Mol. Ther. 2016, 24, 890–902. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Mohammad, G.; Nawaz, M.I.; Siddiquei, M.M.; Van den Eynde, K.; Mousa, A.; Hertogh, G.D.; Opdenakker, G. Relationship between vitreous levels of matrix metalloproteinases and vascular endothelial growth factor in proliferative diabetic retinopathy. PLoS ONE 2013, 8, e85857. [Google Scholar] [CrossRef]
- Tah, V.; Orlans, H.O.; Hyer, J.; Casswell, E.; Din, N.; Sri Shanmuganathan, V.; Ramskold, L.; Pasu, S. Anti-VEGF therapy and the retina: An update. J. Ophthalmol. 2015, 2015, 627674. [Google Scholar] [CrossRef]
- Chen, Q.; Cederbaum, A.I. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol. Pharmacol. 1998, 53, 638–648. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Prakasam, A.; Fant, J.; Fauq, A.; Crosson, C.; Sambamurti, K. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J. Biol. Chem. 2009, 284, 30177–30186. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Locatelli-Hoops, S.; Kenealey, J.; DesJardin, J.; Notari, L.; Becerra, S.P. Pigment epithelium-derived factor (PEDF) prevents retinal cell death via PEDF Receptor (PEDF-R): Identification of a functional ligand binding site. J. Biol. Chem. 2013, 288, 23928–23942. [Google Scholar] [CrossRef] [PubMed]
- Prosser, H.C.; Tan, J.T.; Dunn, L.L.; Patel, S.; Vanags, L.Z.; Bao, S.; Ng, M.K.C.; Bursill, C.A. Multifunctional regulation of angiogenesis by high-density lipoproteins. Cardiovasc. Res. 2014, 101, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cederbaum, A.I. CYP2E1 potentiation of LPS and TNFα-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr. 2010, 5, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; El-Kadi, A.O. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr. Drug Metab. 2009, 10, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, M.T.; Brekken, R.A. Phosphorylation of Akt and ERK1/2 is required for VEGF-A/VEGFR2-induced proliferation and migration of lymphatic endothelium. PLoS ONE 2011, 6, e28947. [Google Scholar] [CrossRef]
- Cao, G.; Chen, M.; Song, Q.; Liu, Y.; Xie, L.; Han, Y.; Liu, Z.; Ji, Y.; Jiang, Q. EGCG protects against UVB-induced apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19 cells. Mol. Med. Rep. 2012, 5, 54–59. [Google Scholar]
- Du, H.; Sun, X.; Guma, M.; Luo, J.; Ouyang, H.; Zhang, H.; Zeng, J.; Quach, J.; Nguyen, D.H.; Shaw, P.X.; et al. JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 2377–2382. [Google Scholar] [CrossRef]
- Papa, S.; Zazzeroni, F.; Pham, C.G.; Bubici, C.; Franzoso, G. Linking JNK signaling to NF-kappaB: A key to survival. J. Cell Sci. 2004, 117, 5197–5208. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Gil, N.; Vidal-Gil, L.; Flores-Bellver, M.; Maisto, R.; Sancho-Pelluz, J.; Diaz-Llopis, M.; M. Barcia, J.; Romero, F.J. Ethanol-Induced Oxidative Stress Modifies Inflammation and Angiogenesis Biomarkers in Retinal Pigment Epithelial Cells (ARPE-19): Role of CYP2E1 and its Inhibition by Antioxidants. Antioxidants 2020, 9, 776. https://doi.org/10.3390/antiox9090776
Martinez-Gil N, Vidal-Gil L, Flores-Bellver M, Maisto R, Sancho-Pelluz J, Diaz-Llopis M, M. Barcia J, Romero FJ. Ethanol-Induced Oxidative Stress Modifies Inflammation and Angiogenesis Biomarkers in Retinal Pigment Epithelial Cells (ARPE-19): Role of CYP2E1 and its Inhibition by Antioxidants. Antioxidants. 2020; 9(9):776. https://doi.org/10.3390/antiox9090776
Chicago/Turabian StyleMartinez-Gil, Natalia, Lorena Vidal-Gil, Miguel Flores-Bellver, Rosa Maisto, Javier Sancho-Pelluz, Manuel Diaz-Llopis, Jorge M. Barcia, and Francisco J. Romero. 2020. "Ethanol-Induced Oxidative Stress Modifies Inflammation and Angiogenesis Biomarkers in Retinal Pigment Epithelial Cells (ARPE-19): Role of CYP2E1 and its Inhibition by Antioxidants" Antioxidants 9, no. 9: 776. https://doi.org/10.3390/antiox9090776
APA StyleMartinez-Gil, N., Vidal-Gil, L., Flores-Bellver, M., Maisto, R., Sancho-Pelluz, J., Diaz-Llopis, M., M. Barcia, J., & Romero, F. J. (2020). Ethanol-Induced Oxidative Stress Modifies Inflammation and Angiogenesis Biomarkers in Retinal Pigment Epithelial Cells (ARPE-19): Role of CYP2E1 and its Inhibition by Antioxidants. Antioxidants, 9(9), 776. https://doi.org/10.3390/antiox9090776





