1. Introduction
Henna and walnut tree bark, locally known as souak, are traditional plant cosmetic products that have been widely used in North Africa, the Middle East, and Western Asia countries for medical and cosmetic purposes [
1,
2,
3,
4,
5]. As these traditional plant cosmetics are not permanent, painless, and cheap, and carry no risk of HIV or hepatitis infections, they are extremely popular among women as cosmetics [
6,
7,
8,
9].
Henna is sourced from the finely pulverized dried leaves of
Lawsonia inermis L. plant; this tree is grown in the tropical and subtropical regions of North Africa, India, Sri Lanka, and the Middle East [
10,
11,
12,
13]. As per ethnobotanical and historical information, henna is among the first plants to be used for cosmetic purposes [
2,
3,
4,
14,
15]. Henna contains an active dye (red-orange pigment), lawsone (2-hydroxy-1,4-naphthoquinone), which can be used to color hair and skin [
1,
6,
16,
17,
18,
19,
20,
21]. Traditionally, it is used as a medicine to remedy several ailments [
22,
23,
24,
25,
26,
27,
28]. Henna has several biological activities [
12,
29,
30,
31,
32,
33,
34].
When used for cosmetic purposes, henna is often mixed with water or oil to make a paste, which can be applied to the skin or hair. Upon the application of the henna paste on the skin, its dye (lawsone) will be adsorbed to the outermost skin layer, creating a red-orange stain [
35]. Marketed henna is often mixed with various herbs and different chemical additives (which contains high levels of trace elements) to make it permanent or stronger [
1,
36,
37,
38,
39,
40]. Natural henna is generally safe and well-tolerated in humans, but henna with additives may have side effects or even life-threatening effects [
37,
41,
42].
Henna is extremely popular in Libya as it is part of the culture and traditions. Various henna products with various colors are available in the Libyan market. Some of them are locally produced, while some are imported from Sudan, Yemen, Tunisia, India, and Pakistan. Unfortunately, over the last 10 years, henna has become a life-threatening material for Libyan women, especially in the eastern region of Libya where more than 550 victims of henna poisoning have been reported, of which 57 died as per the Libyan Ministry of health. Till today, the scientific information about the real reasons that led to this wide scale poisoning and death cases remain unknown. Despite the seriousness of the problem, published studies on henna in Libya are rare [
43].
Walnut tree bark, locally known as souak, is another traditional plant cosmetic material widely used to clean and whiten teeth, which, after use, leaves an orange-brown tint on gums and lips. It is obtained as a natural chewing stick from the bark of a walnut tree (
Juglan regia L.) [
8,
9]. Additionally, it is used as a medicine, improves dental hygiene [
44,
45,
46], and widely applied in pharmaceutical industries [
5,
47,
48,
49,
50,
51,
52]. Walnut tree bark is not locally produced in Libya, but imported from other countries like Algeria, Morocco, Tunisia, Sudan, and India. It is available in herbalist shops as a solid tough and fibrous material in thin pieces or shreds of varying lengths. Walnut tree bark has a feeble order and somewhat acrid bitter taste. Currently, it is extensively used by Libyan women as toothbrush and dye for aesthetics. They detach a piece of walnut tree bark and rinse it with water, then, rub it to their teeth and gums for a few minutes before rinsing their mouth with warm water several times. The process can be repeated more than twice a week. Moreover, walnut tree bark is among the popular materials added for intensifying the color of henna.
Heavy metals toxicity to human is well documented [
53]. Lead (Pb), cadmium (Cd) and arsenic (As), which are extremely toxic to humans, accumulate in internal organs over time [
54,
55,
56,
57]. The human skin has been reported in a study to absorb soluble Pb as evidenced by its increased concentration in sweat, blood, and urine within 6 h of its application on the skin [
58]. Cd is a human carcinogen as per the World Health Organization [
59]. Arsenic, on the other hand, has a high affinity for keratin and as such, can be easily found in the hair and nails. Acute overexposure to arsenic results in skin eruptions, nails striation, and alopecia [
60].
The maximum amounts of metals present in cosmetic ingredients provided by Cosmetica Italia are 20, 5 and 5 for Pb, Cd and As, respectively. Dermal absorption of As is 1.9%. The extent of percutaneous absorption of As was found to be 6.4% for trace doses and 2.0% for high doses in monkeys, and 1.9% when applied in trace doses onto human skin samples. Dermal absorption of Cd is 0.8%; about 0.3–0.8% of Cd is absorbed through the skin. Dermal absorption of Pb is 0.3%. The percutaneous absorption of lead acetate following the use of hair coloring preparations was almost nil, ranging from 0 to 0.3% of the dose applied onto the skin. Moderate absorption was detectable in the case of the damaged epidermis [
61]. In a study conducted by Ibrahim and others in 2016, they investigated heavy metal content in henna and reported that the mean concentration of these metals was higher than the permissible levels [
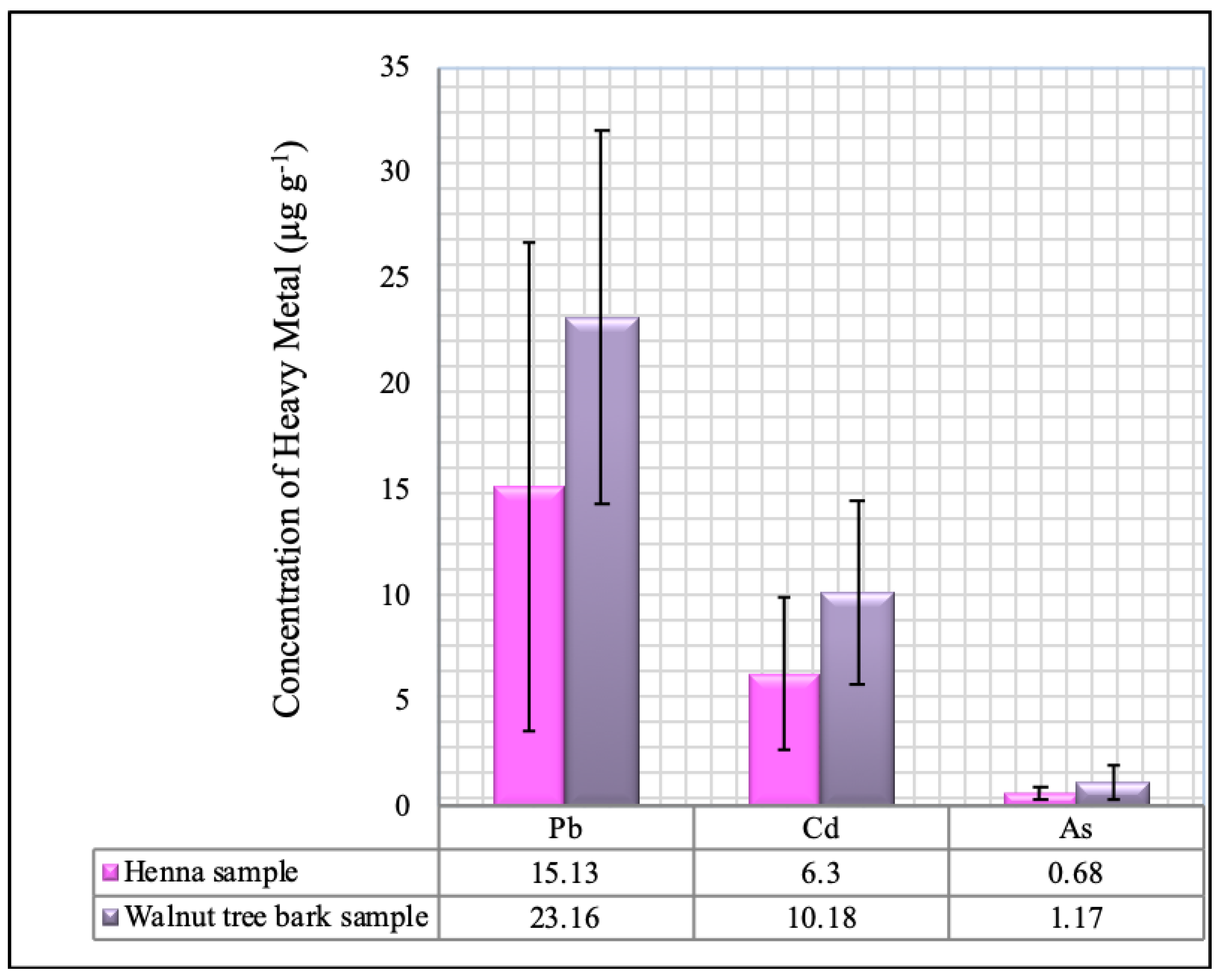
62].
Unfortunately, international standards for impurities in cosmetics are currently unavailable. However, the limits provided by the United States Food and Drug Administration (US FDA) for Pb and As contents of henna (as a color additive for cosmetic products) were 20 and 3 μg·g−1, respectively, while the Canadian regularity limits for certain metals in cosmetics were 10 μg·g−1 for Pb; 3 μg·g−1 for As, and Cd. Other legislation, such as the Danish Statutory Order on Cosmetics number 489, has banned the marketing of all cosmetic products containing Pb, Cd, and, As.
Many traditional cosmetic and medical plants play an important role in the general state of health of a population [
63,
64,
65,
66,
67,
68] and can pose health risks due to the presence of heavy metals [
1,
69,
70,
71]. Therefore, henna and walnut tree bark may be considered as a potential source of heavy metals poisoning as their intensive use may increase the body intake of heavy metals through percutaneous absorption.
Given the increasing concern about the use of henna in the Libyan society and the absence of any legal control of heavy metals in traditional plant cosmetics sold in the Libyan markets, we aim to investigate whether traditional plant cosmetics sold in Libya are possible sources of toxic heavy metals. Our study will contribute to the existing body of knowledge in this area. Therefore, the aims of this study are as follows: To estimate Pb, Cd and As contents in henna and walnut tree bark traditional plant cosmetics sold in Libyan markets, and to compare the obtained results with internal standards for heavy metals in cosmetic products.
The main enthusiasm for the current research is to have an informative vision of the heavy metal concentrations (i.e., Pb, Cd, and As) in the henna and walnut tree bark in Libya. Indeed, the accurate determination of heavy metals in cosmetics is important as they have a narrow range of safety between adequate amounts and excessive consumption. Among the various methods proposed for heavy metals analysis in cosmetic products, inductively coupled plasma mass spectrometry (ICP-MS) is the most frequently used due to its relative simplicity, low sample volume requirements, and low detection limits [
72,
73]. A method using microwave acid digestion, followed by ICP-MS determination for the analysis of heavy metal content in the henna and walnut tree bark samples was used for this study.
2. Material and Methods
2.1. Chemical and Reagent
All chemicals and reagents used were of analytical-reagent grade with no further purification. Nitric acid (HNO3) 69% (w/v) and hydrogen peroxide (H2O2) 30% (w/v) purchased from Merck (Darmstadt, Germany) were used for sample digestion. Ultrapure water (18.2 MΩ.cm quality, model: LPTA/PB/7/1-Maxima Ultrapure Water, ELGA company, Milan, Italy) was used for solution preparation and dilution. The heavy metal standard solutions used for the calibration were prepared by the step dilution of certified stock solutions of 1000 mg·L−1 of each heavy metal (Pb, Cd and As) purchased from Merck (Darmstadt, Germany). For the accuracy tests, a certified reference material (CRM) bush branches and leaves (NCS DC 73,348 CRM) from the China National Analysis Center for Iron and Steel (Beijing, China) were used.
To avoid sample contamination, all glassware, plastic ware, and digestion vessels were washed with Extran® detergent, soaked in 10% (v/v) HNO3 for a minimum of 24 h, washed several times with tap water, rinsed thoroughly with ultrapure water, dried and stored in closed polypropylene containers until use.
2.2. Sample Collection
Convenience sampling technique was implemented in 2018 to collect only henna and walnut tree bark (souak) products that are widely used by Libyan women as traditional plant cosmetics. Samples were collected from well-known local herbalist shops who were conveniently available to participate in this study. These herbalist shops sell traditional plant cosmetic products imported from different countries and those manufactured locally. The sample size ensured that a maximum number of different brands of henna and walnut tree bark products sold were obtained. Notably, all the walnut tree bark products are sold as pieces of plant bark in open polyethylene containers without information labels. Meanwhile, all the henna products are sold without information about their chemical contents.
A total of 15 different brands of powdered henna and 10 unpacked walnut tree bark (souak) samples were purchased from local herbalist shops in Benghazi City, Libya. From each brand of henna and walnut tree bark type, five samples were collected, i.e., 75 and 50 different samples of henna and walnut tree bark (souak) were collected, respectively. About 15 g of each henna brand were separated by quartering sampling method (CAC, 2004) and collected separately in polyethylene ziplock bag previously washed with 10% (v/v) nitric acid (HNO3) and ultrapure water and dried at room temperature before the sample collection. The brand names of the powdered henna were blinded, labeled and given the codes H1–H15. A 200 g of each unpacked walnut tree bark samples of each brand were dried, ground and mixed together. Likewise, 15 g of powdered walnut tree bark samples were separated by quartering sampling method (CAC, 2004) and collected in a pre-cleaned polyethylene ziplock bag, labeled and given the codes S1–S10. The collected samples were kept at room temperature under non-humid conditions until analysis. Analyses were completed within 1 month after collection.
2.3. Samples Pre-Treatment
Prior to analysis, the henna samples were dried at 105 °C in an air ventilated oven for 24 h. The walnut tree bark samples were cut into small pieces, dried at 50 °C in the oven for 48 h, grounded into a fine powder using a mixer grinder, and then sieved through a 250 μm mesh. The obtained powdered walnut tree bark samples were again oven-dried for 24 h at 105 °C. Both dried henna and walnut tree bark samples were allowed to cool over silica gel and stored separately at room temperature in tightly closed Zip lock polyethylene bags until analysis.
2.4. Sample Digestion
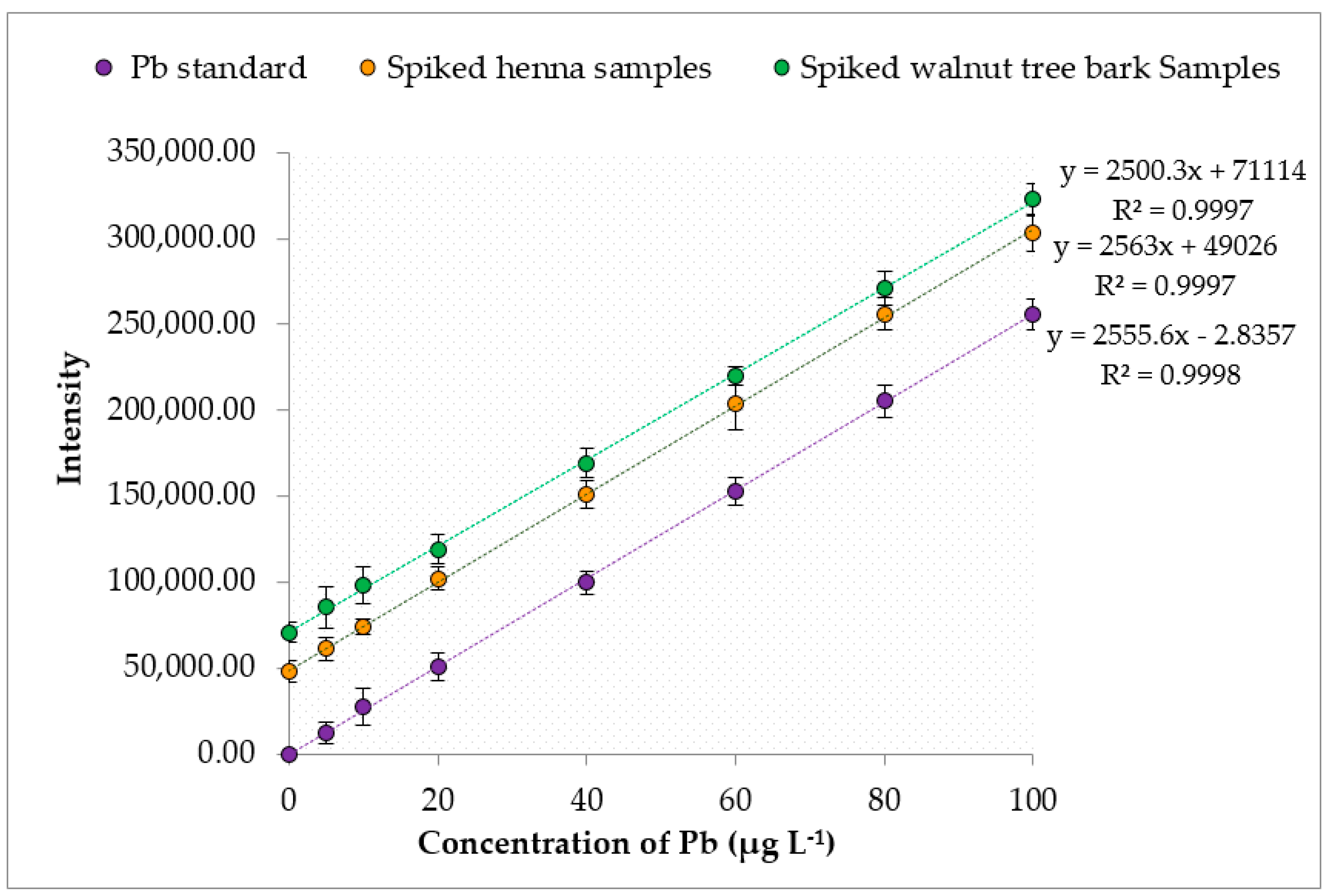
Approximately 0.2 g of each dried henna or walnut tree bark sample was accurately weighed in a dry and clean digestion vessel. Then, 10 mL of HNO
3/H
2O
2 mixture (v/v, 3:1) and 4 mL of ultrapure water were added to the sample [
74]. The digestion process was performed at room temperature for about 15 min to ensure the completion of the initial reaction. Later, the digestion vessel was moved to a laboratory microwave digestion system for the microwave-assisted digestion, as shown in
Table 1. After digestion, the vessel was cooled to room temperature before being opened and sonicated for 30 min at room temperature to eliminate nitrous oxide vapor. The inner side of the vessel lid was rinsed with ultrapure water before transferring the digested solution quantitatively into a volumetric flask using 1% HNO
3. The final volume of the solution was made up to 100 mL using ultrapure water before filtering the samples through a 0.45 μm Millipore membrane syringe filter. The samples were later stored at room temperature in polyethylene bottles and analyzed for heavy metals contents by ICP-MS within a week of storage. The measurements were performed in triplicate, and the mean value was calculated on a dry weight basis (µg·g
−1, dry weight). The blank solutions for the heavy metals determined by ICP-MS were prepared by following all the analytical steps of the proposed method in the absence of any sample or standard. A developed Perkin Elmer method was used for the calculation of the limits of detection (LOD) and quantification (LOQ) of the heavy metals evaluated by the proposed method using ICP-MS. The level of the studied analytes in a certified reference material (CRM) was determined using the proposed method to check for its accuracy. Since henna and walnut tree bark CRM could not be found, bush branches and leaves CRM from the China National Analysis Center for Iron and Steel were used. Both the blank solutions and CRM were similarly analyzed as the samples.
2.5. ICP-MS Determination
The analytical measurements of Pb, Cd and As in the digested henna and walnut tree bark samples were carried out with a Perkin Elmer SCIEX Elan 9000 ICP-MS (Perkin-Elmer SCIEX, Norwalk, CT, USA) after its calibration using the certified stock solutions and optimization according to the recommendation of the manufacturer. The operating conditions of ICP-MS for Pb, Cd, and As determination are provided in
Table 2.
2.6. Statistical Analysis
The results for heavy metals estimation were tabulated, expressed as mean ± standard deviation (SD), and analyzed for statistical significance using Statistical Package for Social Sciences (SPSS version 23.0). Independent sample t-test was used to determine the level of statistical significance between the mean concentration levels of Pb, Cd and As of henna samples and those of walnut tree bark samples; differences at p < 0.05 were considered significant.