Utility and Predictive Value of Human Standard Semen Parameters and Sperm DNA Dispersion for Fertility Potential
Abstract
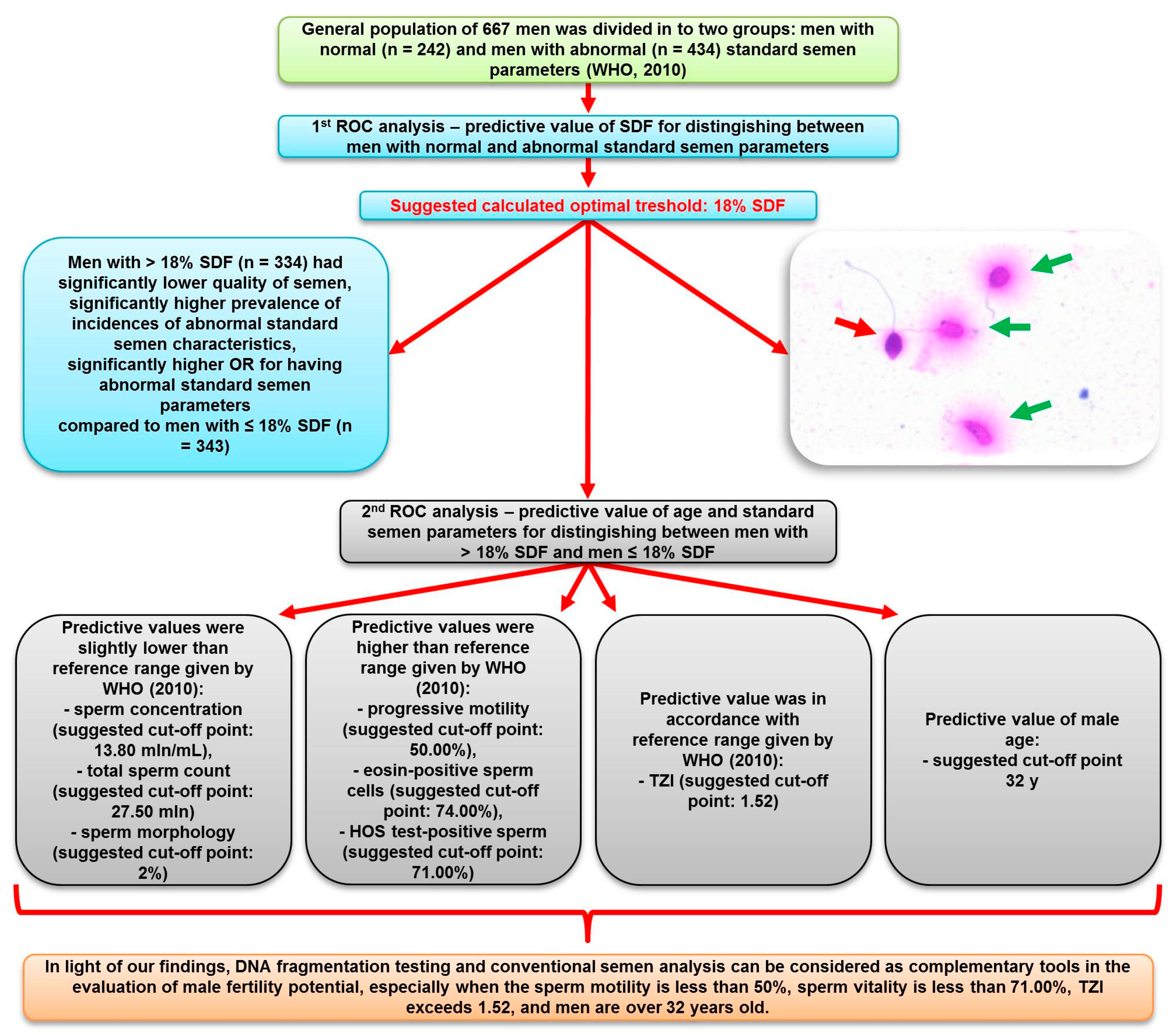
:1. Introduction
2. Subjects
2.1. Sperm Chromatin Dispersion (SCD) Test (Halosperm Test)
2.2. Statistical Analyses
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.M.; Hockenberry, M.S.; Kirby, E.W.; Lipshultz, L.I. Male Infertility Diagnosis and Treatment in the Era of In vitro Fertilization and Intracytoplasmic Sperm Injection. Med. Clin. N. Am. 2018, 102, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Uppangala, S.; Pudakalakatti, S.; D’souza, F.; Salian, S.R.; Kalthur, G.; Kumar, P.; Atreya, H.; Adiga, S.K. Influence of sperm DNA damage on human preimplantation embryo metabolism. Reprod. Biol. 2016, 16, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P. Male reproductive organs are at risk from environmental hazards. Asian J. Androl. 2010, 12, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, R.; Ahmad, G.; Sharma, R.; Ahmad, G. Sperm chromatin assessment. In Textbook of Assisted Reproductive Techniques, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 65–87. [Google Scholar]
- Kazerooni, T.; Asadi, N.; Jadid, L.; Kazerooni, M.; Ghanadi, A.; Ghaffarpasand, F.; Kazerooni, Y.; Zolghadr, J. Evaluation of sperm’s chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J. Assist. Reprod. Genet. 2009, 26, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Rex, A.S.; Aagaard, J.; Fedder, J. DNA fragmentation in spermatozoa: A historical review. Andrology 2017, 5, 622–630. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef]
- Yatsenko, A.N.; Turek, P.J. Reproductive genetics and the aging male. J. Assist. Reprod. Genet. 2018, 35, 933–941. [Google Scholar] [CrossRef]
- Evgeni, E.; Lymberopoulos, G.; Touloupidis, S.; Asimakopoulos, B. Sperm nuclear DNA fragmentation and its association with semen quality in Greek men. Andrologia 2015, 47, 1166–1174. [Google Scholar] [CrossRef]
- Evgeni, E.; Lymberopoulos, G.; Gazouli, M.; Asimakopoulos, B. Conventional semen parameters and DNA fragmentation in relation to fertility status in a Greek population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 18, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Panner Selvam, M.K.; Baskaran, S.; Cho, C.-L. Sperm DNA damage and its impact on male reproductive health: A critical review for clinicians, reproductive professionals and researchers. Expert Rev. Mol. Diagn. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Rosiak, A.; Gaczarzewicz, D.; Jakubik, J.; Kurzawa, R.; Kazienko, A.; Rymaszewska, A.; Laszczynska, M.; Grochans, E.; Piasecka, M. The effect of human sperm chromatin maturity on ICSI outcomes. Hum. Cell 2018, 31, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.V.; Schlegel, P.N. Sperm DNA damage and its role in IVF and ICSI. Basic Clin. Androl. 2016, 26, 15. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Imam, S.N.; Dada, R. Sperm DNA integrity assays: Diagnostic and prognostic challenges and implications in management of infertility. J. Assist. Reprod. Genet. 2011, 28, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, G.; du Plessis, S.S. Not just the marriage of figaro: But the marriage of who/eshre semen analysis criteria with sperm functionality. Adv. Androl. Online 2017, 4, 6–21. [Google Scholar]
- Tan, J.; Taskin, O.; Albert, A.; Bedaiwy, M.A. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: A systematic review and meta-analysis. Reprod. Biomed. Online 2019. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-L.; Agarwal, A. Role of sperm DNA fragmentation in male factor infertility: A systematic review. Arab. J. Urol. 2018, 16, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.-L.; Agarwal, A.; Majzoub, A.; Esteves, S.C. Clinical utility of sperm DNA fragmentation testing: Concise practice recommendations. Transl. Androl. Urol. 2017, 6, 366–373. [Google Scholar] [CrossRef]
- McQueen, D.B.; Zhang, J.; Robins, J.C. Sperm DNA fragmentation and recurrent pregnancy loss: A systematic review and meta-analysis. Fertil. Steril. 2019. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Esteves, S.C.; Ko, E.; Ramasamy, R.; Zini, A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl. Androl. Urol. 2016, 5, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Jerre, E.; Bungum, M.; Evenson, D.; Giwercman, A. Sperm chromatin structure assay high DNA stainability sperm as a marker of early miscarriage after intracytoplasmic sperm injection. Fertil. Steril. 2019. [CrossRef] [PubMed]
- Bungum, M.; Humaidan, P.; Axmon, A.; Spano, M.; Bungum, L.; Erenpreiss, J.; Giwercman, A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum. Reprod. 2007, 22, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Bungum, M.; Bungum, L.; Giwercman, A. Sperm chromatin structure assay (SCSA): A tool in diagnosis and treatment of infertility. Asian J. Androl. 2011, 13, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Erenpreiss, J.; Elzanaty, S.; Giwercman, A. Sperm DNA damage in men from infertile couples. Asian J. Androl. 2008, 10, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Giwercman, A.; Lindstedt, L.; Larsson, M.; Bungum, M.; Spano, M.; Levine, R.J.; Rylander, L. Sperm chromatin structure assay as an independent predictor of fertility in vivo: A case-control study. Int. J. Androl. 2010, 33, e221–e227. [Google Scholar] [CrossRef]
- Evenson, D.P. Evaluation of sperm chromatin structure and DNA strand breaks is an important part of clinical male fertility assessment. Transl. Androl. Urol. 2017, 6, 495–500. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization Press: Geneva, Switzerland, 2010. [Google Scholar]
- Wdowiak, A.; Wdowiak, A.; Bakalczuk, S. Relationship between alcohol consumption and sperm nuclear dna fragmentation and pregnancy. Postep Androl. Online 2016, 3, 14–21. [Google Scholar]
- Gill, K.; Jakubik, J.; Kups, M.; Rosiak-Gill, A.; Kurzawa, R.; Kurpisz, M.; Fraczek, M.; Piasecka, M. The impact of sedentary work on sperm nuclear DNA integrity. Folia Histochem. Cytobiol. 2019, 57, 15–22. [Google Scholar] [CrossRef]
- Bounartzi, T.; Dafopoulos, K.; Anifandis, G.; Messini, C.I.; Koutsonikou, C.; Kouris, S.; Satra, M.; Sotiriou, S.; Vamvakopoulos, N.; Messinis, I.E. Pregnancy prediction by free sperm DNA and sperm DNA fragmentation in semen specimens of IVF/ICSI-ET patients. Hum. Fertil. 2016, 19, 56–62. [Google Scholar] [CrossRef]
- Cissen, M.; van Wely, M.; Scholten, I.; Mansell, S.; de Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm chromatin structure assay: Its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Emery, B.R.; Carrell, D.T. Review: Diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 44, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Arafa, M.; Mahdi, M.; Agarwal, A.; Al Said, S.; Al-Emadi, I.; Alattar, A.; Al Rumaihi, K.; Elbardisi, H. Oxidation-reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab. J. Urol. 2018, 16, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wiweko, B.; Utami, P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin. Androl. 2017, 27, 1. [Google Scholar] [CrossRef]
- Leach, M.; Aitken, R.J.; Sacks, G. Sperm DNA fragmentation abnormalities in men from couples with a history of recurrent miscarriage. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 379–383. [Google Scholar] [CrossRef]
- Abdelbaki, S.A.; Sabry, J.H.; Al-Adl, A.M.; Sabry, H.H. The impact of coexisting sperm DNA fragmentation and seminal oxidative stress on the outcome of varicocelectomy in infertile patients: A prospective controlled study. Arab. J. Urol. 2017, 15, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Al Omrani, B.; Al Eisa, N.; Javed, M.; Al Ghedan, M.; Al Matrafi, H.; Al Sufyan, H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod. Biol. Endocrinol. 2018, 16, 49. [Google Scholar] [CrossRef]
- Menkveld, R.; Wong, W.Y.; Lombard, C.J.; Wetzels, A.M.M.; Thomas, C.M.G.; Merkus, H.M.W.M.; Steegers-Theunissen, R.P.M. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: An effort towards standardization of in-vivo thresholds. Hum. Reprod. 2001, 16, 1165–1171. [Google Scholar] [CrossRef]
- Kaarouch, I.; Bouamoud, N.; Madkour, A.; Louanjli, N.; Saadani, B.; Assou, S.; Aboulmaouahib, S.; Amzazi, S.; Copin, M.; Benkhalifa, M. Paternal age: Negative impact on sperm genome decays and IVF outcomes after 40 years. Mol. Reprod. Dev. 2018, 85, 271–280. [Google Scholar] [CrossRef]
- Petersen, C.G.; Mauri, A.L.; Vagnini, L.D.; Renzi, A.; Petersen, B.; Mattila, M.; Comar, V.; Ricci, J.; Dieamant, F.; Oliveira, J.B.A.; et al. The effects of male age on sperm DNA damage: An evaluation of 2,178 semen samples. JBRA Assist. Reprod. 2018, 22, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rosiak, A.; Gill, K.; Jakubik, J.; Kupś, M.; Patorski, Ł.; Kurzawa, R.; Piasecka, M. [Czy zaawansowany wiek ojcowski ma wpływ na sukces rozrodczy? Część I: Ocena wybranych parametrów seminologicznych] Is advanced paternal age a reproductive risk? Part I: Assessment of selected standard sperm characteristics. Postep Androl. Online 2017, 4, 23–32. [Google Scholar]
- Javed, A.; Talkad, M.S.; Ramaiah, M.K. Evaluation of sperm DNA fragmentation using multiple methods: A comparison of their predictive power for male infertility. Clin. Exp. Reprod. Med. 2019, 46, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Evgeni, E.; Charalabopoulos, K.; Asimakopoulos, B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J. Reprod. Infertil. 2014, 15, 2–14. [Google Scholar]
- Ribas-Maynou, J.; García-Peiró, A.; Fernández-Encinas, A.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013, 1, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Bounartzi, T.; Messini, C.I.; Dafopoulos, K.; Markandona, R.; Sotiriou, S.; Tzavella, A.; Messinis, I.E. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia 2015, 47, 295–302. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Total | Men with >18% SDF | Men with ≤18% SDF |
|---|---|---|---|
| n | n | n | |
| Median (Range) | Median (Range) | Median (Range) | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Age (y) | n = 667 | n = 334 | n = 343 |
| 32.00 (19.00–54.00) | 33.00 (21.00–54.00) ** | 31.00 (19.00–49.00) | |
| 32.20 ± 5.80 | 33.23 ± 5.76 | 31.19 ± 5.66 | |
| Semen volume (mL) | n = 677 | n = 334 | n = 434 |
| 3.00 (0.50–11.50) | 3.25 (0.50–11.50) | 3.00 (0.50–10.00) | |
| 3.59 ± 1.69 | 3.64 ± 1.81 | 3.54 ± 1.57 | |
| Sperm concentration (×106/mL) | n = 677 | n = 334 | n = 343 |
| 19.92 (0.05–283.00) | 14.60 (0.05–166.00) ** | 25.70 (0.25–283.00) | |
| 28.04 ± 30.37 | 22.44 ± 25.21 | 33.49 ± 33.83 | |
| Total number of spermatozoa (×106) | n = 677 | n = 334 | n = 343 |
| 66.00 (0.25–672.00) | 53.32 (0.25–672.00) ** | 75.62 (0.50–660.25) | |
| 92.90 ± 95.45 | 75.48 ± 83.86 | 109.87 ± 102.83 | |
| Morphologically normal spermatozoa (%) | n = 677 | n = 334 | n = 343 |
| 2.00 (0.00–15.00) | 1.00 (0.00–13.00) ** | 4.00 (0.00–15.00) | |
| 3.10 ± 3.07 | 1.86 ± 2.47 | 4.31 ± 3.12 | |
| TZI | n = 677 | n = 334 | n = 343 |
| 1.55 (1.13–2.58) | 1.63 (1.20–2.58) ** | 1.50 (1.13–2.46) | |
| 1.60 ± 0.22 | 1.67 ± 0.24 | 1.62 ± 0.19 | |
| Sperm progressive motility (%) | n = 677 | n = 334 | n = 343 |
| 51.00 (0.00–89.00) | 39.00 (0.00–85.00) ** | 61.00 (2.00–89.00) | |
| 47.68 ± 21.98 | 38.16 ± 21.39 | 56.94 ± 18.31 | |
| Eosin-negative spermatozoa—live cells (%) | n = 677 | n = 334 | n = 343 |
| 77.00 (0.00–96.00) | 70.50 (0.00–94.00) ** | 81.00 (14.00–96.00) | |
| 72.73 ± 17.02 | 65.80 ± 19.11 | 79.48 ± 11.17 | |
| HOS test-positive spermatozoa—live cells (%) | n = 615 | n = 288 | n = 327 |
| 76.00 (0.00–94.00) | 70.00 (0.00–91.00) ** | 80.00 (12.00–94.00) | |
| 71.76 ± 16.97 | 64.77 ± 19.05 | 77.91 ± 11.91 | |
| Peroxidase-positive cells (mln/mL) | n = 677 | n = 334 | n = 343 |
| 0.20 (0.00–27.00) | 0.25 (0.00–10.25) * | 0.12 (0.00–27.00) | |
| 0.49 ± 1.45 | 0.53 ± 1.21 | 0.24 ± 0.00 |
| Group | Standard Semen Parameters | |
|---|---|---|
| Normal N (%) | Abnormal & N (%) | |
| Men with > 18% SDF (n = 334) | 59 (17.66) ** | 275 (82.34) ** |
| Men with ≤ 18% SDF (n = 343) | 184 (53.64) | 159 (46.36) |
| Semen Category | Men with >18% SDF N (%) | Men with ≤18% SDF N (%) | OR (95%CI) |
|---|---|---|---|
| Abnormal standard semen parameters & | 275 (82.34) | 159 (46.36) | 5.394 (3.7922–7.6720) ** |
| Parameter | AUC | SE | CI 95% | Suggested Optimal Cut-Off Point |
|---|---|---|---|---|
| Age (y) | 0.601 ** | 0.021 | 0.563–0.638 | 32.00 |
| Semen volume (mL) | 0.506 | 0.022 | 0.468–0.545 | 6.00 |
| Sperm concentration (×106/mL) | 0.641 ** | 0.021 | 0.603–0.677 | 13.80 |
| Total number of spermatozoa (×106) | 0.625 ** | 0.021 | 0.587–0.661 | 27.75 |
| Morphologically normal spermatozoa (%) | 0.740 ** | 0.018 | 0.705–0.772 | 2.00 |
| TZI | 0.677 ** | 0.020 | 0.641–0.713 | 1.52 |
| Sperm progressive motility (%) | 0.746 ** | 0.018 | 0.711–0.778 | 50.00 |
| Eosine-negative spermatozoa—live cells (%) | 0.743 ** | 0.018 | 0.708–0.775 | 74.00 |
| HOS test-positive spermatozoa—live cells (%) | 0.743 ** | 0.019 | 0.706–0.777 | 71.00 |
| Peroxidase-positive cells (mln/mL) | 0.567 | 0.021 | 0.529–0.605 | 0.00 |
| Parameters | rs |
|---|---|
| Age (y) | 0.211 p < 0.001 |
| Semen volume (mL) | −0.010 p = 0.794 |
| Sperm concentration (×106/mL) | −0.289 p < 0.001 |
| Total number of spermatozoa (×106) | −0.255 p < 0.001 |
| Morphologically normal spermatozoa (%) | −0.457 p < 0.001 |
| TZI | 0.339 p < 0.001 |
| Sperm progressive motility (%) | −0.524 p < 0.001 |
| Eosin-negative spermatozoa—live cells (%) | −0.524 p < 0.001 |
| HOS test-positive spermatozoa—live cells (%) | −0.537 p < 0.001 |
| Peroxidase-positive cells (mln/mL) | 0.125 p = 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, K.; Jakubik, J.; Rosiak-Gill, A.; Kups, M.; Lukaszuk, M.; Kurpisz, M.; Fraczek, M.; Piasecka, M. Utility and Predictive Value of Human Standard Semen Parameters and Sperm DNA Dispersion for Fertility Potential. Int. J. Environ. Res. Public Health 2019, 16, 2004. https://doi.org/10.3390/ijerph16112004
Gill K, Jakubik J, Rosiak-Gill A, Kups M, Lukaszuk M, Kurpisz M, Fraczek M, Piasecka M. Utility and Predictive Value of Human Standard Semen Parameters and Sperm DNA Dispersion for Fertility Potential. International Journal of Environmental Research and Public Health. 2019; 16(11):2004. https://doi.org/10.3390/ijerph16112004
Chicago/Turabian StyleGill, Kamil, Joanna Jakubik, Aleksandra Rosiak-Gill, Michał Kups, Mariusz Lukaszuk, Maciej Kurpisz, Monika Fraczek, and Małgorzata Piasecka. 2019. "Utility and Predictive Value of Human Standard Semen Parameters and Sperm DNA Dispersion for Fertility Potential" International Journal of Environmental Research and Public Health 16, no. 11: 2004. https://doi.org/10.3390/ijerph16112004






