Diffusion and Percolation: How COVID-19 Spread Through Populations
Abstract
1. Introduction
2. Key Concepts: Importation, Diffusion, and Percolation
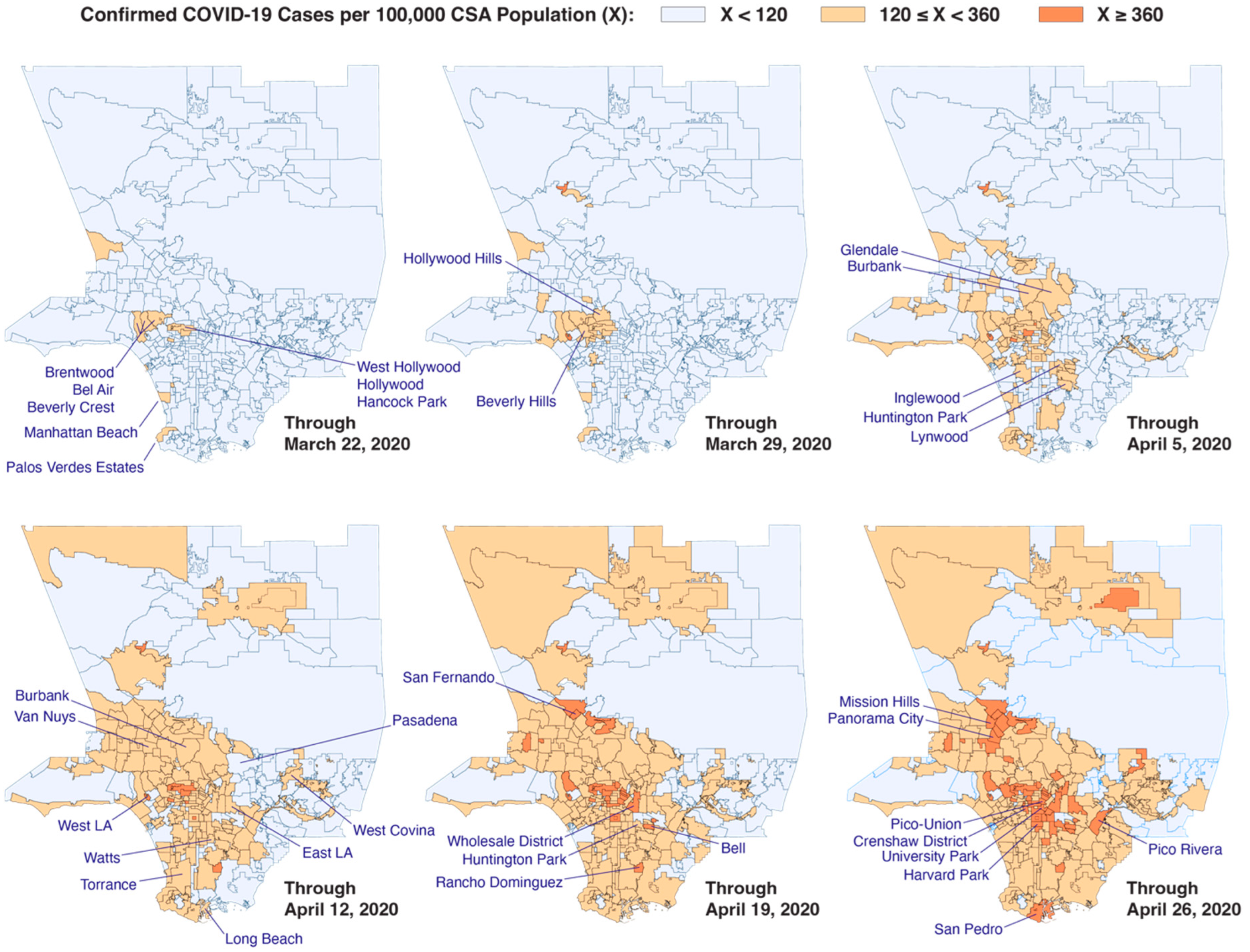
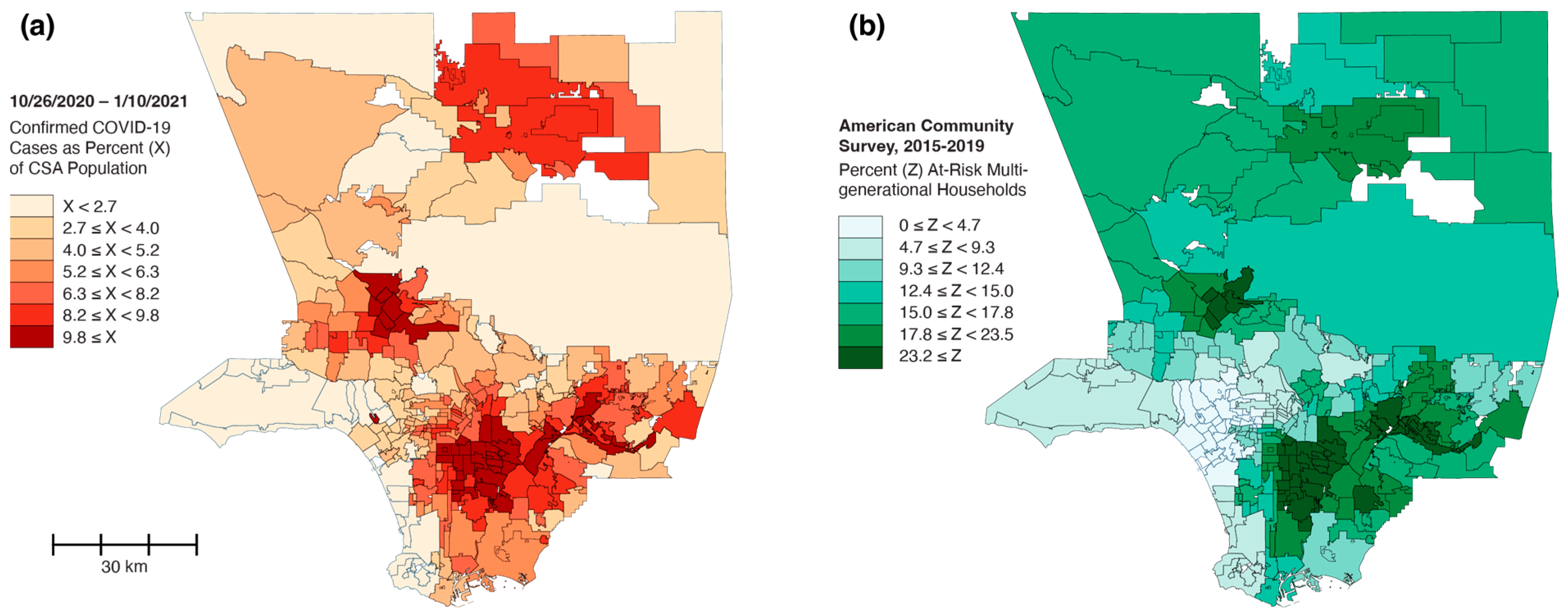
3. Los Angeles County: Radial Diffusion and Percolation Among Multigenerational Households
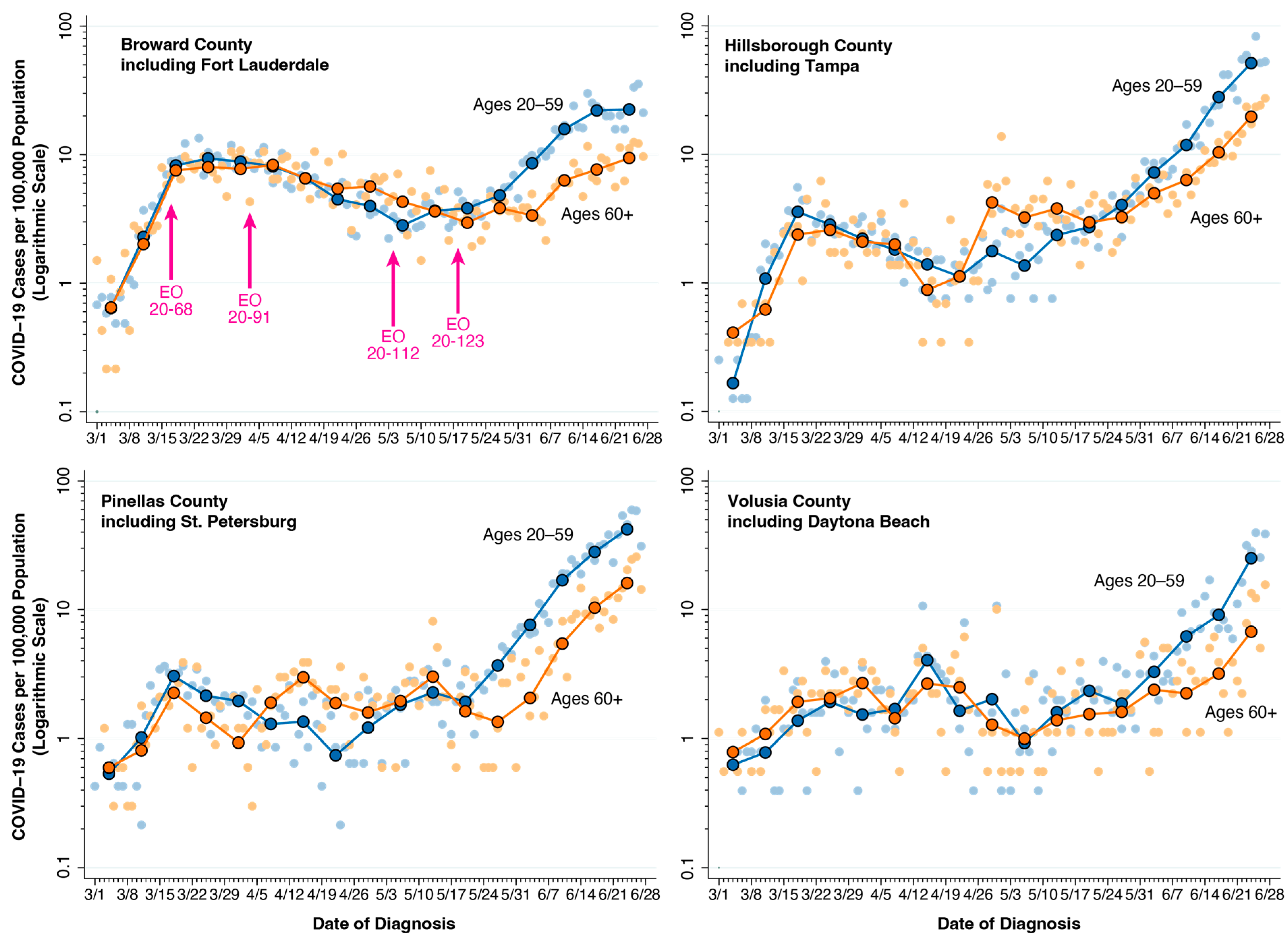
4. Florida Counties: Intergenerational Transmission
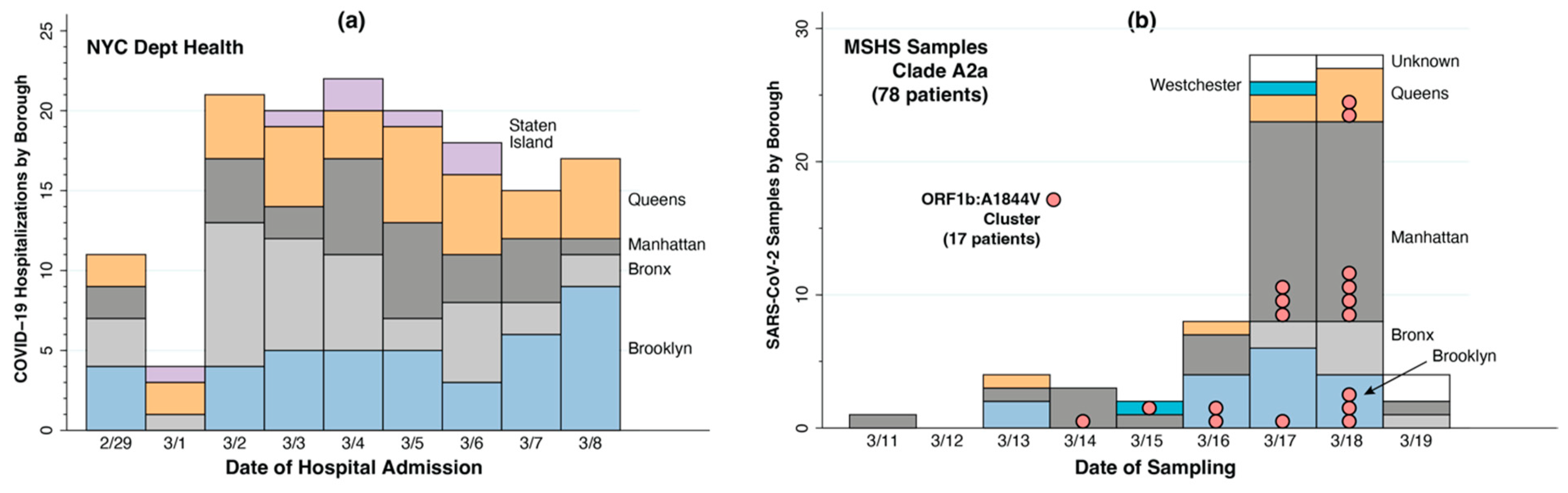
5. New York City: Network Diffusion and Local Percolation
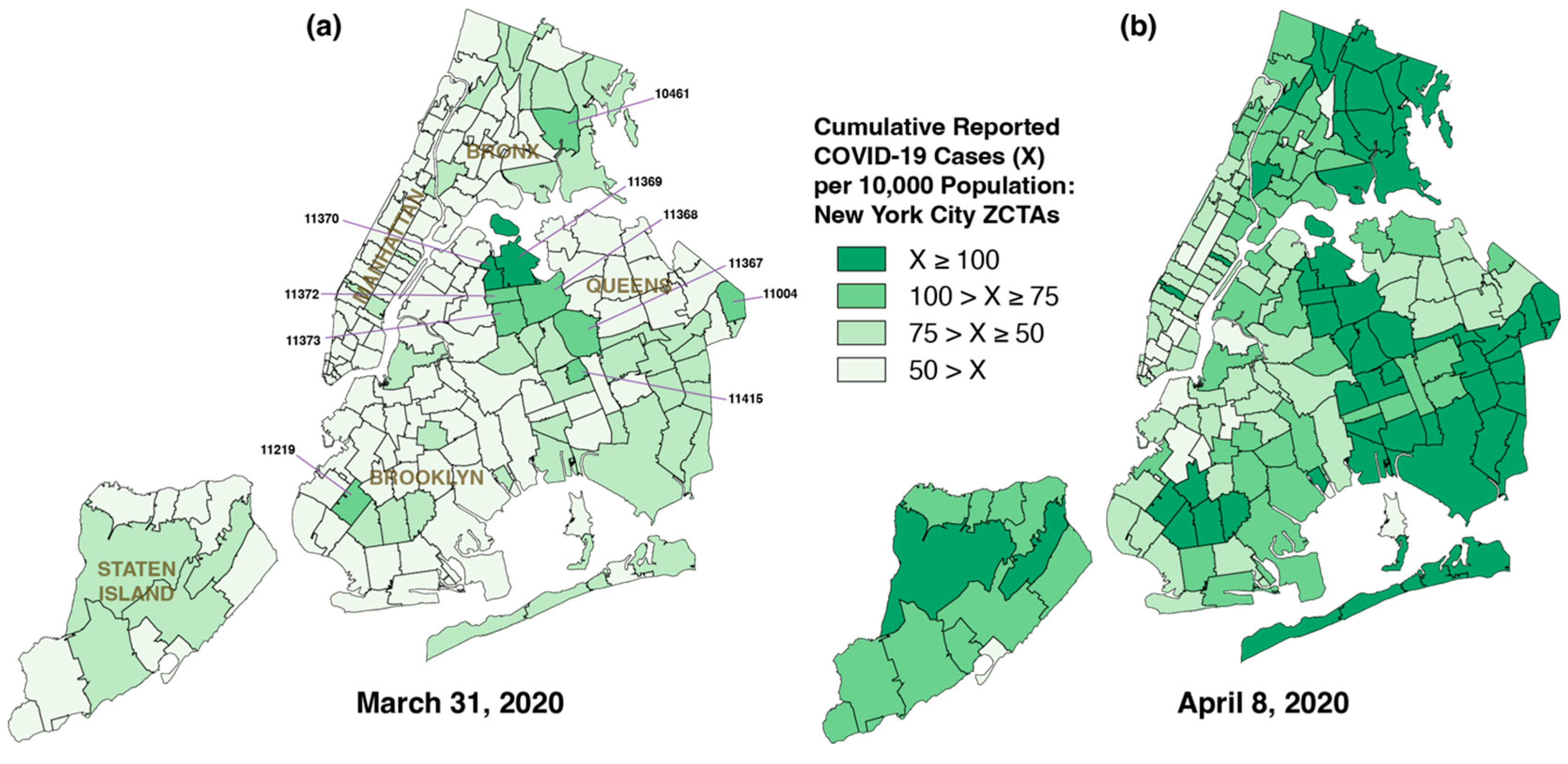
5.1. Widespread Diffusion of Infections Within Days of Importation
5.2. From Diffusion to Percolation
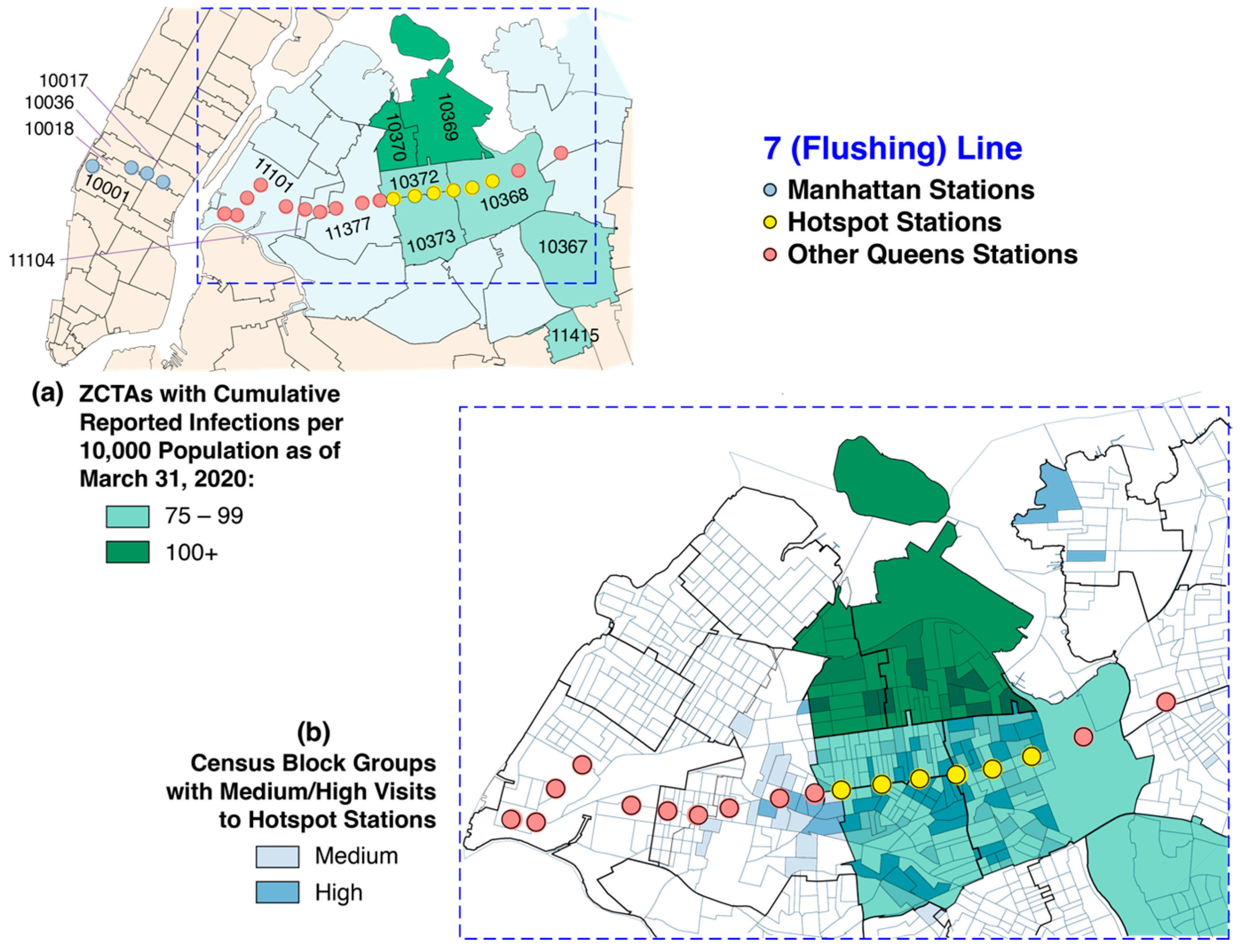
5.3. Unique, Extensive Subway System as a Vehicle for Rapid Diffusion
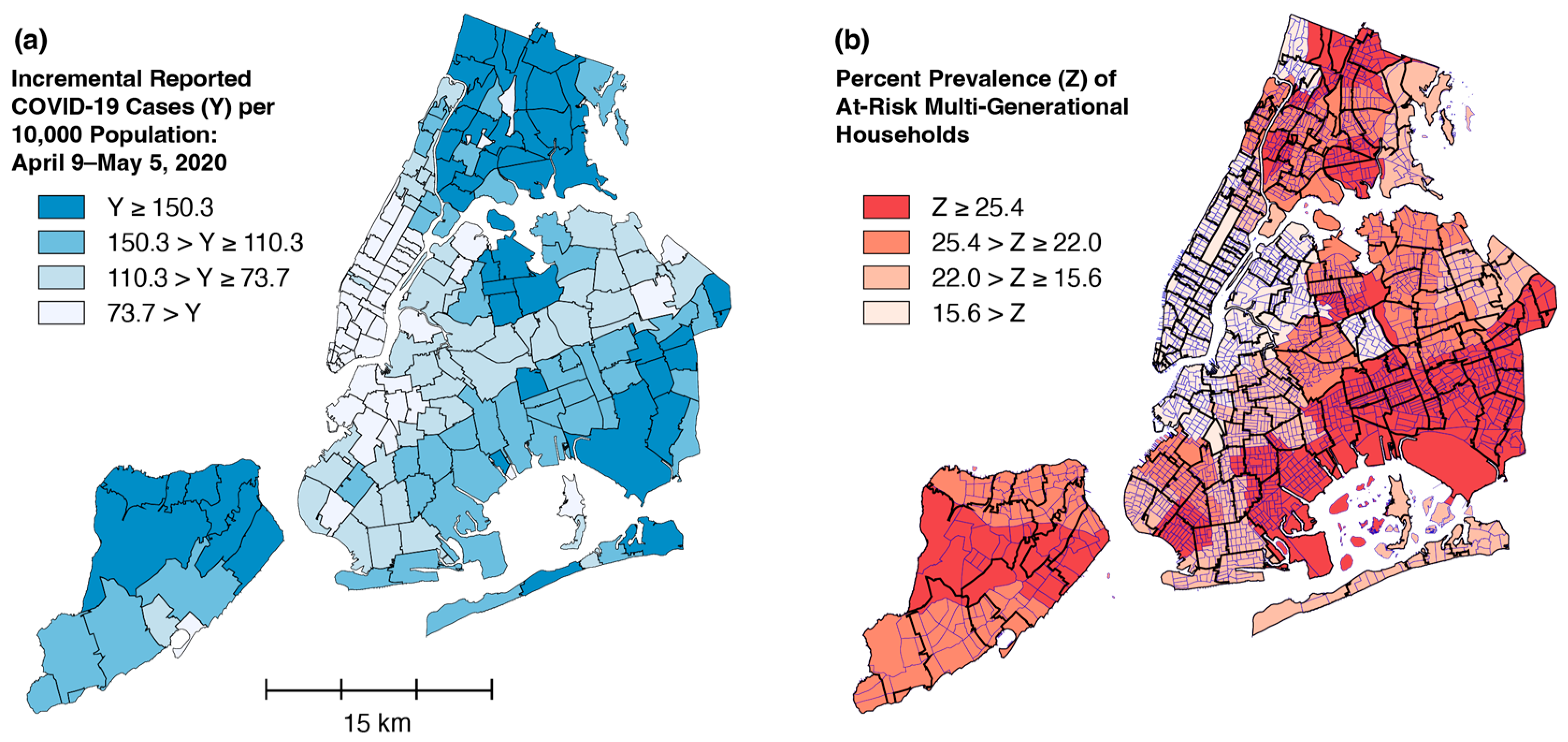
5.4. A Question of Timing
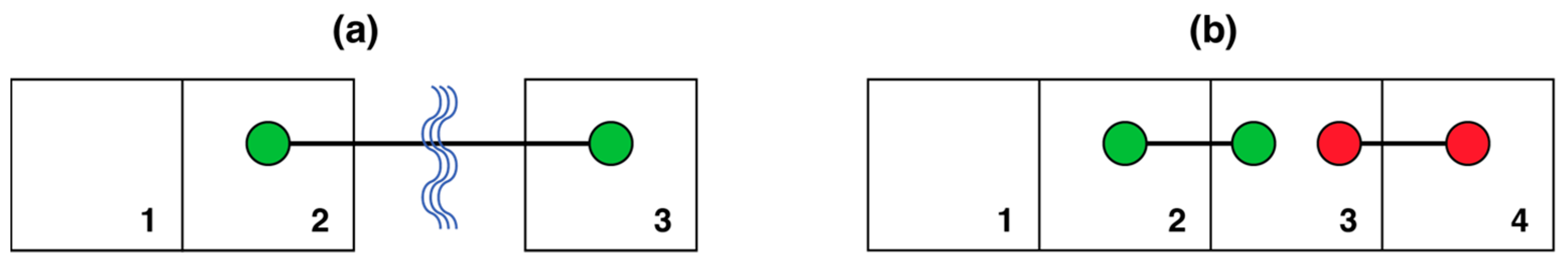
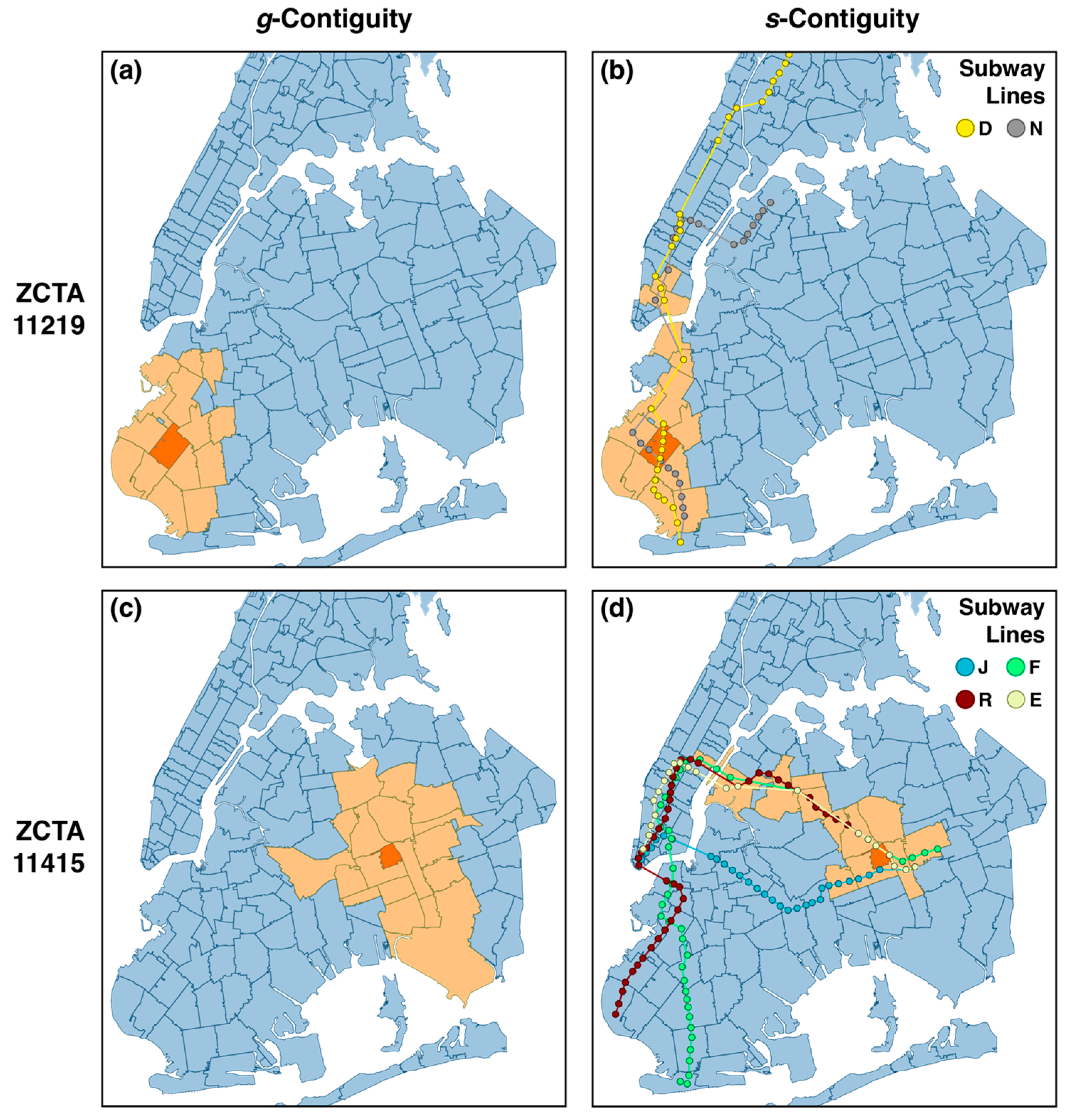
5.5. g- and s-Contiguity
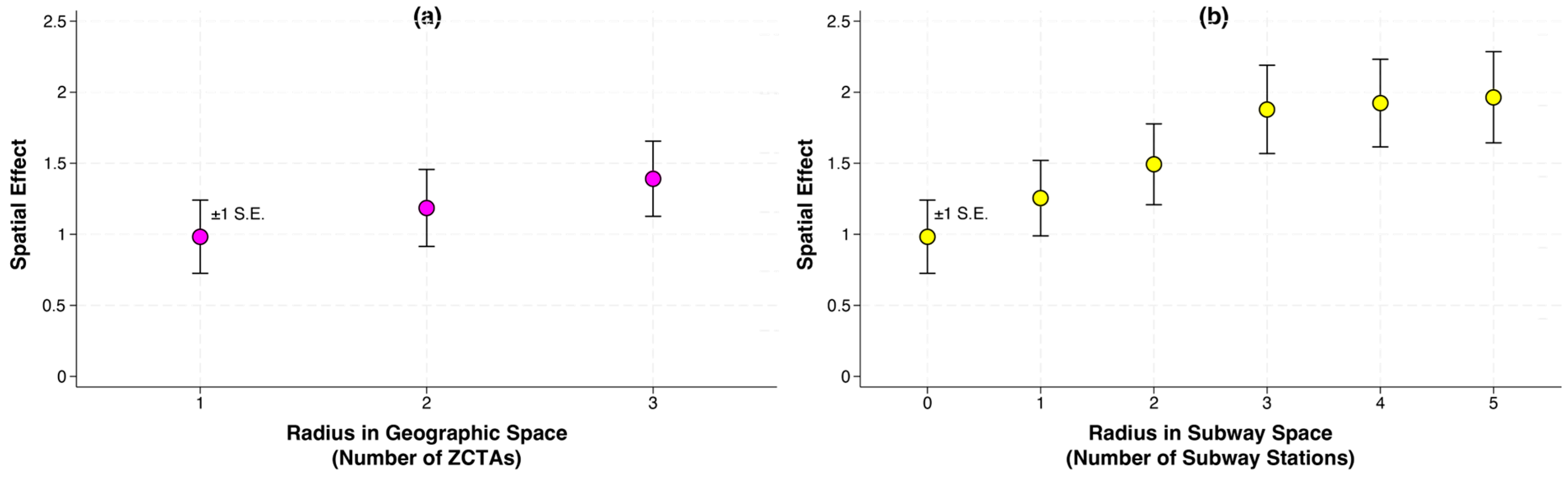
5.6. Modeling Diffusion Through Geographic and Subway Space
6. South Brooklyn: Percolation Through Local Social Mobility
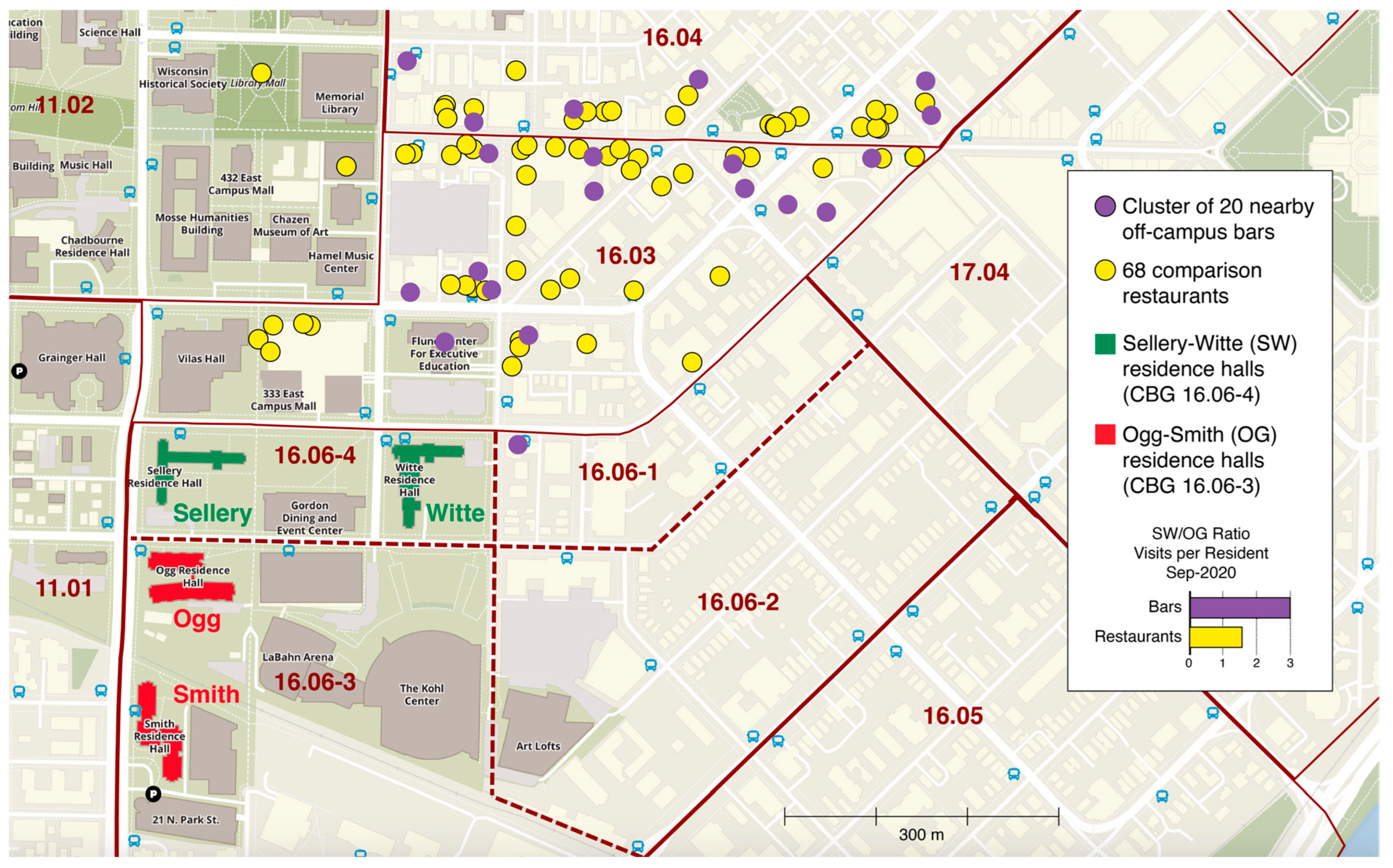
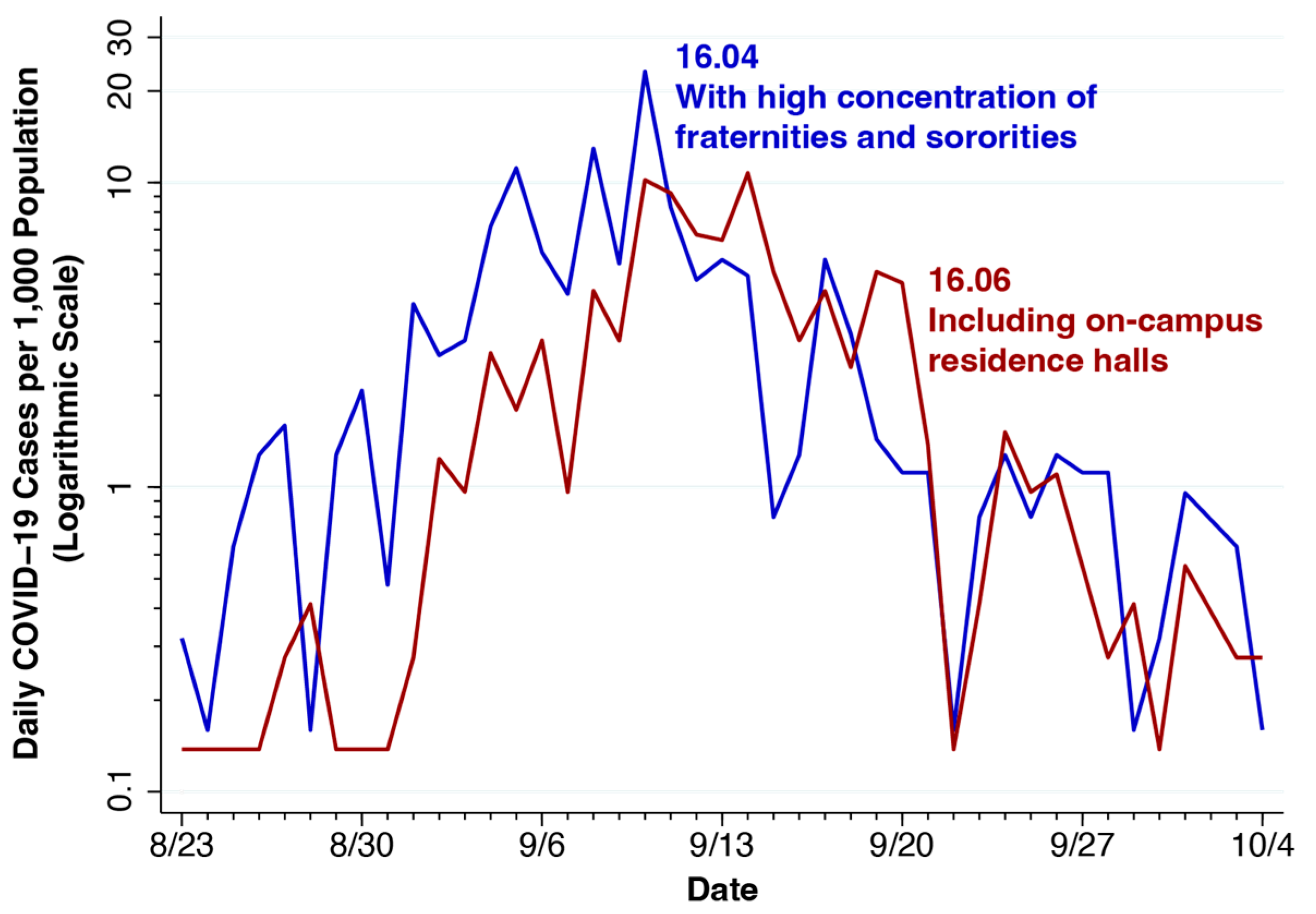
7. University of Wisconsin—Madison: Attractors and Quarantine Policies
8. Discussion and Conclusions
8.1. Limitations of This Review
8.2. Macro, Not Micro
8.3. Viral Transmission Among Subway Passengers: The Subway as Attractor
8.4. Pandemic Control Policy Evaluation: The Details Matter
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Gianino, M.M.; Nurchis, M.C.; Politano, G.; Rousset, S.; Damiani, G. Evaluation of the Strategies to Control COVID-19 Pandemic in Four European Countries. Front. Public. Health 2021, 9, 700811. [Google Scholar] [CrossRef]
- Girum, T.; Lentiro, K.; Geremew, M.; Migora, B.; Shewamare, S.; Shimbre, M.S. Optimal strategies for COVID-19 prevention from global evidence achieved through social distancing, stay at home, travel restriction and lockdown: A systematic review. Arch. Public Health 2021, 79, 150. [Google Scholar] [CrossRef]
- Riaz, M.; Shah, K.; Abdeljawad, T.; Amacha, I.; Al-Jaser, A.; Alqudah, M. A comprehensive analysis of COVID-19 nonlinear mathematical model by incorporating the environment and social distancing. Sci. Rep. 2024, 14, 12238. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. A tight fit of the SIR dynamic epidemic model to daily cases of COVID-19 reported during the 2021–2022 Omicron surge in New York City: A novel approach. Stat. Methods Med. Res. 2024, 33, 1877–1898. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. COVID-19 Incidence and Hospitalization During the Delta Surge Were Inversely Related to Vaccination Coverage Among the Most Populous U.S. Counties. Health Policy Technol. 2021, 11, 100583. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. The repeated setbacks of HIV vaccine development laid the groundwork for SARS-CoV-2 vaccines. Health Policy Technol. 2022, 11, 100619. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.T.; Perry, M.; Linden, W.; Croft, R.; Wolff, R.; Kleijnen, J. The quality of COVID-19 systematic reviews during the coronavirus 2019 pandemic: An exploratory comparison. Syst. Rev. 2024, 13, 126. [Google Scholar] [CrossRef]
- McDonald, S.; Turner, S.L.; Nguyen, P.Y.; Page, M.J.; Turner, T. Are COVID-19 systematic reviews up to date and can we tell? A cross-sectional study. Syst. Rev. 2023, 12, 85. [Google Scholar] [CrossRef]
- Delmelle, E.; Bearman, N.; Yao, X.A. Developing geospatial analytical methods to explore and communicate the spread of COVID19. Cartogr. Geogr. Inf. Sci. 2024, 51, 193–199. [Google Scholar] [CrossRef]
- McMahon, T.; Chan, A.; Havlin, S.; Gallos, L.K. Spatial correlations in geographical spreading of COVID-19 in the United States. Sci. Rep. 2022, 12, 699. [Google Scholar] [CrossRef]
- Sander, L.M.; Warren, C.P.; Sokolov, I.M.; Simon, C.; Koopman, J. Percolation on heterogeneous networks as a model for epidemics. Math. Biosci. 2002, 180, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Eames, K.T. Networks and epidemic models. J. R. Soc. Interface 2005, 2, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Kenah, E.; Miller, J.C. Epidemic percolation networks, epidemic outcomes, and interventions. Interdiscip. Perspect. Infect. Dis. 2011, 2011, 543520. [Google Scholar] [CrossRef]
- Stegehuis, C.; van der Hofstad, R.; van Leeuwaarden, J.S. Epidemic spreading on complex networks with community structures. Sci. Rep. 2016, 6, 29748. [Google Scholar] [CrossRef]
- Li, R.; Song, Y.; Wang, H.; Jiang, G.P.; Xiao, M. Reactive-diffusion epidemic model on human mobility networks: Analysis and applications to COVID-19 in China. Phys. A 2023, 609, 128337. [Google Scholar] [CrossRef]
- Wang, L.; Khan, A.A.; Ullah, S.; Haider, N.; AlQahtani, S.A.; Saqib, A.B. A rigorous theoretical and numerical analysis of a nonlinear reaction-diffusion epidemic model pertaining dynamics of COVID-19. Sci. Rep. 2024, 14, 7902. [Google Scholar] [CrossRef]
- Harris, J.E. Los Angeles County SARS-CoV-2 Epidemic: Critical Role of Multi-generational Intra-household Transmission. J. Bioecon. 2021, 23, 55–83. [Google Scholar] [CrossRef]
- Worobey, M.; Watts, T.D.; McKay, R.A.; Suchard, M.A.; Granade, T.; Teuwen, D.E.; Koblin, B.A.; Heneine, W.; Lemey, P.; Jaffe, H.W. 1970s and ‘Patient 0’ HIV-1 genomes illuminate early HIV/AIDS history in North America. Nature 2016, 539, 98–101. [Google Scholar] [CrossRef]
- Los Angeles County. Countywide Statistical Areas (CSAs). Available online: https://data.lacounty.gov/datasets/lacounty::countywide-statistical-areas-csas/about (accessed on 8 December 2024).
- Los Angeles County Department of Public Health. LA County COVID-19 Surveillance Dashboard. Available online: http://dashboard.publichealth.lacounty.gov/covid19_surveillance_dashboard/ (accessed on 8 December 2024).
- U.S Census Bureau. Accessing PUMS Data. Available online: https://www.census.gov/programs-surveys/acs/microdata/access.html (accessed on 14 January 2021).
- Desantis, R. Executive Order Number 20-68: Emergency Management-COVID-19, Effective March 17, 2020; State of Florida, Office of the Governor, 17 March 2020. Available online: https://www.flgov.com/wp-content/uploads/orders/2020/EO_20-68.pdf (accessed on 6 January 2025).
- Desantis, R. Executive Order Number 20-91: Essential Services and Activities During COVID-19 Emergency, Effective April 3, 2020; State of Florida, Office of the Governor, 1 April 2020. Available online: https://www.flgov.com/wp-content/uploads/orders/2020/EO_20-91-compressed.pdf (accessed on 6 January 2025).
- Desantis, R. Executive Order Number 20-112. Phase 1: Safe. Smart. Step-by-Step. Plan for Florida’s Recovery, Effective May 4, 2020; State of Florida, Office of the Governor, 29 April 2020. Available online: https://www.flgov.com/wp-content/uploads/orders/2020/EO_20-112.pdf (accessed on 6 January 2025).
- Desantis, R. Executive Order Number 20-123: Full Phase I: Safe. Smart. Step-by-Step. Plan for Florida’s Recovery, Effective May 18, 2020; State of Florida, Office of the Governor, 14 May 2020. Available online: https://www.flgov.com/wp-content/uploads/orders/2020/EO_20-123.pdf (accessed on 6 January 2025).
- Harris, J.E. Data from the COVID-19 epidemic in Florida suggest that younger cohorts have been transmitting their infections to less socially mobile older adults. Rev. Econ. Househ. 2020, 18, 1019–1037. [Google Scholar] [CrossRef] [PubMed]
- New York Department of Health and Mental Hygiene. NYC Coronavirus Disease 2019 (COVID-19) Data. Available online: https://github.com/nychealth/coronavirus-data (accessed on 3 July 2022).
- Gonzalez-Reiche, A.S.; Hernandez, M.M.; Sullivan, M.J.; Ciferri, B.; Alshammary, H.; Obla, A.; Fabre, S.; Kleiner, G.; Polanco, J.; Khan, Z.; et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science 2020, 369, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Maurano, M.T.; Ramaswami, S.; Zappile, P.; Dimartino, D.; Boytard, L.; Ribeiro-dos-Santos, A.M.; Vulpescu, N.A.; Westby, G.; Shen, G.; Feng, X. Sequencing identifies multiple early introductions of SARS-CoV-2 to the New York City Region. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.04.15.20064931v4 (accessed on 19 August 2020). [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Updated Guidance on Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19); Distributed via the CDC Health Alert Network, 08 March 2020, 8:20 PM ET, CDCHAN-00429. 2020. Available online: https://emergency.cdc.gov/han/2020/han00429.asp (accessed on 6 January 2025).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Stadlbauer, D.; Tan, J.; Jiang, K.; Hernandez, M.M.; Fabre, S.; Amanat, F.; Teo, C.; Arunkumar, G.A.; McMahon, M.; Capuano, C.; et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature 2021, 590, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Metropolitan Transportation Authority. About Us. The MTA Network: Public Transportation for the New York Region; Last accessed 27 December 2020. Available online: http://web.mta.info/mta/network.htm (accessed on 6 January 2025).
- Metropolitan Transportation Authority. Subway and bus ridership for 2019. Updated 14 April 2020. Available online: https://new.mta.info/agency/new-york-city-transit/subway-bus-ridership-2019 (accessed on 6 January 2025).
- Washington Metropolitan Area Transit Authority. Rail Ridership Data Viewer; Last accessed 27 December 2020. Available online: https://www.wmata.com/initiatives/ridership-portal/Rail-Data-Portal.cfm (accessed on 6 January 2025).
- Harris, J.E. Critical Role of the Subways in the Initial Spread of SARS-CoV-2 in New York City. Front. Public Health 2021, 9, 754767. [Google Scholar] [CrossRef] [PubMed]
- Metropolitan Transportation Authority (MTA). Turnstile Data. Available online: http://web.mta.info/developers/turnstile.html (accessed on 6 January 2025).
- SafeGraph Inc. Social Distancing Metrics. Available online: https://docs.safegraph.com/docs/social-distancing-metrics (accessed on 6 January 2025).
- Harris, J.E. Mobility was a significant determinant of reported COVID-19 incidence during the Omicron Surge in the most populous U.S. Counties. BMC Infect. Dis. 2022, 22, 691. [Google Scholar] [CrossRef] [PubMed]
- New York Governor. Governor Cuomo Announces New Cluster Action Initiative. Press Release, 6 October 2020. Available online: https://www.governor.ny.gov/news/governor-cuomo-announces-new-cluster-action-initiative (accessed on 6 January 2025).
- Harris, J.E. Concentric regulatory zones failed to halt surging COVID-19: Brooklyn 2020. Front. Public Health 2022, 10, 970363. [Google Scholar] [CrossRef]
- Viviani, N. Parisi Urges UW On-Campus Students Home and Go to Online Classes; 15 WMTV, 9 September 2020. Available online: https://www.wmtv15news.com/2020/09/09/parisi-urges-uw-to-send-all-undergraduates-home-for-the-semester/ (accessed on 6 January 2025).
- Traub, A. Rebecca Blank, Who Changed How Poverty Is Measured, Dies at 67; New York Times,9 March (Updated March 11). 2023. Available online: https://www.nytimes.com/2023/03/09/us/rebecca-blank-dead.html (accessed on 8 January 2024).
- University of Wisconsin-Madison. University Shifts to Two Weeks of Remote Instruction, Quarantines Two Residence Halls. University of Wisconsin-Madison, Press Release, 9 September 2020. Available online: https://news.wisc.edu/university-shifts-to-two-weeks-remote-instruction/ (accessed on 6 January 2025).
- University of Wisconsin-Madison. Sellery & Witte Requirements; 15 September 2020. Available online: https://www.housing.wisc.edu/2020/09/sellery-witte-requirements/ (accessed on 6 January 2025).
- Olstad, C. Message to Students: COVID-19 Clarifications and Reminders. University of Wisconsin-Madison, 18 September 2020. Available online: https://news.wisc.edu/message-to-students-covid-19-clarifications-and-reminders/ (accessed on 6 January 2025).
- Blank, R. Chancellor Blank to County Executive: Partner with Us in Enforcing Safe Behavior Off-Campus. University of Wisconsin-Madison, 21 September 2020. Available online: https://news.wisc.edu/chancellor-blank-to-county-executive-partner-with-us-in-enforcing-safe-behavior-off-campus/ (accessed on 6 January 2025).
- Blank, R. Campus to Resume Some In-Person Activities, Maintain Strict Health Measures; University of Wisconsin-Madison, 26 September 2020. Available online: https://covidresponse.wisc.edu/campus-to-resume-some-in-person-activities-maintain-strict-health-measures/ (accessed on 6 January 2025).
- Blank, R. Chancellor Blank Statement on Dane County Board of Supervisors. University of Wisconsin-Madison, 1 October 2020. Available online: https://news.wisc.edu/chancellor-blank-statement-on-dane-county-board-of-supervisors/ (accessed on 6 January 2025).
- Harris, J.E. Geospatial Analysis of a COVID-19 Outbreak at the University of Wisconsin-Madison: Potential Role of a Cluster of Local Bars. Epidemiol. Infect. 2022, 150, e76. [Google Scholar] [CrossRef]
- Worobey, M.; Pekar, J.; Larsen, B.B.; Nelson, M.I.; Hill, V.; Joy, J.B.; Rambaut, A.; Suchard, M.A.; Wertheim, J.O.; Lemey, P. The emergence of SARS-CoV-2 in Europe and North America. Science 2020, 370, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Tripadvosir. State Street & Downtown Madison: Fun Place to Bar Hop…Even as an Adult; 22 January 2018. Available online: https://www.tripadvisor.com/ShowUserReviews-g60859-d281199-r555577961-State_Street_Downtown_Madison-Madison_Wisconsin.html (accessed on 6 January 2025).
- Kwang-tae, K.S. Korea Reports 34 New Virus Cases, Biggest Single-Day Jump in One Month. Yonhap News Agency, 10 May 202. Available online: https://en.yna.co.kr/view/AEN20200510001251320 (accessed on 6 January 2025).
- Kang, C.R.; Lee, J.Y.; Park, Y.; Huh, I.S.; Ham, H.J.; Han, J.K.; Kim, J.I.; Na, B.J.; Seoul Metropolitan Government, C.-R.R.T. Coronavirus Disease Exposure and Spread from Nightclubs, South Korea. Emerg. Infect. Dis. 2020, 26, 2499–2501. [Google Scholar] [CrossRef]
- James, A.; Plank, M.J.; Hendy, S.; Binny, R.; Lustig, A.; Steyn, N.; Nesdale, A.; Verrall, A. Successful contact tracing systems for COVID-19 rely on effective quarantine and isolation. PLoS ONE 2021, 16, e0252499. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Lucas, T.C.D.; Borlase, A.; Pollington, T.M.; Abbott, S.; Ayabina, D.; Crellen, T.; Hellewell, J.; Pi, L.; Group, C.C.-W.; et al. Contact tracing is an imperfect tool for controlling COVID-19 transmission and relies on population adherence. Nat. Commun. 2021, 12, 5412. [Google Scholar] [CrossRef] [PubMed]
- Auranen, K.; Shubin, M.; Erra, E.; Isosomppi, S.; Kontto, J.; Leino, T.; Lukkarinen, T. Efficacy and effectiveness of case isolation and quarantine during a growing phase of the COVID-19 epidemic in Finland. Sci. Rep. 2023, 13, 298. [Google Scholar] [CrossRef]
- Fauver, J.R.; Petrone, M.E.; Hodcroft, E.B.; Shioda, K.; Ehrlich, H.Y.; Watts, A.G.; Vogels, C.B.F.; Brito, A.F.; Alpert, T.; Muyombwe, A.; et al. Coast-to-Coast Spread of SARS-CoV-2 during the Early Epidemic in the United States. Cell 2020, 181, 990–996.E5. [Google Scholar] [CrossRef]
- Gwee, S.X.W.; Chua, P.E.Y.; Wang, M.X.; Pang, J. Impact of travel ban implementation on COVID-19 spread in Singapore, Taiwan, Hong Kong and South Korea during the early phase of the pandemic: A comparative study. BMC Infect. Dis. 2021, 21, 799. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, V.; Aarnio, M.; Alava, M.; Alopaeus, V.; Atanasova, N.; Auvinen, M.; Balasubramanian, N.; Bordbar, H.; Erasto, P.; Grande, R.; et al. Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Saf. Sci. 2020, 130, 104866. [Google Scholar] [CrossRef] [PubMed]
- Romano-Bertrand, S.; Aho-Glele, L.S.; Grandbastien, B.; Gehanno, J.F.; Lepelletier, D. Sustainabilit of SARS-CoV-2 in aerosols: Should we worry about airborne transmission? J. Hosp. Infect. 2020, 105, 601–603. [Google Scholar] [CrossRef]
- Drossinos, Y.; Weber, T.P.; Stilianakis, N.I. Droplets and aerosols: An artificial dichotomy in respiratory virus transmission. Health Sci. Rep. 2021, 4, e275. [Google Scholar] [CrossRef] [PubMed]
- Bahl, P.; Doolan, C.; de Silva, C.; Chughtai, A.A.; Bourouiba, L.; MacIntyre, C.R. Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019? J. Infect. Dis. 2022, 225, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Meneveau, C.; Wu, W. A mathematical framework for estimating risk of airborne transmission of COVID-19 with application to face mask use and social distancing. Phys. Fluids 2020, 32, 101903. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Z.; Bobrovitz, N.; Premji, Z.; Koopmans, M.; Fisman, D.N.; Gu, F.X. Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Lin, H.; Wang, J.; Xu, C.; Tatem, A.J.; Meng, B.; Zhang, X.; Liu, Y.; Wang, P.; Wu, G.; et al. The risk of COVID-19 transmission in train passengers: An epidemiological and modelling study. Clin. Infect. Dis. 2020, 72, 604–610. [Google Scholar] [CrossRef]
- Du, Z.; Wang, L.; Cauchemez, S.; Xu, X.; Wang, X.; Cowling, B.J.; Meyers, L.A. Risk for Transportation of Coronavirus Disease from Wuhan to Other Cities in China. Emerg. Infect. Dis. 2020, 26, 1049–1052. [Google Scholar] [CrossRef]
- Zheng, R.; Xu, Y.; Wang, W.; Ning, G.; Bi, Y. Spatial transmission of COVID-19 via public and private transportation in China. Travel Med. Infect. Dis. 2020, 34, 101626. [Google Scholar] [CrossRef]
- Sy, K.T.L.; Martinez, M.E.; Rader, B.; White, L.F. Socioeconomic Disparities in Subway Use and COVID-19 Outcomes in New York City. Am. J. Epidemiol. 2020, 190, 1234–1242. [Google Scholar] [CrossRef]
- Glaeser, E.L.; Gorback, C.; Redding, S.J. JUE insight: How much does COVID-19 increase with mobility? Evidence from New York and four other U.S. cities. J. Urban Econ. 2022, 127, 103292. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0094119020300632?via%3Dihub (accessed on 21 October 2020). [CrossRef] [PubMed]
- Fathi-Kazerooni, S.; Rojas-Cessa, R.; Dong, Z.; Umpaichitra, V. Correlation of subway turnstile entries and COVID-19 incidence and deaths in New York City. Infect. Dis. Model. 2021, 6, 183–194. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Ma, C.; Zhu, C.; Lu, W. Risk assessment of COVID-19 infection for subway commuters integrating dynamic changes in passenger numbers. Environ. Sci. Pollut. Res. Int. 2022, 29, 74715–74724. [Google Scholar] [CrossRef]
- Edwards, N.J.; Widrick, R.; Wilmes, J.; Breish, B.; Gershefske, M.; Sullivan, J.; Potember, R.; Espinoza-Calvio, A. Reducing COVID-19 airborne transmission risks on public transportation buses: An empirical study on aerosol dispersion and control. Aerosol Sci. Technol. 2021, 55, 1378–1397. [Google Scholar] [CrossRef]
- Zhao, S.; Zhuang, Z.; Ran, J.; Lin, J.; Yang, G.; Yang, L.; He, D. The association between domestic train transportation and novel coronavirus (2019-nCoV) outbreak in China from 2019 to 2020: A data-driven correlational report. Travel Med. Infect. Dis. 2020, 33, 101568. [Google Scholar] [CrossRef] [PubMed]
- SAGE—Environmental and Modelling Group 18052020. Evidence for Transmission of SARS-CoV-2 on Ground Public Transport and Potential Effectiveness of Mitigation Measures. United Kingdom. Available online: https://assets.publishing.service.gov.uk/media/5f215948e90e071a603d33c0/S0441_EMG_-_Evidence_for_transmission_of_SARS-COV-2_on_ground_public_transport.pdf (accessed on 1 April 2020).
- Metropolitan Transportation Authority (MTA). Letter from Chairman Patrick J. Foye to Senator Charles E. Schumer and Congresswoman Nita Lowey; 16 April 2020. Available online: https://apps.cio.ny.gov/apps/mediaContact/public/download.cfm?attachment_uuid=24982848-D532-BD18-F4767900C446F08C (accessed on 6 January 2025).
- Martinez, J. NYC Subway Crews Hit Hardest by Coronavirus, MTA Numbers Show. The City, 1 June 2020. Available online: https://www.thecity.nyc/2020/6/1/21277407/nyc-subway-crews-hit-hardest-by-coronavirus-pandemic-mta-numbers-show (accessed on 6 January 2025).
- Harris, J.E. The Subways Seeded the Massive Coronavirus Epidemic in New York City; National Bureau of Economic Research Working Paper No. 27021, Updated August 2020. Available online: https://www.nber.org/papers/w27021 (accessed on 6 January 2025).
- Chikina, M.; Pegden, W. Modeling strict age-targeted mitigation strategies for COVID-19. PLoS ONE 2020, 15, e0236237. [Google Scholar] [CrossRef] [PubMed]
- Iverson, T.; Karp, L.S.; Peri, A. Protecting the Vulnerable during a Pandemic with Uncertain Vaccine Arrival and Reinfection. Soc. Sci. Res. Netw. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3648714 (accessed on 6 January 2025). [CrossRef]
- Gollier, C. Cost-benefit analysis of age-specific deconfinement strategies. Toulouse Sch. Econ 2020. Available online: https://www.institutlouisbachelier.org/wp-content/uploads/2020/05/cost-benefit-analysis-of-age-specific-deconfinement-strategies-christian-gollier-tse.pdf (accessed on 6 January 2025). [CrossRef]
- Acemoglu, D.; Chernozhukov, V.; Werning, I.; Whinston, M.D. Optimal Targeted Lockdowns in a Multigroup SIR Model. Am. Econ. Rev. Insights 2021, 3, 487–502. [Google Scholar] [CrossRef]
- Harris, J.E. The Coronavirus Epidemic Curve is Already Flattening in New York City; National Bureau of Economic Research, Working Paper 26917, Posted April 2020. Available online: https://www.nber.org/papers/w26917 (accessed on 6 January 2025).
- Office of the Mayor. Statement From Mayor de Blasio on Bars, Restaurants, and Entertainment Venues; City of New York, 15 March 2020. Available online: https://www1.nyc.gov/office-of-the-mayor/news/152-20/statement-mayor-de-blasio-bars-restaurants-entertainment-venues (accessed on 6 January 2025).
- Vielkind, J.; Grayce, M. Amid Coronavirus Fears, Officials Say New York City Subway Is Safe. Wall Str. J. 2020. Available online: https://www.wsj.com/amp/articles/amid-coronavirus-fears-officials-say-new-york-city-subway-is-safe-11583272664 (accessed on 6 January 2025).
- Harris, J.E. COVID-19, bar crowding, and the Wisconsin Supreme Court: A non-linear tale of two counties. Res. Int. Bus. Financ. 2020, 54, 101310. [Google Scholar] [CrossRef]
- Kephart, J.L.; Delclos-Alio, X.; Rodriguez, D.A.; Sarmiento, O.L.; Barrientos-Gutierrez, T.; Ramirez-Zea, M.; Quistberg, D.A.; Bilal, U.; Diez Roux, A.V. The effect of population mobility on COVID-19 incidence in 314 Latin American cities: A longitudinal ecological study with mobile phone location data. Lancet Digit. Health 2021, 3, e716–e722. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.Y.; Khuong, Q.L.; Markuns, J.F.; Bullen, C.; Barrios, O.E.A.; Hwang, S.S.; Suh, Y.S.; McCool, J.; Kachur, S.P.; et al. Mobility restrictions were associated with reductions in COVID-19 incidence early in the pandemic: Evidence from a real-time evaluation in 34 countries. Sci. Rep. 2021, 11, 13717. [Google Scholar] [CrossRef] [PubMed]
- Nouvellet, P.; Bhatia, S.; Cori, A.; Ainslie, K.E.C.; Baguelin, M.; Bhatt, S.; Boonyasiri, A.; Brazeau, N.F.; Cattarino, L.; Cooper, L.V.; et al. Reduction in mobility and COVID-19 transmission. Nat. Commun. 2021, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lee, J.; Langworthy, B.; Xin, J.; James, P.; Yang, Y.; Wang, M. Delay in the Effect of Restricting Community Mobility on the Spread of COVID-19 During the First Wave in the United States. Open Forum Infect. Dis. 2022, 9, ofab586. [Google Scholar] [CrossRef] [PubMed]
- Manzira, C.K.; Charly, A.; Caulfield, B. Assessing the impact of mobility on the incidence of COVID-19 in Dublin City. Sustain. Cities Soc. 2022, 80, 103770. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. Community Health Centers Can Help Stamp out COVID-19. STAT News, 3 February 2021. Available online: https://www.statnews.com/2021/02/03/community-health-centers-can-help-stamp-out-covid-19/ (accessed on 6 January 2025).
- Romero, L.; Pao, L.Z.; Clark, H.; Riley, C.; Merali, S.; Park, M.; Eggers, C.; Campbell, S.; Bui, C.; Bolton, J.; et al. Health Center Testing for SARS-CoV-2 During the COVID-19 Pandemic-United States, 5 June–2 October 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Demeke, H.B.; Pao, L.Z.; Clark, H.; Romero, L.; Neri, A.; Shah, R.; McDow, K.B.; Tindall, E.; Iqbal, N.J.; Hatfield-Timajchy, K.; et al. Telehealth Practice Among Health Centers During the COVID-19 Pandemic-United States, 11–17 July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1902–1905. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, J.E. Diffusion and Percolation: How COVID-19 Spread Through Populations. Populations 2025, 1, 5. https://doi.org/10.3390/populations1010005
Harris JE. Diffusion and Percolation: How COVID-19 Spread Through Populations. Populations. 2025; 1(1):5. https://doi.org/10.3390/populations1010005
Chicago/Turabian StyleHarris, Jeffrey E. 2025. "Diffusion and Percolation: How COVID-19 Spread Through Populations" Populations 1, no. 1: 5. https://doi.org/10.3390/populations1010005
APA StyleHarris, J. E. (2025). Diffusion and Percolation: How COVID-19 Spread Through Populations. Populations, 1(1), 5. https://doi.org/10.3390/populations1010005






