Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of G. uralensis Extract
2.2. Microbial Preparation
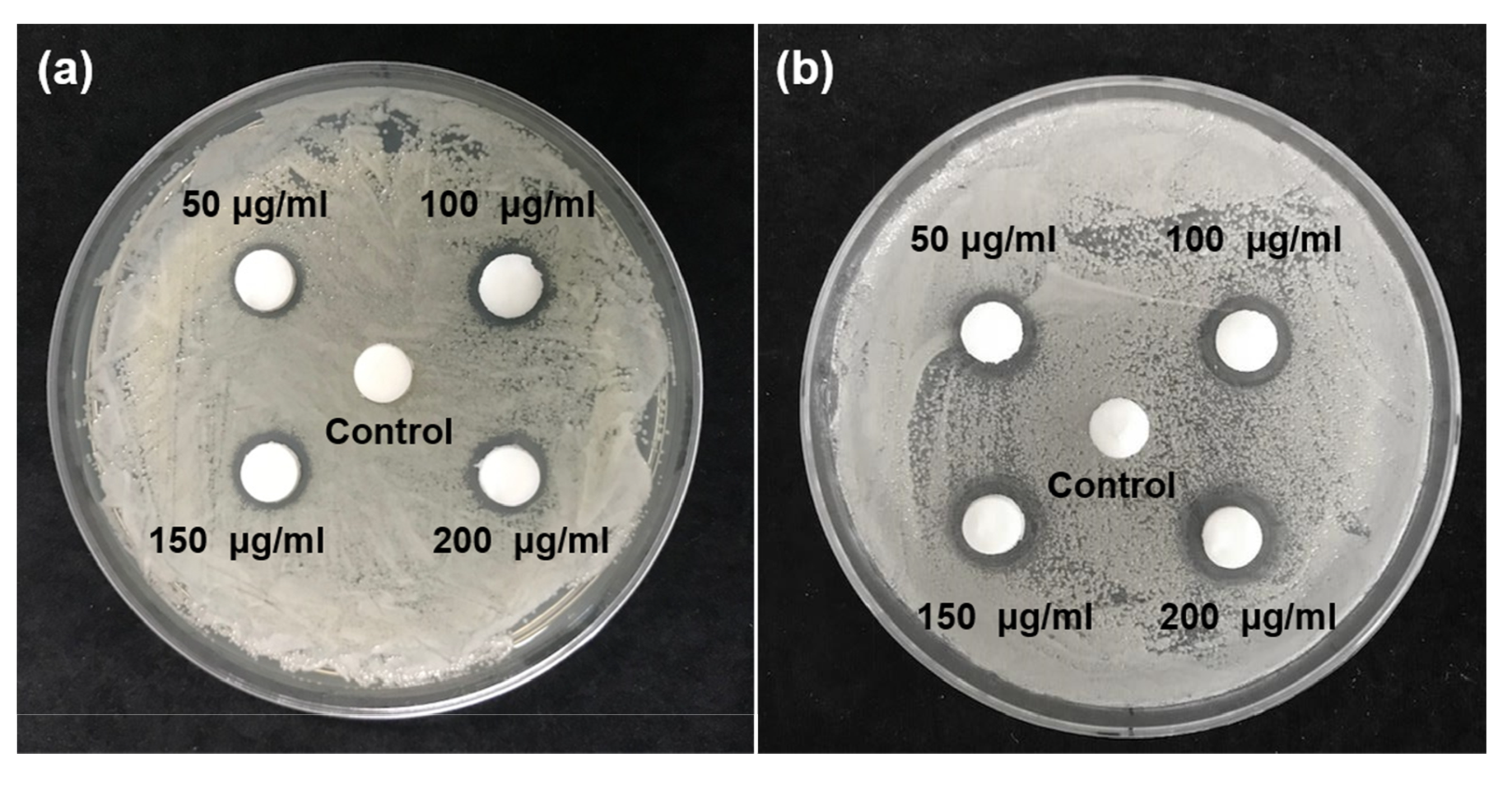
2.3. Inhibition Zone Test
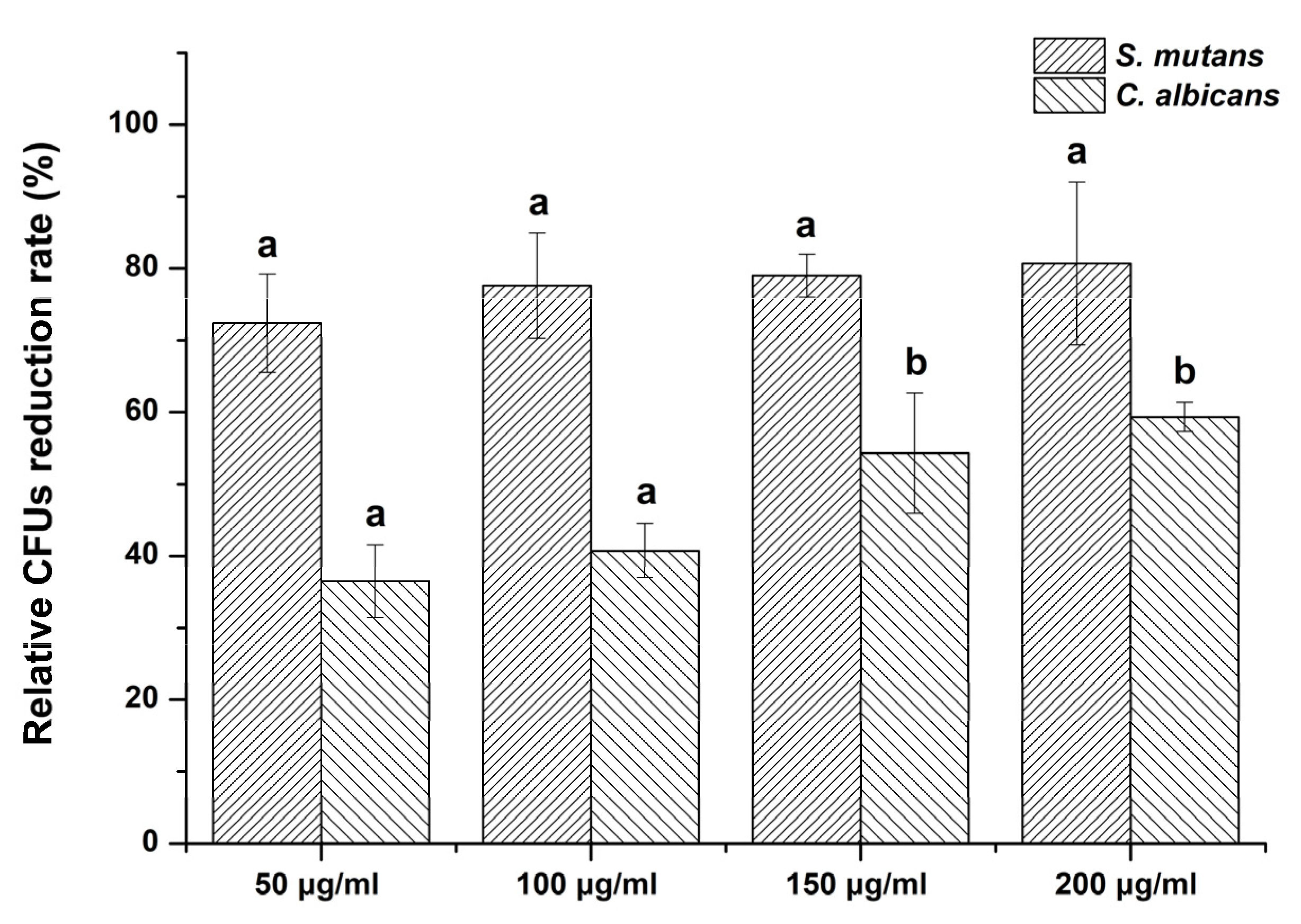
2.4. Evaluation of Colony-Forming Units (CFUs)
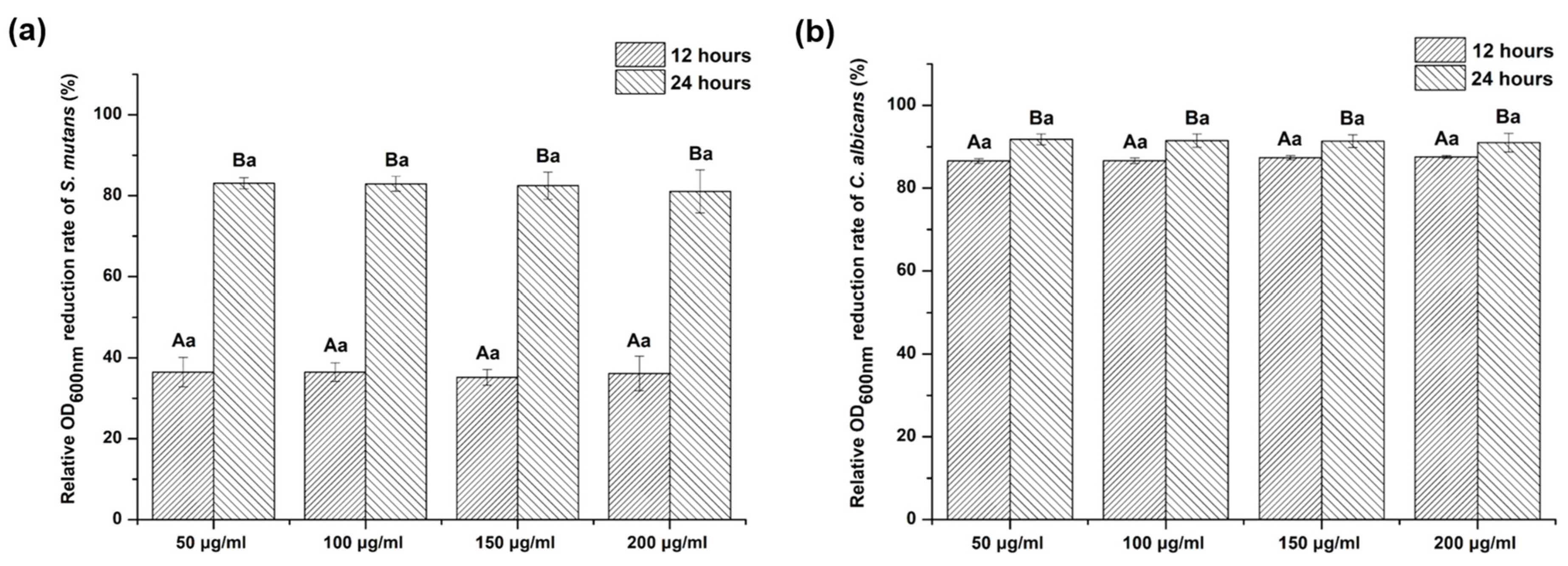
2.5. Evaluation of Optical Density (OD)
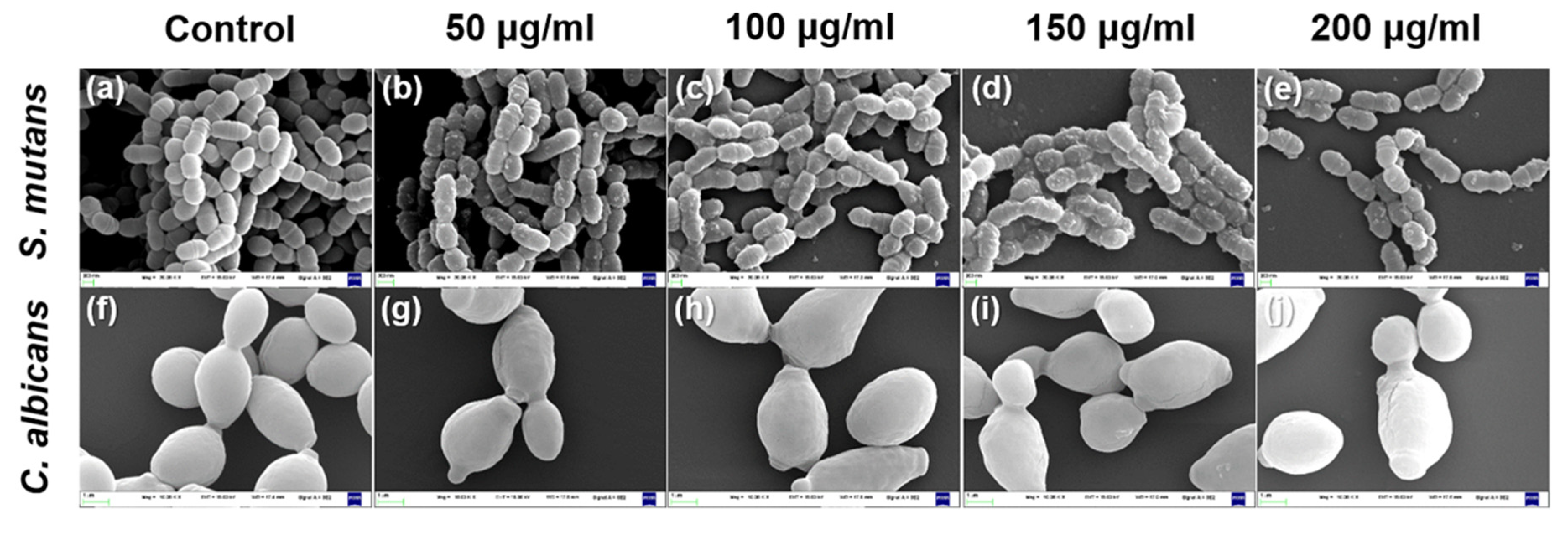
2.6. Morphological Observations
2.7. Measurement of Polyphenol and Flavonoid Contents
2.8. Cytotoxicity Tests
2.9. Statistical Analysis
3. Results
3.1. Inhibition Zone
3.2. Colony-Forming Units
3.3. Growth Inhibitory Effect
3.4. Characterization of Microbial Morphology
3.5. Polyphenol and Flavonoid Contents
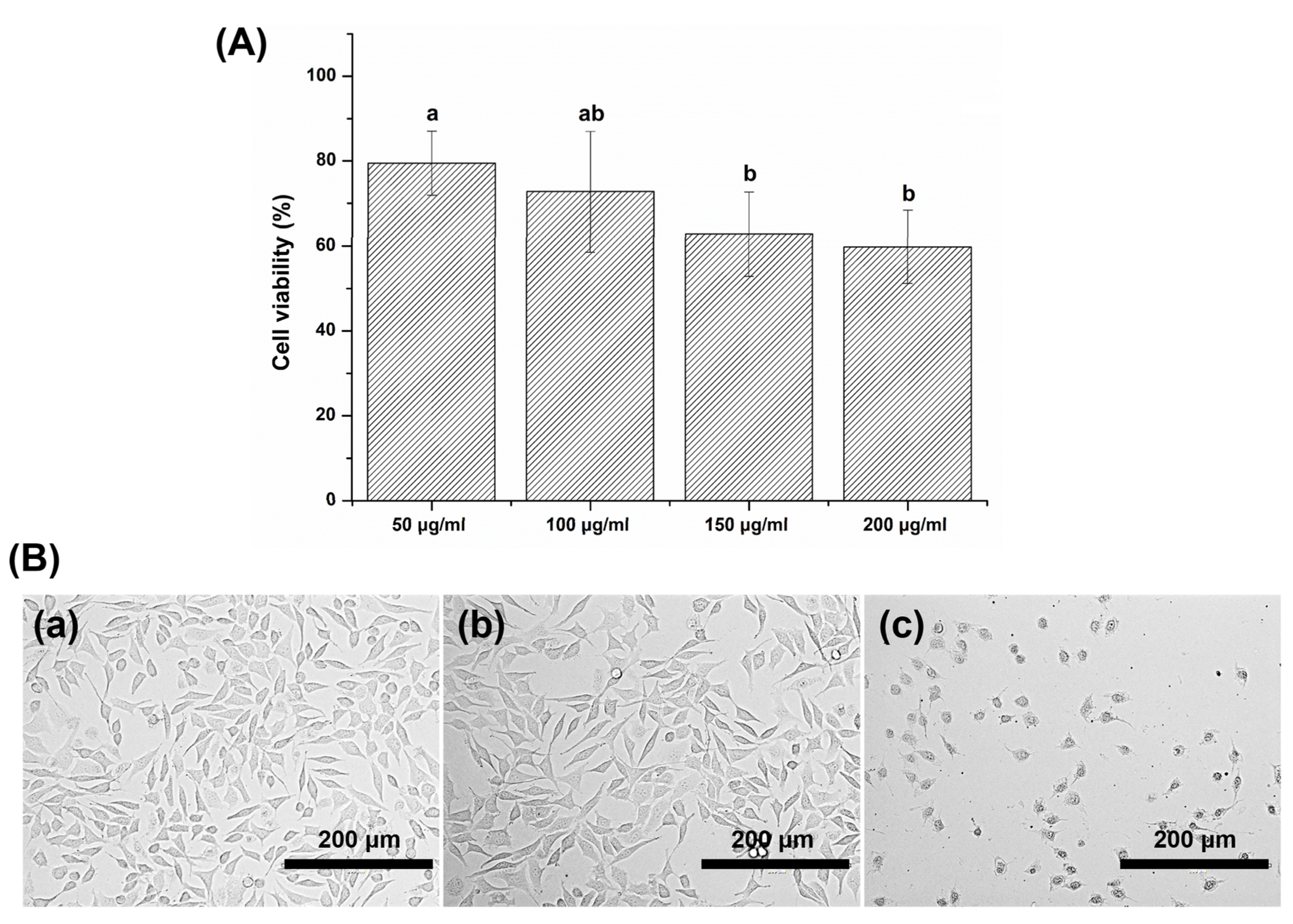
3.6. Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hammad, M.; Sallal, A.K.; Darmani, H. Inhibition of Streptococcus mutans adhesion to buccal epithelial cells by an aqueous extract of Thymus vulgaris. Int. J. Dent. Hyg. 2007, 5, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Rosan, B.; Lamont, R.J. Dental plaque formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Cateau, E.; Berjeaud, J.M.; Rodier, M.H.; Imbert, C. Fungal biofilm inhibition by a component naturally produced by Candida albicans yeasts growing as a biofilm. Int. J. Antimicrob. Agents 2008, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.W.; Lee, S.Y.; Kim, S.G.; Heo, Y.R.; Son, M.K. Antimicrobial, Antioxidant and Cytotoxic Activities of Dendropanax morbifera Léveille extract for mouthwash and denture cleaning solution. J. Adv. Prosthodont. 2016, 8, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Jagger, D.C.; Harrison, A. Denture cleansing—The best approach. Br. Dent. J. 1995, 178, 413–417. [Google Scholar] [CrossRef]
- Lee, H.; Li, C.; Chang, H.; Yang, Y.; Wu, J. Effects of different denture cleaning methods to remove Candida albicans from acrylic resin denture based material. J. Dent. Sci. 2011, 6, 216–220. [Google Scholar] [CrossRef]
- Almas, K.; Skaug, N.; Ahmad, I. An in vitro antimicrobial comparison of miswak extract with commercially available non-alcohol mouthrinses. Int. J. Dent. Hyg. 2005, 3, 18–24. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.J.; Hong, M.; Choi, H.G.; Kim, J.A.; Lee, S. Investigation of selective inhibitory effects of glycyrol on human CYP 1A1 and 2C9. Xenobiotica 2016, 46, 857–861. [Google Scholar] [CrossRef]
- Hatano, T.; Aga, Y.; Shintani, Y.; Ito, H.; Okuda, T.; Yoshida, T. Minor flavonoids from licorice. Phytochemistry 2000, 55, 959–963. [Google Scholar] [CrossRef]
- Tsukiyama, R.; Katsura, H.; Tokuriki, N.; Kobayashi, M. Antibacterial activity of licochalcone a against spore-forming bacteria. Antimicrob. Agents Chemother. 2002, 46, 1226–1230. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.Y. Antibacterial compounds from Glycyrrhiza uralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Agnello, M.; Dinis, M.; Chien, K.C.; Wang, J.; Hu, W.; Shi, W.; He, X.; Zou, J. Lollipop containing Glycyrrhiza uralensis extract reduces Streptococcus mutans colonization and maintains oral microbial diversity in Chinese preschool children. PLoS ONE 2019, 14, e0221756. [Google Scholar] [CrossRef] [PubMed]
- Villinski, J.R.; Bergeron, C.; Cannistra, J.C.; Gloer, J.B.; Coleman, C.M.; Ferreira, D.; Azelmat, J.; Grenier, D.; Gafner, S. Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J. Nat. Prod. 2014, 77, 521–526. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 10993-12: 2012 Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- ISO. ISO 10993-5: 2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Campos, K.P.L.; Viana, G.M.; Cabral, L.M.; Portela, M.B.; Hirata Junior, R.; Cavalcante, L.M.; Lourenço, E.J.V.; Telles, D.M. Self-cured resin modified by quaternary ammonium methacrylates and chlorhexidine: Cytotoxicity, antimicrobial, physical, and mechanical properties. Dent. Mater. 2020, 36, 68–75. [Google Scholar] [CrossRef]
- Guandalini Cunha, B.; Duque, C.; Sampaio Caiaffa, K.; Massunari, L.; Araguê Catanoze, I.; Dos Santos, D.M.; de Oliveira, S.H.P.; Guiotti, A.M. Cytotoxicity and antimicrobial effects of citronella oil (Cymbopogon nardus) and commercial mouthwashes on S. aureus and C. albicans biofilms in prosthetic materials. Arch. Oral Biol. 2020, 109, 104577. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of plant roots to spectral quality of light and UV-B radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Shin, D.H.; Baek, N.I.; Oh, J.A. Isolation and identification of antimicrobial activity substance from Glycyrrhiza uralensis FISCH. Korean J. Food Sci. Technol. 1998, 30, 680–687. [Google Scholar]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, A.; Hisaka, S.; Hayashi, H.; Nose, M. Effect of hot water extract of a glycyrrhizin-deficient strain of Glycyrrhiza uralensis on contact hypersensitivity in mice. J. Nat. Med. 2020, 74, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiao, Y.; Sun, Y.; Gao, S. In vitro production and distribution of flavonoids in Glycyrrhiza uralensis Fisch. J. Food Sci. Technol. 2020, 57, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ji, Y.J.; Yu, M.H.; Bo, M.H.; Seo, H.J.; Lee, S.P.; Lee, I.S. Antimicrobial effect and resistant regulation of Glycyrrhiza uralensis on methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 2009, 23, 101–111. [Google Scholar] [CrossRef]
- Gafner, S.; Bergeron, C.; Villinski, J.R.; Godejohann, M.; Kessler, P.; Cardellina, J.H.; Ferreira, D.; Feghali, K.; Grenier, D. Isoflavonoids and coumarins from Glycyrrhiza uralensis: Antibacterial activity against oral pathogens and conversion of isoflavans into isoflavan-quinones during purification. J. Nat. Prod. 2011, 74, 2514–2519. [Google Scholar] [CrossRef]
- Park, C.G.; Bang, K.H.; Lee, S.E.; Cha, M.S.; Seong, J.S.; Park, S.U.; Seong, N.S. Antimicrobial effect of various medicinal herb on Staphylococcus aureus. Korean J. Med. Crop Sci. 2001, 9, 251–258. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Kwan Hwang, J.K. The effects of xanthorrhizol on the morphology of Candida cells examined by scanning electron microscopy. Microbiol. Indones. 2007, 1, 98–100. [Google Scholar] [CrossRef][Green Version]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, K.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef]
- Tao, W.W.; Duan, J.A.; Yang, N.Y.; Tang, Y.P.; Liu, M.Z.; Qian, Y.F. Antithrombotic phenolic compounds from Glycyrrhiza uralensis. Fitoterapia 2012, 83, 422–425. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial Activity of the Phenolic Compounds of Prunus mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In vitro antimicrobial activity of some phenolic compounds (coumarin and quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar] [CrossRef]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Özcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Geurtsen, W.; Lehmann, F.; Spahl, W.; Leyhausen, G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J. Biomed. Mater. Res. 1998, 41, 474–480. [Google Scholar] [CrossRef]
- Jevremović, D.; Kojić, V.; Bogdanović, G.; Puškar, T.; Eggbeer, D.; Thomas, D.; Williams, R. A selective laser melted Co-Cr alloy used for the rapid manufacture of removable partial denture frameworks: Initial screening of biocompatibility. J. Serb. Chem. Soc. 2011, 76, 43–52. [Google Scholar] [CrossRef]
| Experimental Group | Polyphenol Content (μg/mL) | Flavonoid Content (μg/mL) |
|---|---|---|
| 50 μg/mL | 13.1 ± 3.4 a | 21.7 ± 1.6 a |
| 100 μg/mL | 13.8 ± 1.4 a | 21.8 ± 1.0 a |
| 150 μg/mL | 16.2 ± 0.8 a | 21.8 ± 0.9 a |
| 200 μg/mL | 17.7 ± 3.9 a | 25.0 ± 1.6 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-Y.; Choi, Y.-R.; Lee, M.-J.; Kang, M.-K. Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract. Plants 2020, 9, 838. https://doi.org/10.3390/plants9070838
Yang S-Y, Choi Y-R, Lee M-J, Kang M-K. Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract. Plants. 2020; 9(7):838. https://doi.org/10.3390/plants9070838
Chicago/Turabian StyleYang, Song-Yi, Yu-Ri Choi, Myung-Jin Lee, and Min-Kyung Kang. 2020. "Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract" Plants 9, no. 7: 838. https://doi.org/10.3390/plants9070838
APA StyleYang, S.-Y., Choi, Y.-R., Lee, M.-J., & Kang, M.-K. (2020). Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract. Plants, 9(7), 838. https://doi.org/10.3390/plants9070838






