Polyacrylamide Microspheres-Derived Fe3C@N-doped Carbon Nanospheres as Efficient Catalyst for Oxygen Reduction Reaction
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Synthesis of Fe3C@N-Doped Carbon Nanospheres
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nat. Cell Boil. 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.J.; Seo, D.-J.; Woo, J.; Lim, J.T.; Cheon, J.Y.; Yang, S.Y.; Lee, J.M.; Kang, D.; Shin, T.J.; Shin, H.S.; et al. A General Approach to Preferential Formation of Active Fe-Nx Sites in Fe-N/C Electrocatalysts for Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 15046–15056. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B: Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nat. Cell Boil. 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Kühl, S.; Strasser, P.; Cuenya, B.R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016, 1, 16009. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Kumar, G.G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Kumar, G.G.; Yoon, J.-M.; Yoo, D.J. Toward improved mechanical strength, oxidative stability and proton conductivity of an aligned quadratic hybrid (SPEEK/FPAPB/Fe 3 O 4 -FGO) membrane for application in high temperature and low humidity fuel cells. RSC Adv. 2017, 7, 39034–39048. [Google Scholar] [CrossRef]
- Niu, W.; Li, L.; Liu, X.; Wang, N.; Liu, J.; Zhou, W.; Tang, Z.; Chen, S. Mesoporous N-Doped Carbons Prepared with Thermally Removable Nanoparticle Templates: An Efficient Electrocatalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 5555–5562. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Karuppannan, M.; Vinothkannan, M.; Ramachandran, K.; Kwon, O.J.; Yoo, D.J. Ultrafine Pt Nanoparticles Stabilized by MoS2/N-Doped Reduced Graphene Oxide as a Durable Electrocatalyst for Alcohol Oxidation and Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2019, 11, 12504–12515. [Google Scholar] [CrossRef] [PubMed]
- Arukula, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Cumulative effect of bimetallic alloy, conductive polymer and graphene toward electrooxidation of methanol: An efficient anode catalyst for direct methanol fuel cells. J. Alloy. Compd. 2019, 771, 477–488. [Google Scholar] [CrossRef]
- Kang, Y.J.; Ye, X.C.; Chen, J.; Cai, Y.; Diaz, R.E.; Adzic, R.R.; Stach, E.A.; Murrzy, C.B. Design of Pt-Pd Binary Superlattices Exploiting Shape Effects and Synergistic Effects for Oxygen Reduction Reactions. J. Am. Chem. Soc. 2013, 135, 42–45. [Google Scholar] [CrossRef]
- Proch, S.; Wirth, M.; White, H.S.; Anderson, S.L. Strong Effects of Cluster Size and Air Exposure on Oxygen Reduction and Carbon Oxidation Electrocatalysis by Size-Selected Pt-n (n≤ 11) on glassy carbon electrodes. J. Am. Chem. Soc. 2013, 135, 3073–3086. [Google Scholar] [CrossRef]
- Yang, H.; Geng, L.; Zhang, Y.; Chang, G.; Zhang, Z.; Liu, X.; Lei, M.; He, Y. Graphene-templated synthesis of palladium nanoplates as novel electrocatalyst for direct methanol fuel cell. Appl. Surf. Sci. 2019, 466, 385–392. [Google Scholar] [CrossRef]
- Liang, H.-W.; Wei, W.; Wu, Z.-S.; Feng, X.; Müllen, K. Mesoporous Metal–Nitrogen-Doped Carbon Electrocatalysts for Highly Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, C.; Liu, F.; Hou, Y. Hybrid of Iron Nitride and Nitrogen-Doped Graphene Aerogel as Synergistic Catalyst for Oxygen Reduction Reaction. Adv. Funct. Mater. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Atanassov, P. Fe-N-C Oxygen Reduction Fuel Cell Catalyst Derived from Carbendazim: Synthesis, Structure, and Reactivity. Adv. N.a. Mater. 2014, 4, 1301735. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Xu, A.-W. Noble-Metal-Free Fe–N/C Catalyst for Highly Efficient Oxygen Reduction Reaction under Both Alkaline and Acidic Conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.T.; Won, J.H.; Zelenay, P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat. Commun. 2013, 4, 1922. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Li, Y.; Lü, X.; Wang, Q.; Zhao, S.; Yuan, K.; Cui, Z.; Li, X.; Xin, S.; et al. Durable and Efficient Hollow Porous Oxide Spinel Microspheres for Oxygen Reduction. Joule 2018, 2, 337–348. [Google Scholar] [CrossRef]
- Wu, Z.; Lv, Y.; Xia, Y.; Webley, P.A.; Zhao, D. Ordered Mesoporous Platinum@Graphitic Carbon Embedded Nanophase as a Highly Active, Stable, and Methanol-Tolerant Oxygen Reduction Electrocatalyst. J. Am. Chem. Soc. 2012, 134, 2236–2245. [Google Scholar] [CrossRef]
- Silva, R.; Voiry, D.; Chhowalla, M.; Asefa, T. Efficient Metal-Free Electrocatalysts for Oxygen Reduction: Polyaniline-Derived N- and O-Doped Mesoporous Carbons. J. Am. Chem. Soc. 2013, 135, 7823–7826. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Diao, P.; Chang, W.; Hong, G.; Li, Y.; Gong, M.; Xie, L.; Zhou, J.; Wang, J.; et al. Oxygen Reduction Electrocatalyst Based on Strongly Coupled Cobalt Oxide Nanocrystals and Carbon Nanotubes. J. Am. Chem. Soc. 2012, 134, 15849–15857. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Liu, X.; Wang, Y.; Wan, J.; Chen, S. Nitrogen Self-Doped Porous Carbon from Surplus Sludge as Metal-Free Electrocatalysts for Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2014, 6, 14911–14918. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Marinovic, A.; Jorge, A.B.; Mansor, N.; Brett, D.J.L.; Fuertes, A.B.; Solis, M.S.; Titirici, M.-M. Fe–N-Doped Carbon Capsules with Outstanding Electrochemical Performance and Stability for the Oxygen Reduction Reaction in Both Acid and Alkaline Conditions. ACS Nano 2016, 10, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.J.; Wang, J.Q.; Hu, J.S.; Wei, Z.D.; Wan, L.J. Understanding the High Activity of Fe-NC Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe-Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, Y.; Hu, Y.X.; Kong, B.A.; Simon, G.P.; Zhang, J.; Jiang, S.P.; Wang, H.T. A Versatile Iron-Tannin-Framework Ink Coating Strategy to Fabricate Biomass-Derived Iron Carbide/Fe-N-Carbon Catalysts for Efficient Oxygen Reduction. Angew Chem. Int. Ed. 2016, 55, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Xu, X.-X.; Hu, B.-C.; Liang, H.-W.; Lin, Y.; Chen, L.-F.; Yu, S.-H. Iron Carbide Nanoparticles Encapsulated in Mesoporous Fe-N-Doped Carbon Nanofibers for Efficient Electrocatalysis. Angew. Chem. 2015, 127, 8297–8301. [Google Scholar] [CrossRef]
- Yang, W.X.; Liu, X.J.; Yue, X.Y.; Jia, J.B.; Guo, S.J. Bamboo-like Carbon Nanotube/Fe3C Nanoparticle Hybrids and Their Highly Efficient Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Galeano, C.; Meier, J.C.; Peinecke, V.; Bongard, H.; Katsounaros, I.; Topalov, A.A.; Lu, A.; Mayrhofer, K.J.J.; Schuth, F. Toward Highly Stable Electrocatalysts via Nanoparticle Pore Confinement. J. Am. Chem. Soc. 2012, 134, 20457–20465. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow Spheres of Iron Carbide Nanoparticles Encased in Graphitic Layers as Oxygen Reduction Catalysts. Angew. Chem. 2014, 126, 3749–3753. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, J.; Feng, L.; Liu, C.; Xing, W. Meso/Macroporous Nitrogen-Doped Carbon Architectures with Iron Carbide Encapsulated in Graphitic Layers as an Efficient and Robust Catalyst for the Oxygen Reduction Reaction in Both Acidic and Alkaline Solutions. Adv. Mater. 2015, 27, 2521–2527. [Google Scholar] [CrossRef]
- Ren, G.; Lu, X.; Li, Y.; Zhu, Y.; Dai, L.; Jiang, L. Porous Core–Shell Fe 3 C Embedded N-doped Carbon Nanofibers as an Effective Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 4118–4125. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Wu, Y.X.; Liang, Z.B.; Guo, W.H.; Zhi, C.X.; Qu, C.; Gao, S.; Zhu, B.J.; Zhang, H.; Zou, R.Q. Fabricating hierarchically porous and Fe3C-embeded nitrogen-rich carbon nanofibersas exceptional electocatalysts for oxygen reduction. Carbon 2018, 142, 115–122. [Google Scholar] [CrossRef]
- Aijaz, A.; Masa, J.; Rösler, C.; Antoni, H.; Fischer, R.A.; Schuhmann, W.; Muhler, M. MOF-Templated Assembly Approach for Fe3 C Nanoparticles Encapsulated in Bamboo-Like N-Doped CNTs: Highly Efficient Oxygen Reduction under Acidic and Basic Conditions. Chem. A Eur. J. 2017, 23, 12125–12130. [Google Scholar] [CrossRef]
- He, Z.B.; Maurice, J.L.; Gohier, A.; Lee, C.S.; Pribat, D.; Cojocaru, C.S. Iron catalysts for the growth of carbon nanofibers: Fe, Fe3C or both? Chem. Mater. 2010, 23, 5379–5387. [Google Scholar] [CrossRef]
- Yoshida, H.; Takeda, S.; Uchiyama, T.; Kohno, H.; Homma, Y. Atomic-Scale In-situ Observation of Carbon Nanotube Growth from Solid State Iron Carbide Nanoparticles. Nano Lett. 2008, 8, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Manzo, J.A.; Terrones, M.; Terrones, H.; Kroto, H.W.; Sun, L.; Banhart, F. In situ nucleation of carbon nanotubes by the injection of carbon atoms into metal particles. Nat. Nanotechnol. 2007, 2, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zeng, Y.; Zeng, Y.; Wang, R.; Cai, S.; Liao, C.; Cai, H.; Lu, X.; Tsiakaras, P. Iron-embedded nitrogen doped carbon frameworks as robust catalyst for oxygen reduction reaction in microbial fuel cells. Appl. Catal. B Environ. 2017, 202, 550–556. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, C.H.; Woo, S.I. Heteroatom doped carbons prepared by the pyrolysis of bio-derived amino acids as highly active catalysts for oxygen electro-reduction reactions. Green Chem. 2011, 13, 406–412. [Google Scholar]
- Wang, Y.; Liu, Y.; Wang, K.; Song, S.; Tsiakaras, P.; Liu, H. Preparation and characterization of a novel KOH activated graphite felt cathode for the electro-Fenton process. Appl. Catal. B Environ. 2015, 165, 360–368. [Google Scholar] [CrossRef]
- Zhao, Y.; Watanabe, K.; Hashimoto, K. Self-Supporting Oxygen Reduction Electrocatalysts Made from a Nitrogen-Rich Network Polymer. J. Am. Chem. Soc. 2012, 134, 19528–19531. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. 2012, 124, 11664–11668. [Google Scholar] [CrossRef]
- Wohlgemuth, S.-A.; Fellinger, T.-P.; Jäker, P.; Antonietti, M. Tunable nitrogen-doped carbon aerogels as sustainable electrocatalysts in the oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 4002. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Cai, S.; Zeng, Y.; Zhang, H.; Cai, H.; Tang, H. Facile Synthesis of Fe3 C@Graphene Hybrid Nanorods as an Efficient and Robust Catalyst for Oxygen Reduction Reaction. ChemPlusChem 2016, 81, 646–651. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Y.; Chen, Z.; Wu, N.; Wang, Y.; Wang, B.; Wang, Y. Fe/Fe 3 C@C nanoparticles encapsulated in N-doped graphene–CNTs framework as an efficient bifunctional oxygen electrocatalyst for robust rechargeable Zn–air batteries. J. Mater. Chem. A 2018, 6, 516–526. [Google Scholar] [CrossRef]
- Chakrapani, K.; Sampath, S. The morphology dependent electrocatalytic activity of Ir nanostructures towards oxygen reduction. Phys. Chem. Chem. Phys. 2014, 16, 16815–16823. [Google Scholar] [CrossRef]
- Wu, G.; More, K.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Larouche, N.; Jaouen, F.; Lefevre, M.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe 3 O 4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Tao, N.; Wang, B.; Lin, S.; Lei, M. Graphite carbon coated TiN nanoparticles as high durable oxygen reduction reaction catalyst in alkaline electrolyte. Micro Nano Lett. 2018, 13, 1260–1264. [Google Scholar] [CrossRef]
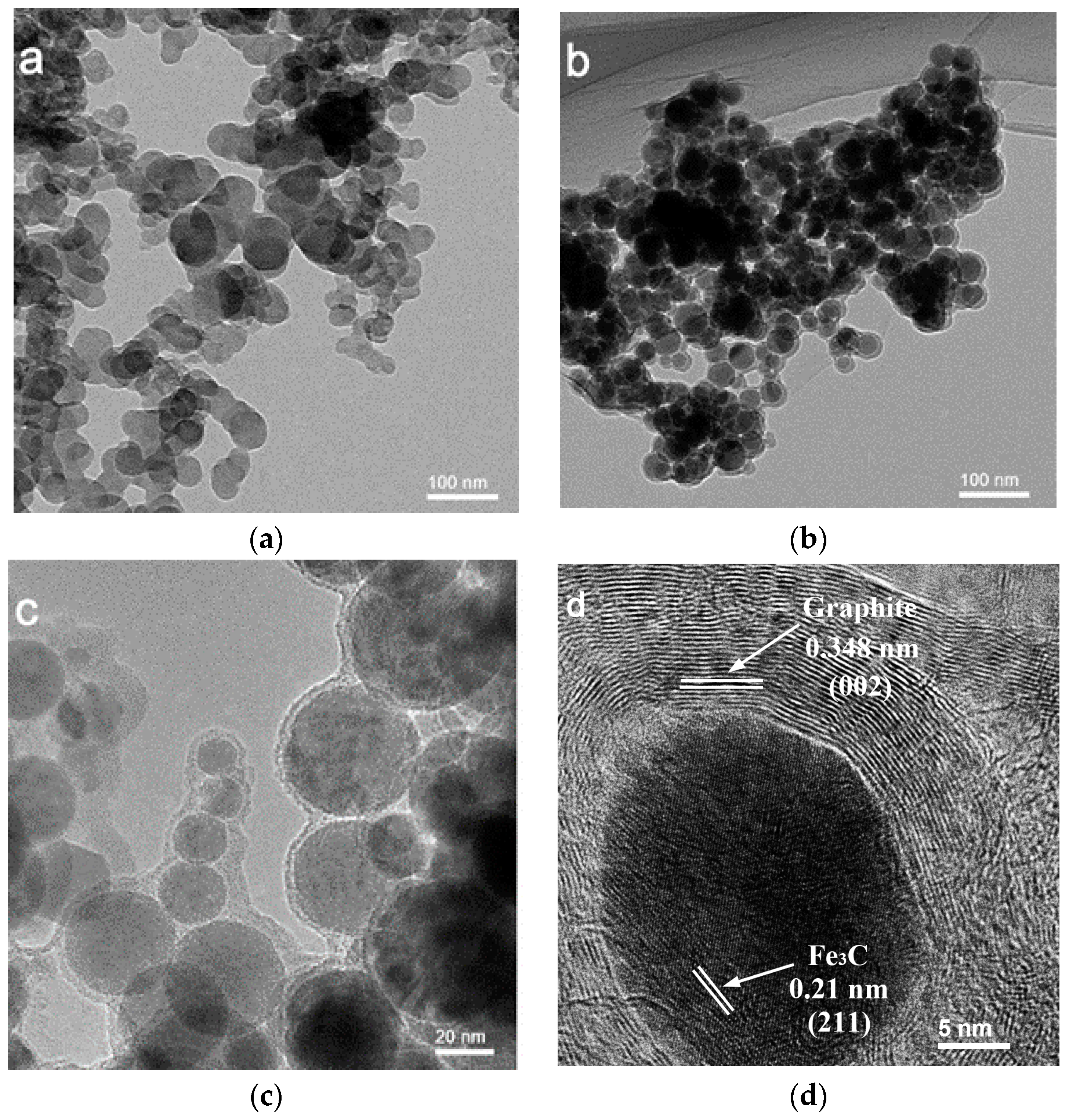
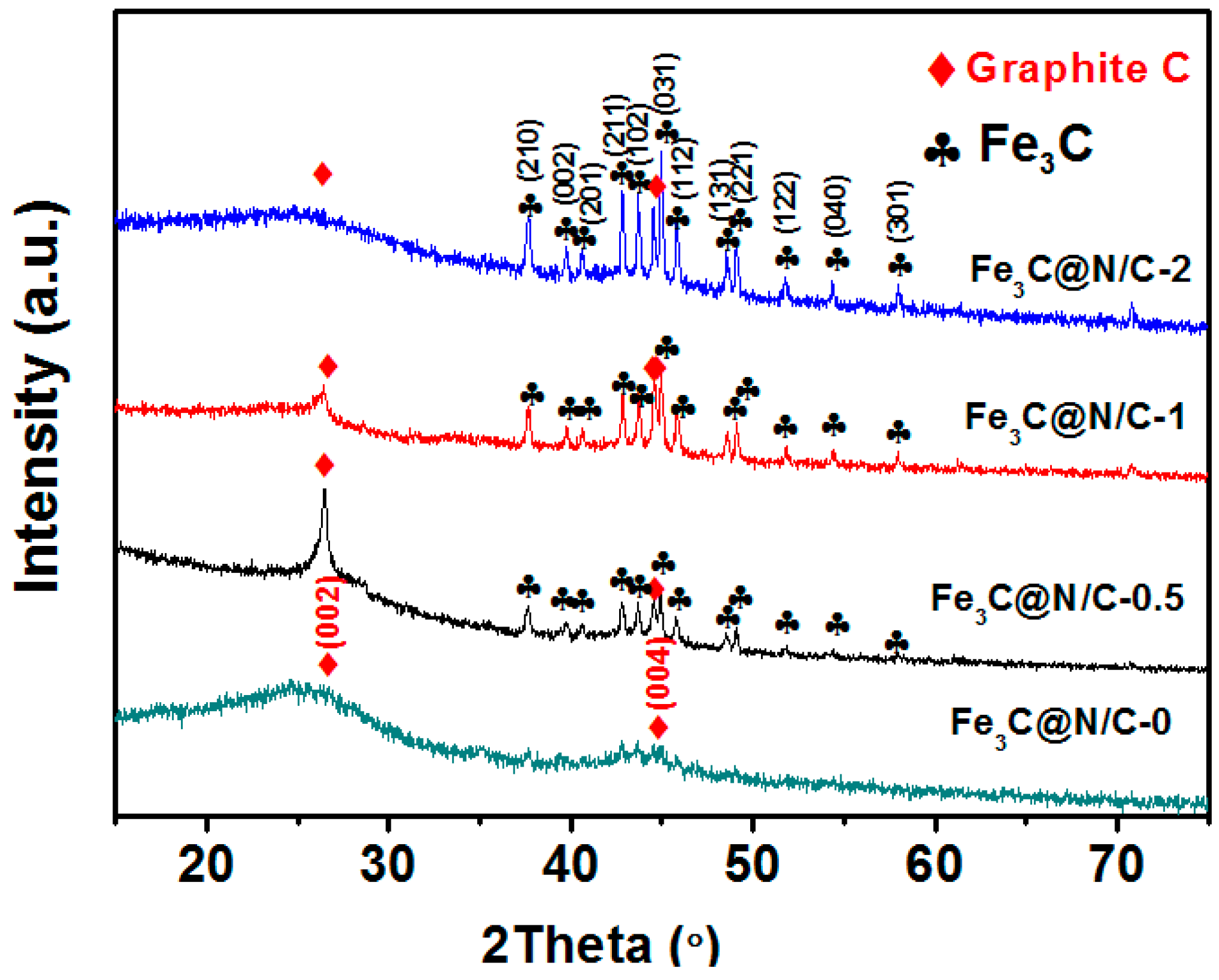
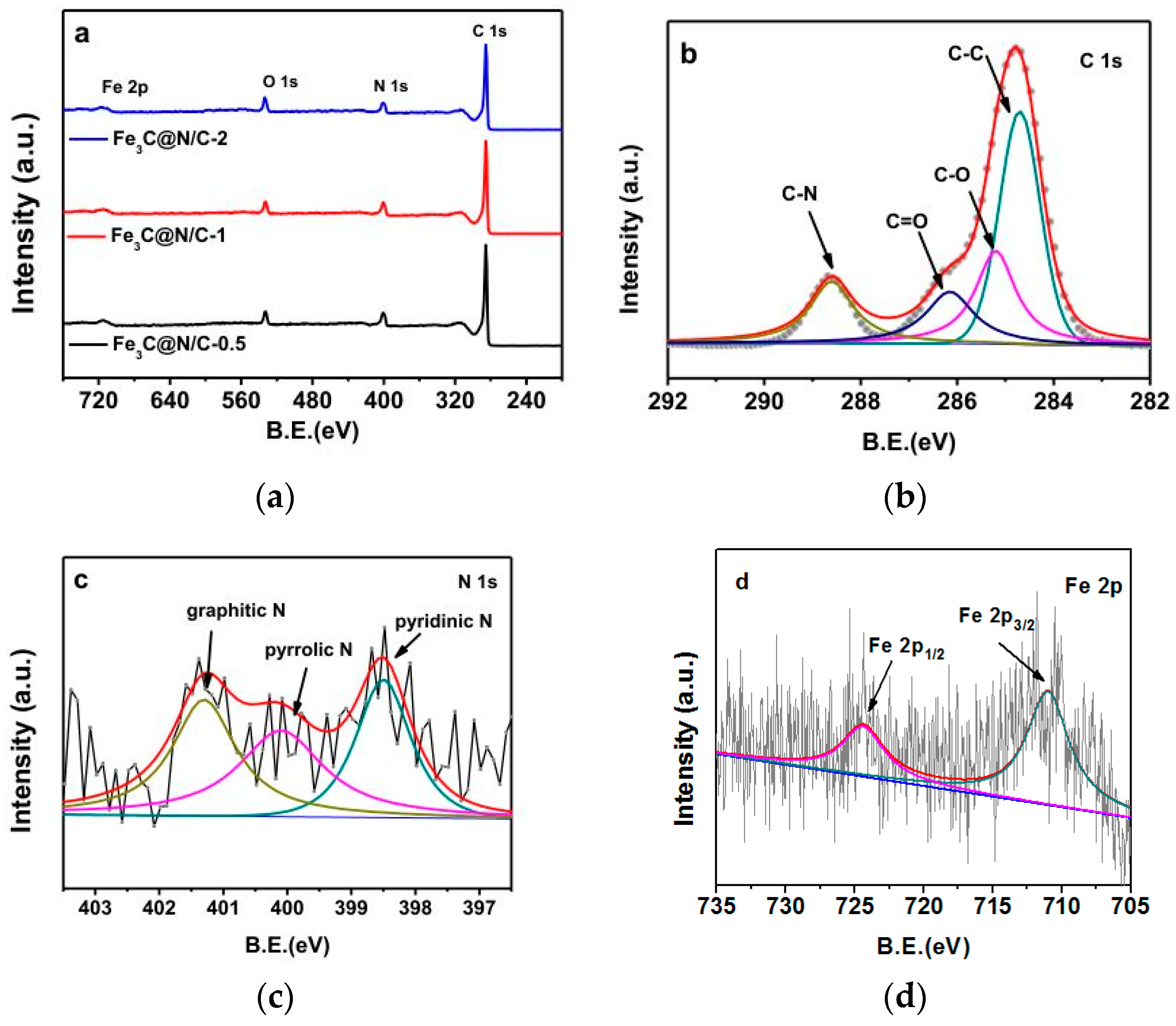
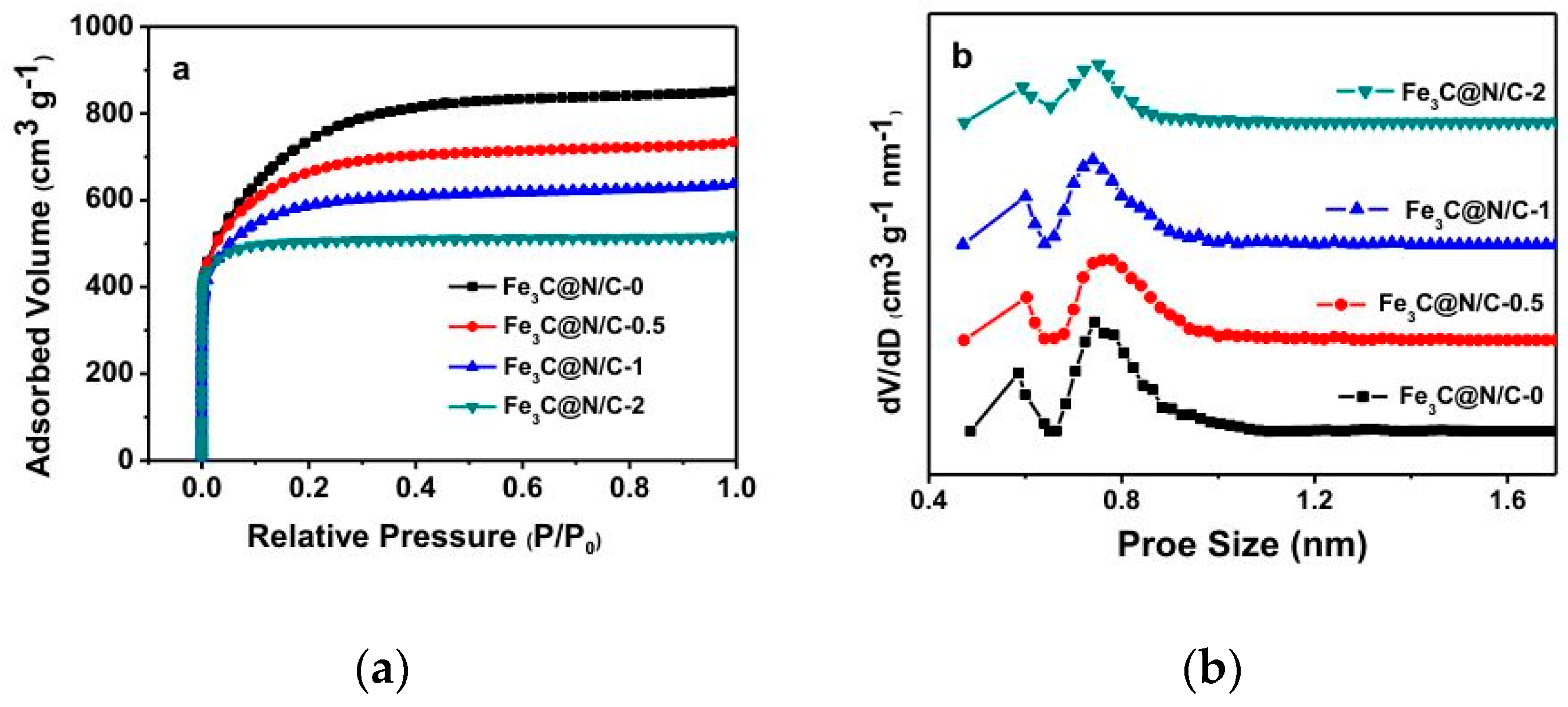
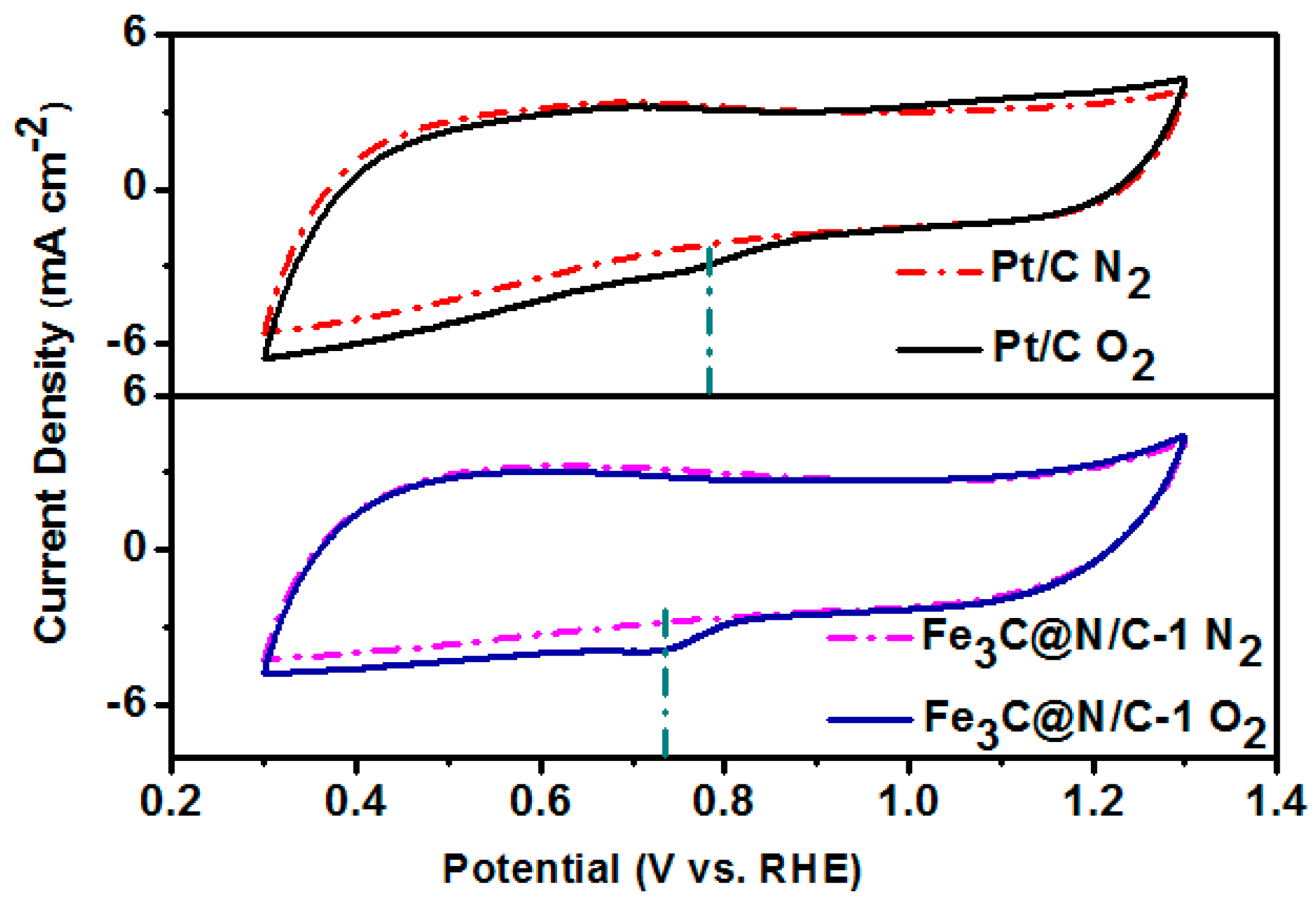
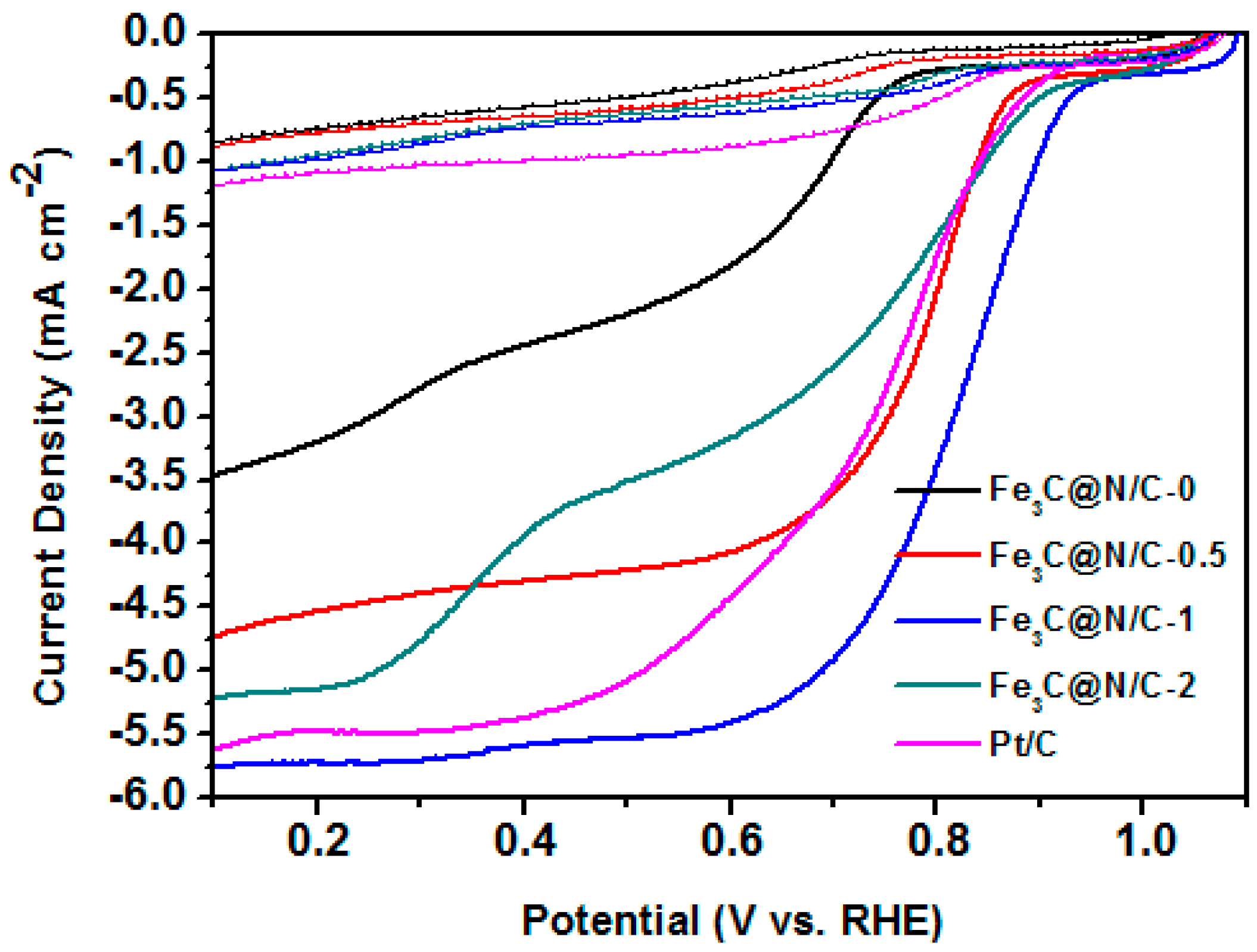
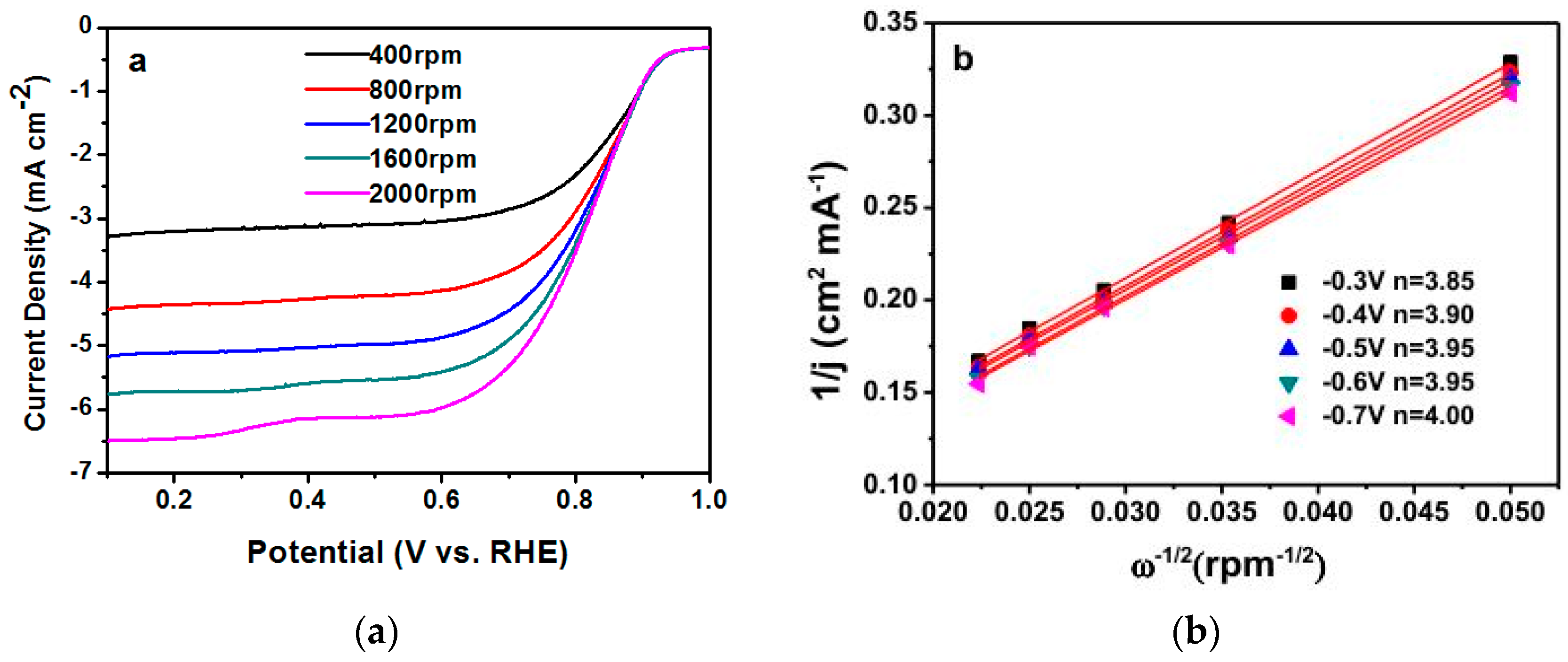

| Sample | Weight Content (%) | N/C Weight Ratio | Relative Atomic Amount of N Species | ||||
|---|---|---|---|---|---|---|---|
| C | N | Fe | Pyridinic N | Pyrrolic N | Graphitic N | ||
| Fe3C@N/C-0 | 87.03 | 8.26 | 0.00 | 0.094 | 0.32 | 0.27 | 0.41 |
| Fe3C@N/C-0.5 | 87.96 | 7.18 | 0.78 | 0.082 | 0.35 | 0.26 | 0.39 |
| Fe3C@N/C-1 | 88.68 | 6.82 | 1.02 | 0.080 | 0.36 | 0.25 | 0.39 |
| Fe3C@N/C-2 | 89.72 | 6.13 | 1.25 | 0.068 | 0.37 | 0.22 | 0.41 |
| Sample | SBET | Vmicro |
|---|---|---|
| (m2 g−1) | (cm3g−1) | |
| Fe3C@N/C-0 | 2484.37 | 0.76 |
| Fe3C@N/C-0.5 | 2121.87 | 0.71 |
| Fe3C@N/C-1 | 1967.83 | 0.68 |
| Fe3C@N/C-2 | 1687.45 | 0.58 |
| Catalyst | Electrolyte | Rotation Speed/rpm | Onset Potential/V vs. RHE | Diffusion Limiting Current (mA cm−2) vs. RHE | Ref. |
|---|---|---|---|---|---|
| PMF-800 | 0.1M KOH | 1600 | 0.95 | 5.78 | [29] |
| Fe3C/C-700 | 0.1M KOH | 1600 | 0.89 | 4.21 | [31] |
| Fe3C@NCNF-900 | 0.1M KOH | 1600 | 0.93 | 4.51 | [33] |
| Fe3C/NCNF | 0.1M KOH | 1600 | 1.012 | 4.81 | [34] |
| Fe3C/b-NCNT | 0.1M KOH | 1600 | 0.96 | 6.25 | [35] |
| Fe/C HN-700C-60M | 0.1M KOH | 1600 | 0.98 | 5.95 | [45] |
| Fe@C-NG/NCNTs | 0.1M KOH | 1600 | 0.93 | 5.11 | [46] |
| Fe3C@N/C-1 | 0.1M KOH | 1600 | 0.94 | 5.78 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Jiang, Y.; Mei, P.; Zhang, Y.; Zheng, X.; Xiao, W.; You, Q.; Yan, X.; Tang, H. Polyacrylamide Microspheres-Derived Fe3C@N-doped Carbon Nanospheres as Efficient Catalyst for Oxygen Reduction Reaction. Polymers 2019, 11, 767. https://doi.org/10.3390/polym11050767
Chen M, Jiang Y, Mei P, Zhang Y, Zheng X, Xiao W, You Q, Yan X, Tang H. Polyacrylamide Microspheres-Derived Fe3C@N-doped Carbon Nanospheres as Efficient Catalyst for Oxygen Reduction Reaction. Polymers. 2019; 11(5):767. https://doi.org/10.3390/polym11050767
Chicago/Turabian StyleChen, Ming, Yu Jiang, Ping Mei, Yan Zhang, Xianfeng Zheng, Wei Xiao, Qinliang You, Xuemin Yan, and Haolin Tang. 2019. "Polyacrylamide Microspheres-Derived Fe3C@N-doped Carbon Nanospheres as Efficient Catalyst for Oxygen Reduction Reaction" Polymers 11, no. 5: 767. https://doi.org/10.3390/polym11050767
APA StyleChen, M., Jiang, Y., Mei, P., Zhang, Y., Zheng, X., Xiao, W., You, Q., Yan, X., & Tang, H. (2019). Polyacrylamide Microspheres-Derived Fe3C@N-doped Carbon Nanospheres as Efficient Catalyst for Oxygen Reduction Reaction. Polymers, 11(5), 767. https://doi.org/10.3390/polym11050767






