Mucosal Vaccination with Lactococcus lactis-Secreting Surface Immunological Protein Induces Humoral and Cellular Immune Protection against Group B Streptococcus in a Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mouse Strains
2.3. Production of Recombinant L. lactis that Secretes rSIP from GBS
2.4. Secretion of SIP from GBS in L. lactis NZ9000
2.5. Oral Immunization and GBS Vaginal Colonization
2.6. Opsonophagocytic Assay
2.7. Analysis of Immunoglobulins from Small Intestine and Feces
2.8. Measures of Immunoglobulins by ELISA
2.9. Analysis of Small Intestinal Mucosal Cells and T Cell Activation
2.10. Analysis of Regulatory T cells (Tregs)
2.11. Relative Quantification of Transcriptions Factors by Real-Time PCR
2.12. Restimulation of Splenocytes
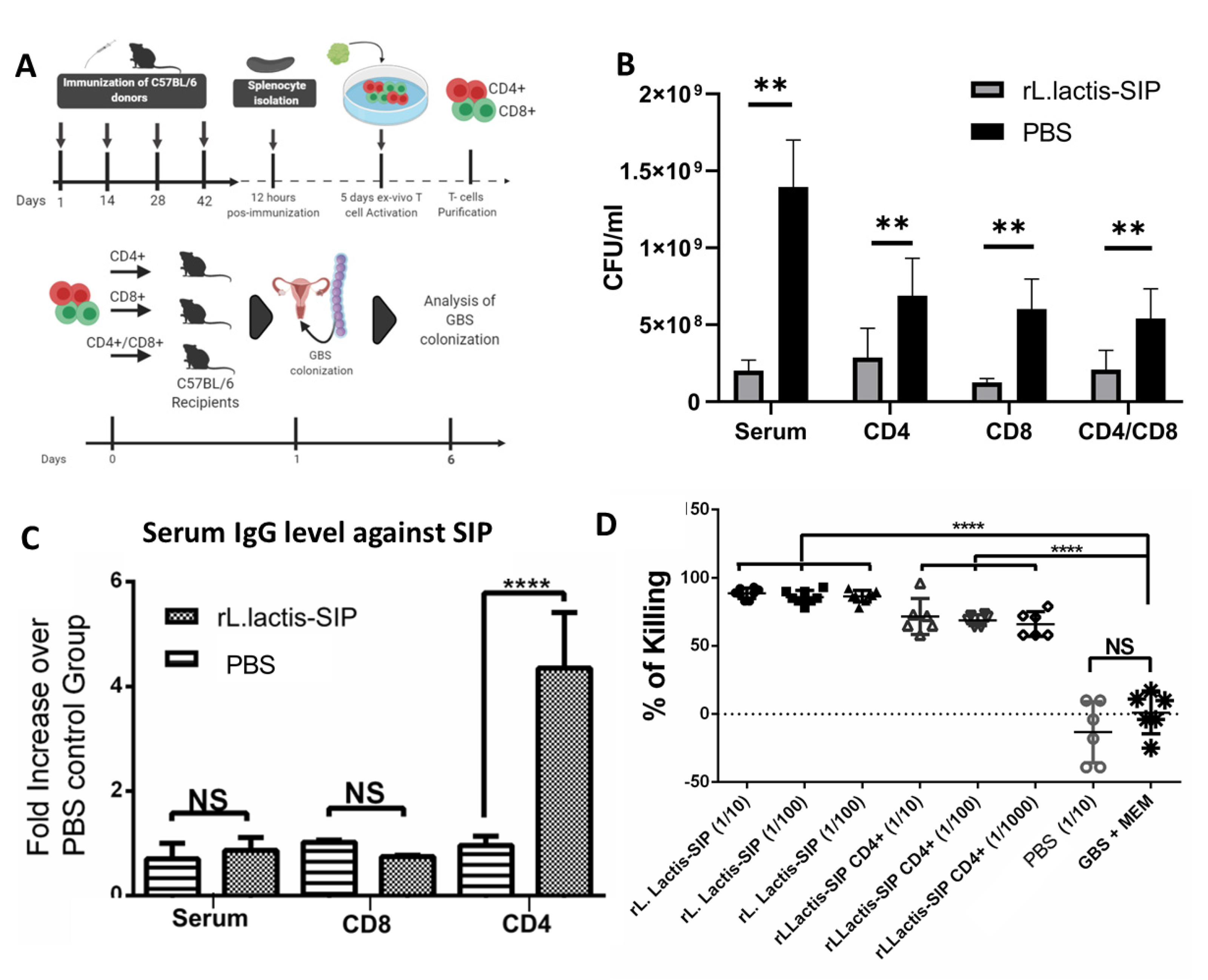
2.13. Passive Transfer of Immune IgG and T Cells (CD4+ and CD8+) from rL. lactis-Vaccinated Mice to Naïve Animals
2.14. Statistical Analyses
3. Results
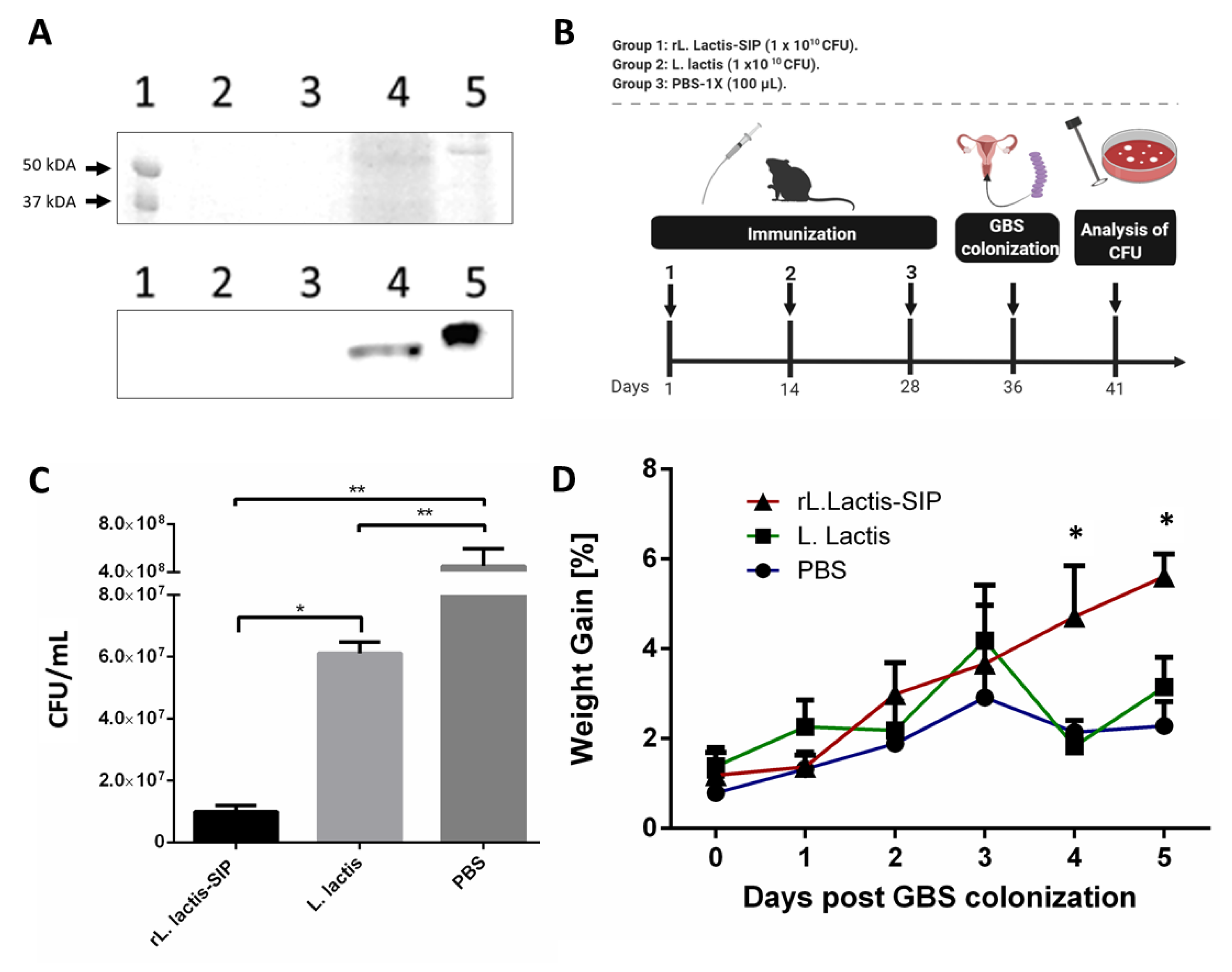
3.1. Secretion of rSIP by Recombinant L. lactis
3.2. Oral Immunization with rL. lactis-SIP Decreased Vaginal-Tract Colonization by GBS in Mice
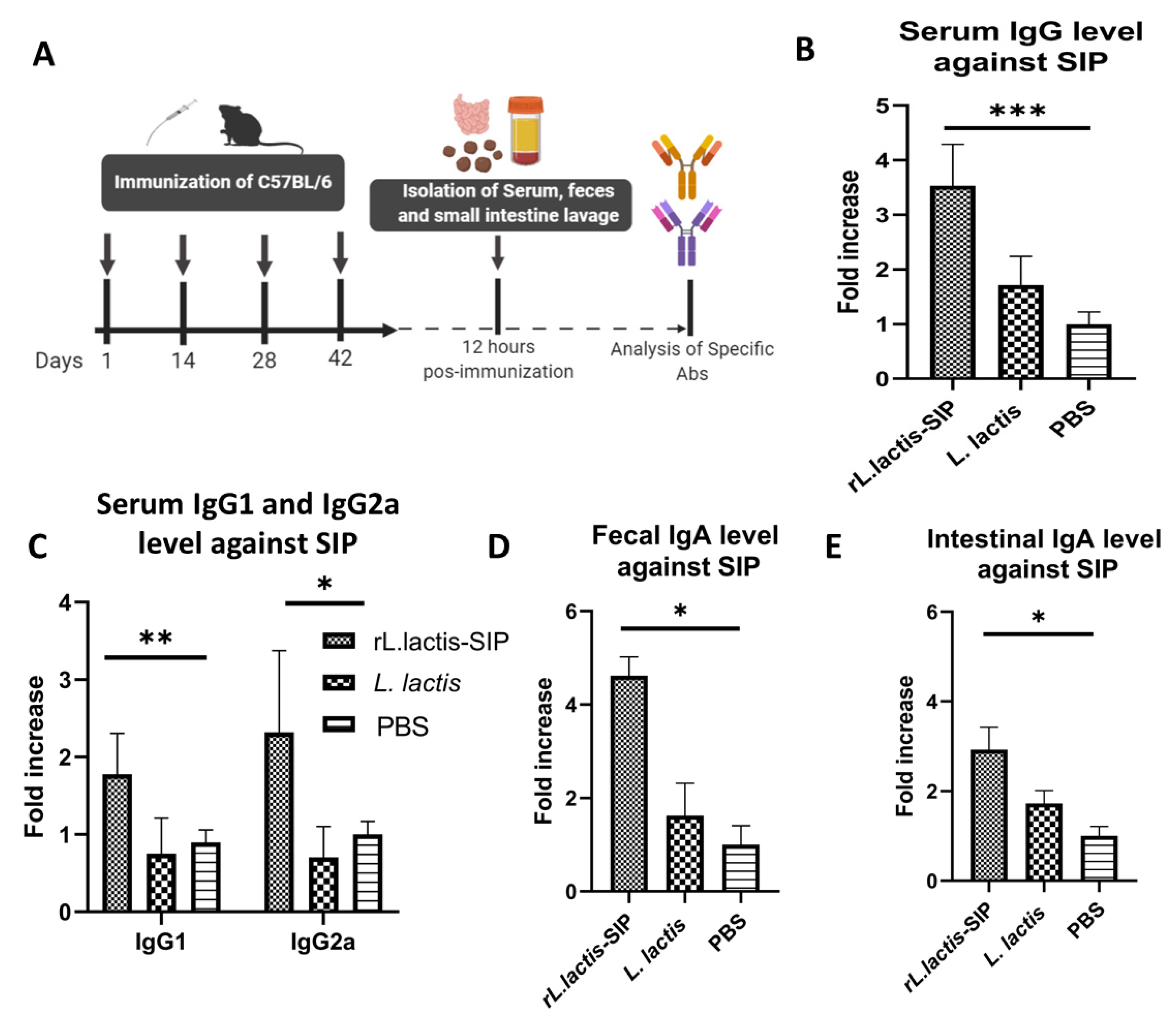
3.3. Oral Immunization with rL. Lactis-SIP Stimulated Secretions of Mucosal and Systemic Anti–SIP IgG and IgA
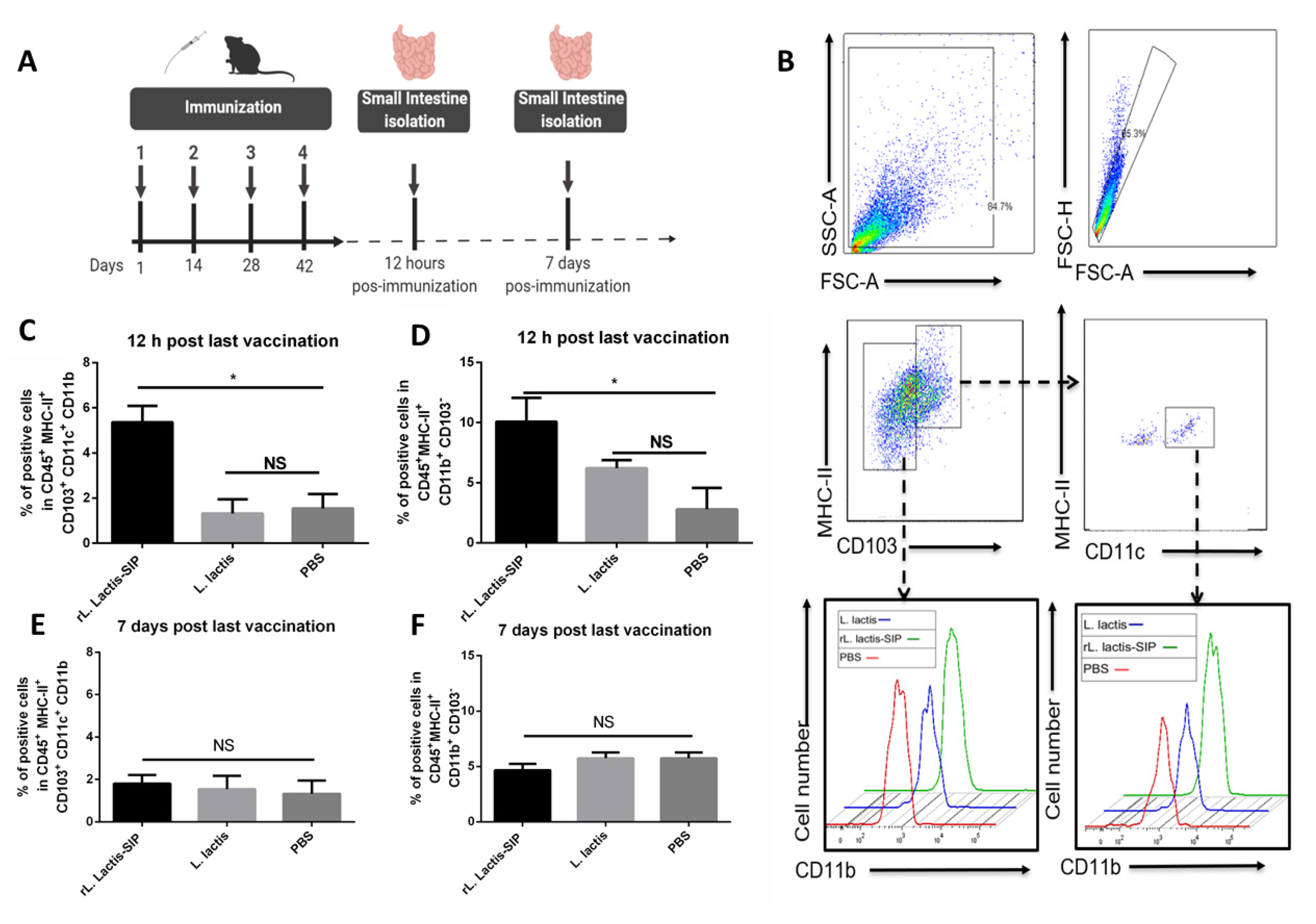
3.4. Oral Vaccine Using rL. Lactis-SIP Increased Intestinal Antigen-Presenting Cells
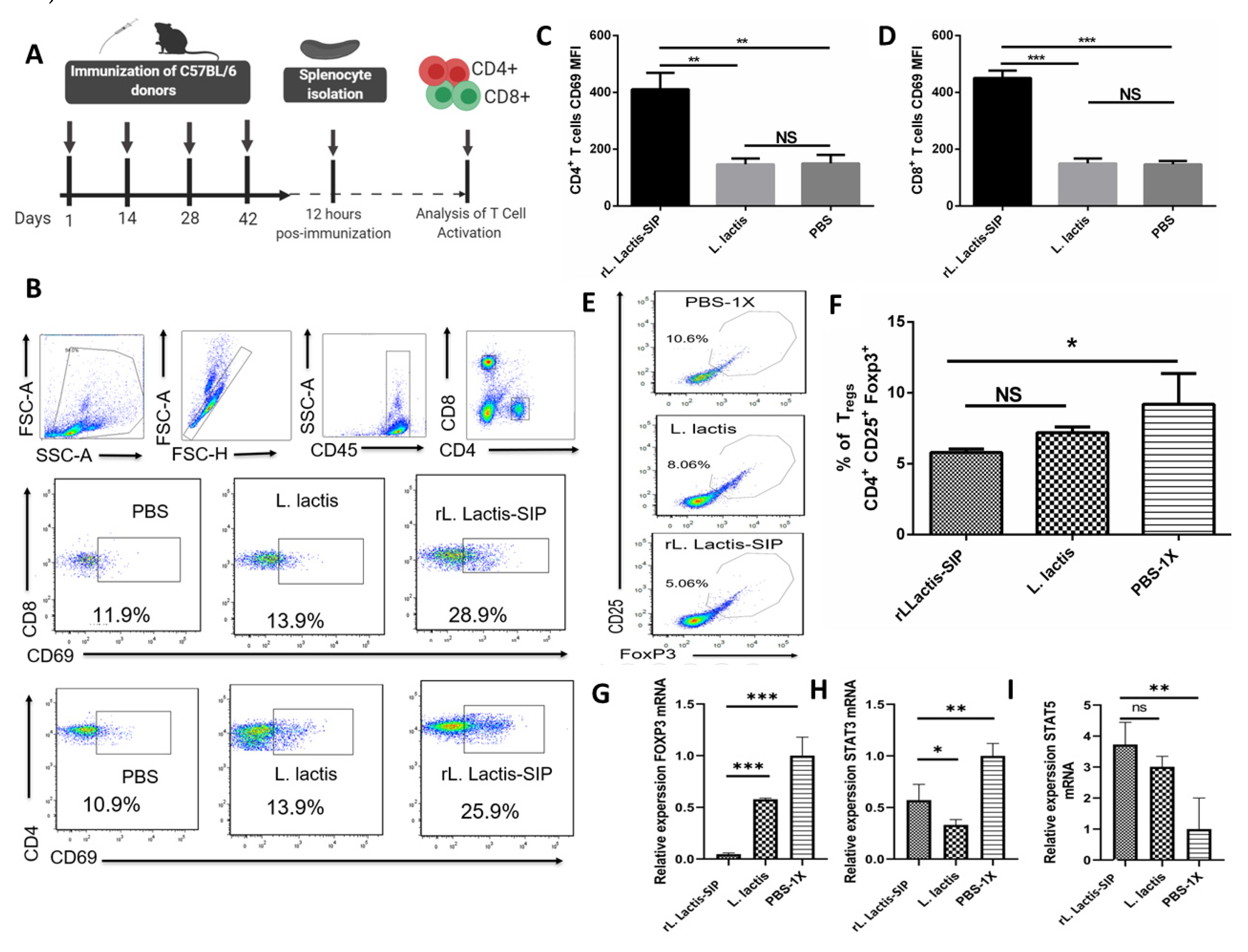
3.5. Immunization with rL. lactis-SIP Induced T cell Activation and Decreased Regulatory T cells
3.6. Adoptive Humoral and T Cell Transfer from rL. lactis-SIP- Immunized Mice Decreased GBS Colonization
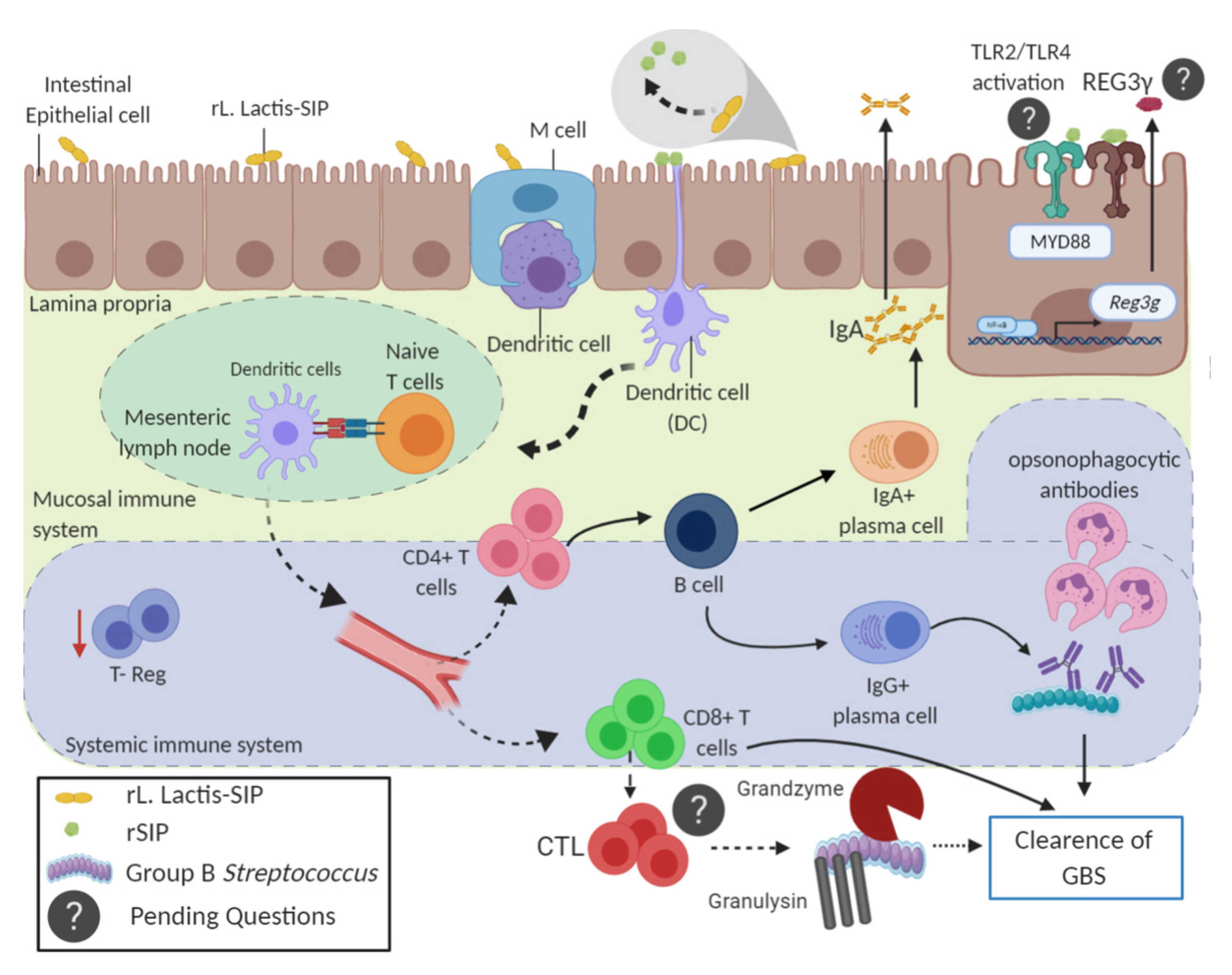
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bliss, S.J.; Manning, S.D.; Tallman, P.; Baker, C.J.; Pearlman, M.D.; Marrs, C.F.; Foxman, B. Group B Streptococcus colonization in male and nonpregnant female university students: A cross-sectional prevalence study. Clin. Infect. Dis. 2002, 34, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landwehr-Kenzel, S.; Henneke, P. Interaction of Streptococcus agalactiae and cellular innate immunity in colonization and disease. Front. Immunol. 2014, 5, 519. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J.; Gravett, M.G. Maternal colonization with group B Streptococcus and serotype distribution worldwide: Systematic review and meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. 2), S100–S111. [Google Scholar] [CrossRef]
- Farías, M.; Rodriguez, A.; Diaz-Dinamarca, D.; Soto, D.; Bastias, D.; Quezada, M.; Vasquez, A.E. Estudio de la portación de Streptococcus agalactiae en mujeres embarazadas atendidas en el Hospital Clínico de la Pontificia Universidad Católica de Chile durante 2011-17. Revista del Inst. de Salud Pública de Chile 2018, 2. [Google Scholar] [CrossRef]
- Seale, A.C.; Blencowe, H.; Bianchi-Jassir, F.; Embleton, N.; Bassat, Q.; Ordi, J.; Lawn, J.E. Stillbirth with group B Streptococcus disease worldwide: Systematic review and meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. 2), S125–S132. [Google Scholar] [CrossRef]
- Davies, H.G.; Carreras-Abad, C.; Le Doare, K.; Heath, P.T. Group B Streptococcus: Trials and tribulations. Pediatr. Infect. Dis. J. 2019, 38, S72–S76. [Google Scholar] [CrossRef]
- Bidmos, F.A.; Siris, S.; Gladstone, C.A.; Langford, P.R. Bacterial vaccine antigen discovery in the reverse vaccinology 2.0 Era: Progress and challenges. Front. Immunol. 2018, 9, 2315. [Google Scholar] [CrossRef] [Green Version]
- Bellais, S.; Six, A.; Fouet, A.; Longo, M.; Dmytruk, N.; Glaser, P.; Poyart, C. Capsular switching in group B Streptococcus CC17 hypervirulent clone: A future challenge for polysaccharide vaccine development. J. Infect. Dis. 2012, 206, 1745–1752. [Google Scholar] [CrossRef] [Green Version]
- Vekemans, J.; Moorthy, V.; Friede, M.; Alderson, M.R.; Sobanjo-Ter Meulen, A.; Baker, C.J.; Schrag, S. Maternal immunization against group B streptococcus: World Health Organization research and development technological roadmap and preferred product characteristics. Vaccine 2019, 37, 7391–7393. [Google Scholar] [CrossRef]
- Pietrocola, G.; Rindi, S.; Rosini, R.; Buccato, S.; Speziale, P.; Margarit, I. The group B Streptococcus–secreted protein CIP interacts with C4, preventing C3b deposition via the Lectin and classical complement pathways. J. Immunol. 2016, 196, 385–394. [Google Scholar] [CrossRef]
- Ali, S.R.; Fong, J.J.; Carlin, A.F.; Busch, T.D.; Linden, R.; Angata, T.; Nizet, V. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J. Exp. Med. 2014, 211, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, B.R.; Boyer, M.; Charlebois, I.; Hamel, J.; Couture, F.; Rioux, C.R.; Martin, D. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect. Immun. 2000, 68, 5610–5618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rioux, S.; Martin, D.; Ackermann, H.-W.; Dumont, J.; Hamel, J.; Brodeur, B.R. Localization of surface immunogenic protein on group b streptococcus. Infect. Immun. 2001, 69, 5162–5165. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Rioux, S.; Gagnon, E.; Boyer, M.; Hamel, J.; Charland, N.; Brodeur, B.R. Protection from group B streptococcal infection in neonatal mice by maternal immunization with recombinant sip protein. Infect. Immun. 2002, 70, 4897–4901. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Dinamarca, D.A.; Manzo, R.A.; Soto, D.A.; Avendaño-Valenzuela, M.J.; Bastias, D.N.; Soto, P.; Wilson, C.A. Surface immunogenic protein of streptococcus group B is an agonist of toll-like receptors 2 and 4 and a potential immune adjuvant. Vaccines 2020, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Yang, Q.; Huang, L.; Wang, K.; Wang, X.; Chen, D.; Lai, W. Effectivity of oral recombinant DNA vaccine against Streptococcus agalactiae in Nile tilapia. Dev. Comp. Immunol. 2017, 77, 77–87. [Google Scholar] [CrossRef]
- Díaz-Dinamarca, D.A.; Jerias, J.I.; Soto, D.A.; Soto, J.A.; Díaz, N.V.; Leyton, Y.Y.; Kalergis, A.M.; Vásquez, A.E. The optimisation of the expression of recombinant surface immunogenic protein of group B Streptococcus in Escherichia coli by response surface methodology improves humoral immunity. Mol. Biotechnol. 2018, 60, 215–225. [Google Scholar] [CrossRef]
- Soto, J.A.; Diaz-Dinamarca, D.A.; Soto, D.A.; Barrientos, M.J.; Carrión, F.; Kalergis, A.M.; Vasquez, A.E. Cellular immune response induced by surface immunogenic protein with AbISCO-100 adjuvant vaccination decreases group B Streptococcus vaginal colonization. Mol. Immunol. 2019, 111, 198–204. [Google Scholar] [CrossRef]
- Church, J.A.; Parker, E.P.; Kirkpatrick, B.D.; Grassly, N.C.; Prendergast, A.J. Interventions to improve oral vaccine performance: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Sit, B.; Zhang, T.; Fakoya, B.; Akter, A.; Biswas, R.; Ryan, E.T.; Waldor, M.K. Oral immunization with a probiotic cholera vaccine induces broad protective immunity against Vibrio cholerae colonization and disease in mice. PLoS Negl. Trop. Dis. 2019, 13, e0007417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, P.M.; Brown, H.W.G.; Le Page, R.W.F. The immune response to Lactococcus lactis: Implications for its use as a vaccine delivery vehicle. FEMS Microbiol. Lett. 1994, 120, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sáez, D.; Fernández, P.; Rivera, A.; Andrews, E.; Oñate, A. Oral immunization of mice with recombinant Lactococcus lactis expressing Cu, Zn superoxide dismutase of Brucella abortus triggers protective immunity. Vaccine 2012, 30, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, D.; Sun, B.; Yu, M.; Wang, Y.; Ru, Y.; Li, Y. Oral vaccination with the porcine circovirus type 2 (PCV-2) capsid protein expressed by Lactococcus lactis induces a specific immune response against PCV-2 in mice. J. Appl. Microbiol. 2020, 128, 74–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klijn, N.; Weerkamp, A.H.; De Vos, W.M. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl. Environ. Microbiol. 1995, 61, 2771–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohseni, A.H.; Taghinezhad-S, S.; Keyvani, H. The first clinical use of a recombinant Lactococcus lactis expressing human papillomavirus type 16 E7 oncogene oral vaccine: A phase I safety and immunogenicity trial in healthy women volunteers. Mol. Cancer Ther. 2020, 19, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Taghinezhad-S, S.; Mohseni, A.H.; Keyvani, H.; Razavi, M.R. Phase 1 safety and immunogenicity trial of recombinant lactococcus lactis expressing human papillomavirus type 16 E6 oncoprotein vaccine. Mol. Ther. Meth. Clin. D 2019, 15, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Medina, M.S.; Vintiñi, E.O.; Villena, J.; Raya, R.R.; Alvarez, S.G. Lactococcus lactis as an adjuvant and delivery vehicle of antigens against pneumococcal respiratory infections. Bioeng. Bugs 2010, 1, 313–325. [Google Scholar] [CrossRef] [Green Version]
- Van Asseldonk, M.; Simons, A.; Visser, H.; de Vos, W.M.; Simons, G. Cloning, nucleotide sequence, and regulatory analysis of the Lactococcus lactis dnaJ gene. J. Bacteriol. 1993, 175, 1637–1644. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Dinamarca, D.A.; Soto, D.A.; Leyton, Y.Y.; Altamirano-Lagos, M.J.; Avendaño, M.J.; Kalergis, A.M.; Vasquez, A.E. Oral vaccine based on a surface immunogenic protein mixed with alum promotes a decrease in Streptococcus agalactiae vaginal colonization in a mouse model. Mol. Immunol. 2018, 103, 63–70. [Google Scholar] [CrossRef]
- Todryk, S.M. T cell memory to vaccination. Vaccines 2018, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Guttormsen, H.-K.; Liu, Y.; Paoletti, L.C. Functional activity of antisera to group B streptococcal conjugate vaccines measured with an opsonophagocytosis assay and HL-60 effector cells. Hum. Vaccine 2008, 4, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Romero-Saavedra, F.; Laverde, D.; Budin-Verneuil, A.; Muller, C.; Bernay, B.; Benachour, A.; Hartke, A.; Huebner, J. Characterization of two metal binding lipoproteins as vaccine candidates for Enterococcal infections. PLoS ONE 2015, 10, e0136625. [Google Scholar] [CrossRef] [Green Version]
- Harusato, A.; Geem, D.; Denning, T.L. Macrophage isolation from the mouse small and large intestine. Methods Mol. Biol. 2016, 1422, 171–180. [Google Scholar] [PubMed] [Green Version]
- Ruas, L.P.; Bernardes, E.S.; Fermino, M.L.; de Oliveira, L.L.; Hsu, D.K.; Liu, F.T.; Roque-Barreira, M.C. Lack of galectin-3 drives response to Paracoccidioides brasiliensis toward a Th2-biased immunity. PLoS ONE 2009, 4, e4519. [Google Scholar] [CrossRef] [PubMed]
- Bueno, S.M.; González, P.A.; Cautivo, K.M.; Mora, J.E.; Leiva, E.D.; Tobar, H.E.; Kalergis, A.M. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc. Natl. Acad. Sci. USA 2008, 105, 20822–20827. [Google Scholar] [CrossRef] [Green Version]
- Azizpour, M.; Hosseini, S.D.; Jafari, P.; Akbary, N. Lactococcus lactis: A new strategy for vaccination. Avicenna J. Med. Biotechnol. 2017, 9, 163. [Google Scholar]
- Ruane, D.T.; Lavelle, E.C. The role of CD103+ dendritic cells in the intestinal mucosal immune system. Front. Immunol. 2011, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Uematsu, S.; Fujimoto, K.; Jang, M.H.; Yang, B.-G.; Jung, Y.-J.; Nishiyama, M.; Akira, S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing toll-like receptor 5. Nat. Immunol. 2008, 9, 769–776. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of oral tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Ciucci, T.; Vacchio, M.S.; Bosselut, R. A STAT3-dependent transcriptional circuitry inhibits cytotoxic gene expression in T cells. Proc. Natl. Acad. Sci. USA 2017, 114, 13236–13241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.J.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Lin, S.-M.; Zhi, Y.; Song, J.Y. Development of a multiplexed opsonophagocytic killing assay (Mopa) for group B Streptococcus. Hum. Vaccines Immunother. 2018, 14, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Rossi, C.; Guidotti, I.; Vellani, G.; Lugli, L.; Reggiani, M.L.B.; Ferrari, F. Factors associated with intrapartum transmission of group B Streptococcus. Pediatr. Infect. Dis. J. 2014, 33, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Verani, J.R.; McGee, L.; Schrag, S.J. Prevention of perinatal group B streptococcal disease. Morbidity and Mortality Weekly Report (MMWR), Revised Guidelines from CDC. Recomm. Rep. 2010, 59, 1–32. [Google Scholar]
- Giorgakoudi, K.; O’Sullivan, C.; Heath, P.T.; Ladhani, S.; Lamagni, T.; Ramsay, M.; Trotter, C. Cost-effectiveness analysis of maternal immunisation against group B Streptococcus (GBS) disease: A modelling study. Vaccine 2018, 36, 7033–7042. [Google Scholar] [CrossRef]
- Lin, S.M.; Zhi, Y.; Ahn, K.B.; Lim, S.; Seo, H.S. Status of group B streptococcal vaccine development. Clin. Exp. Vaccine Res. 2018, 7, 76–81. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Cortes-Perez, N.G.; Lefèvre, F.; Guimarães, V.; Rabot, S.; Alcocer-Gonzalez, J.M.; Langella, P. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 2005, 175, 7297–7302. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Kharrat, P.; Chatel, J.-M.; Langella, P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA Vaccines. Microb. Cell Fact. 2011, 10 (Suppl. 1), S4. [Google Scholar]
- Coria, L.M.; Risso, G.S.; Guaimas, F.F.; Ferrero, M.C.; Bruno, L.; Pasquevich, K.A.; Cassataro, J. Oral co-administration of a bacterial protease inhibitor in the vaccine formulation increases antigen delivery at the intestinal epithelial barrier. J. Control. Release 2019, 293, 158–171. [Google Scholar] [CrossRef]
- Steidler, L.; Robinson, K.; Chamberlain, L.; Schofield, K.M.; Remaut, E.; Le Page, R.W.; Wells, J.M. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 1998, 66, 3183–3189. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, Y.; Le Berre, R.; Barbier, G.; Le Blay, G. Screening of Lactobacillus spp. for the prevention of Pseudomonas aeruginosa pulmonary infections. BMC Microbiol. 2014, 14, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racedo, S.; Villena, J.; Medina, M.; Agüero, G.; Rodríguez, V.; Alvarez, S. Lactobacillus casei administration reduces lung injuries in a Streptococcus pneumoniae infection in mice. Microbes Infect. 2006, 8, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.R.; Soothill, J.F.; Turner, M.W. Immune exclusion is a function of IgA. Nature 1975, 255, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.-E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Neutra, M.R.; Mantis, N.J.; Kraehenbuhl, J.P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001, 2, 1004–1009. [Google Scholar] [CrossRef]
- Hays, C.; Touak, G.; Bouaboud, A.; Fouet, A.; Guignot, J.; Poyart, C.; Tazi, A. Perinatal hormones favor CC17 group B Streptococcus intestinal translocation through M cells and hypervirulence in neonates. eLife 2019, 8, e48772. [Google Scholar] [CrossRef]
- Ouwehand, A.; Isolauri, E.; Salminen, S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur. J. Nutr. 2002, 41, i32–i37. [Google Scholar] [CrossRef]
- Coombes, J.L.; Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef]
- Schulz, O.; Jaensson, E.; Persson, E.K.; Liu, X.; Worbs, T.; Agace, W.W.; Pabst, O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 2009, 206, 3101–3114. [Google Scholar] [CrossRef]
- Gupalova, T.; Leontieva, G.; Kramskaya, T.; Grabovskaya, K.; Bormotova, E.; Korjevski, D.; Suvorov, A. Development of experimental GBS vaccine for mucosal immunization. PLoS ONE 2018, 13, e0196564. [Google Scholar] [CrossRef] [Green Version]
- Morse, K.; Norimine, J.; Palmer, G.H.; Sutten, E.L.; Baszler, T.V.; Brown, W.C. Association and evidence for linked recognition of type IV secretion system proteins VirB9-1, VirB9-2, and VirB10 in Anaplasma marginale. Infect. Immun. 2012, 80, 215–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotiwala, F.; Lieberman, J. Granulysin: Killer lymphocyte safeguard against microbes. Curr. Opin. Immunol. 2019, 60, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; van Baarlen, P.; Wells, J.M. Host-recognition of pathogens and commensals in the mammalian intestine. Curr. Top. Microbiol. 2011, 358, 291–321. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Dinamarca, D.A.; Hernandez, C.; Escobar, D.F.; Soto, D.A.; Muñoz, G.A.; Badilla, J.F.; Manzo, R.A.; Carrión, F.; Kalergis, A.M.; Vasquez, A.E. Mucosal Vaccination with Lactococcus lactis-Secreting Surface Immunological Protein Induces Humoral and Cellular Immune Protection against Group B Streptococcus in a Murine Model. Vaccines 2020, 8, 146. https://doi.org/10.3390/vaccines8020146
Diaz-Dinamarca DA, Hernandez C, Escobar DF, Soto DA, Muñoz GA, Badilla JF, Manzo RA, Carrión F, Kalergis AM, Vasquez AE. Mucosal Vaccination with Lactococcus lactis-Secreting Surface Immunological Protein Induces Humoral and Cellular Immune Protection against Group B Streptococcus in a Murine Model. Vaccines. 2020; 8(2):146. https://doi.org/10.3390/vaccines8020146
Chicago/Turabian StyleDiaz-Dinamarca, Diego A., Carlos Hernandez, Daniel F. Escobar, Daniel A. Soto, Guillermo A. Muñoz, Jesús F. Badilla, Ricardo A. Manzo, Flavio Carrión, Alexis M. Kalergis, and Abel E. Vasquez. 2020. "Mucosal Vaccination with Lactococcus lactis-Secreting Surface Immunological Protein Induces Humoral and Cellular Immune Protection against Group B Streptococcus in a Murine Model" Vaccines 8, no. 2: 146. https://doi.org/10.3390/vaccines8020146





