Sunshine Duration, Genetic Predisposition, and Incident Depression: Findings from a Prospective Cohort
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Environmental Exposure
2.3. Measurement of Peripheral Markers
2.4. Assessment of Sleep Characteristics
2.5. Genotyping and Imputation
2.6. Follow-Up and Ascertainment of Outcomes
2.7. Covariates
2.8. Statistical Analyses
2.8.1. Association Between Sunshine Duration and Depressive Disorders
2.8.2. Polygenic Risk Score (PRS) and Interaction
- (1)
- Recoding SNPs as 0, 1, or 2 based on the risk allele;
- (2)
- Calculating PRS with the following formula:
- (3)
- Standardizing the PRSs.
2.8.3. Mediation Analysis
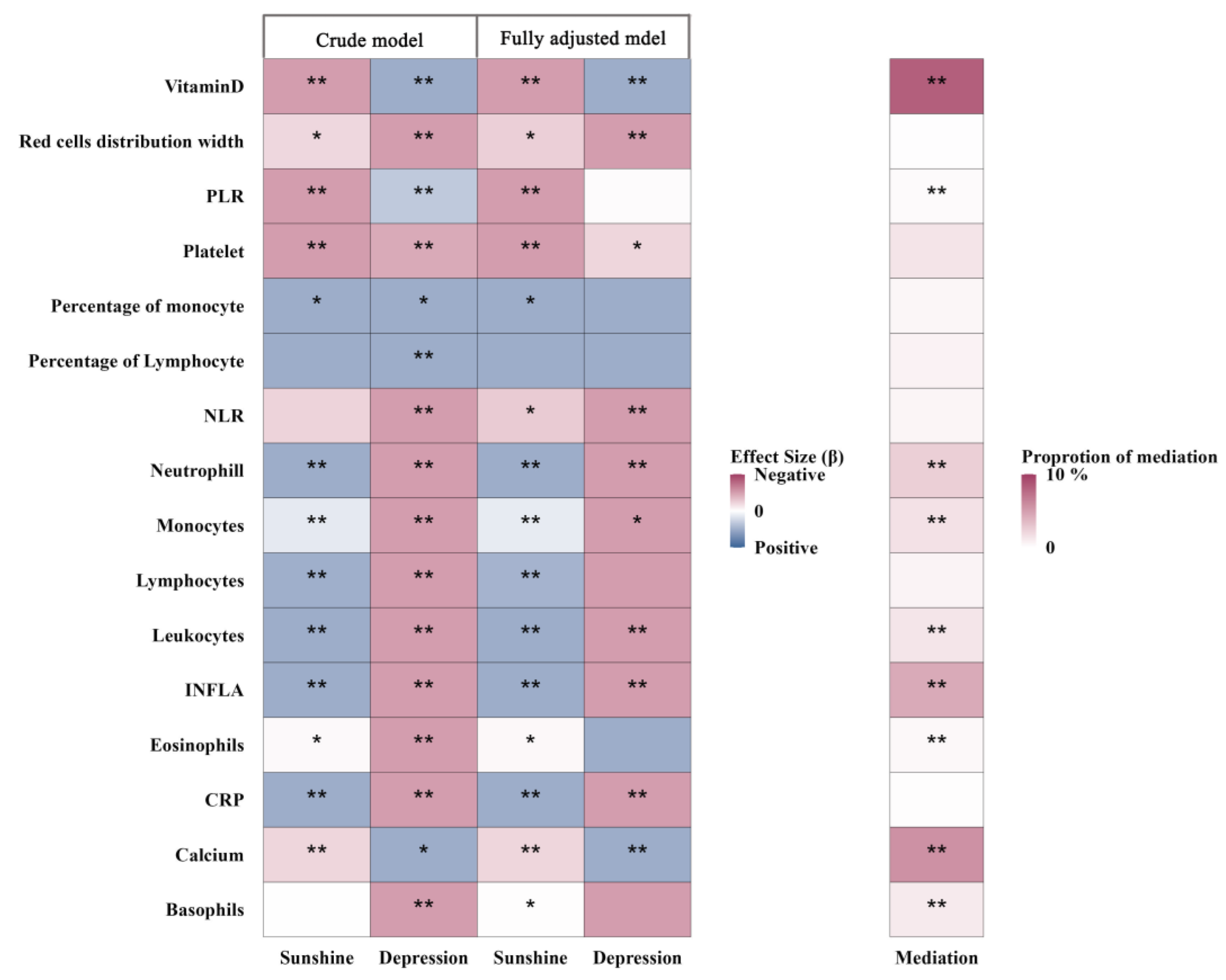
Biochemical Markers
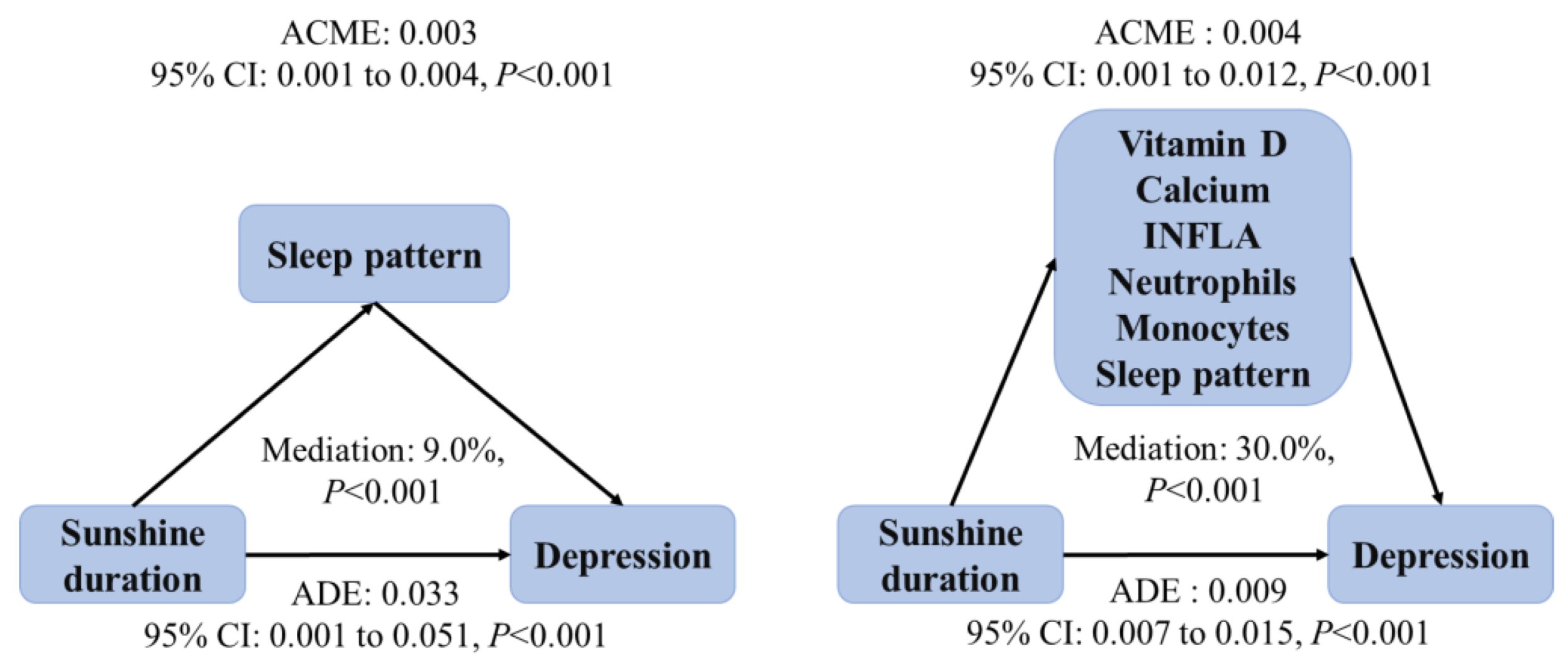
Sleep Pattern
2.8.4. Parallel Mediation Analysis
2.9. Stratification and Interaction Analysis
2.10. Sensitivity Analysis
3. Results
3.1. Descriptive Results
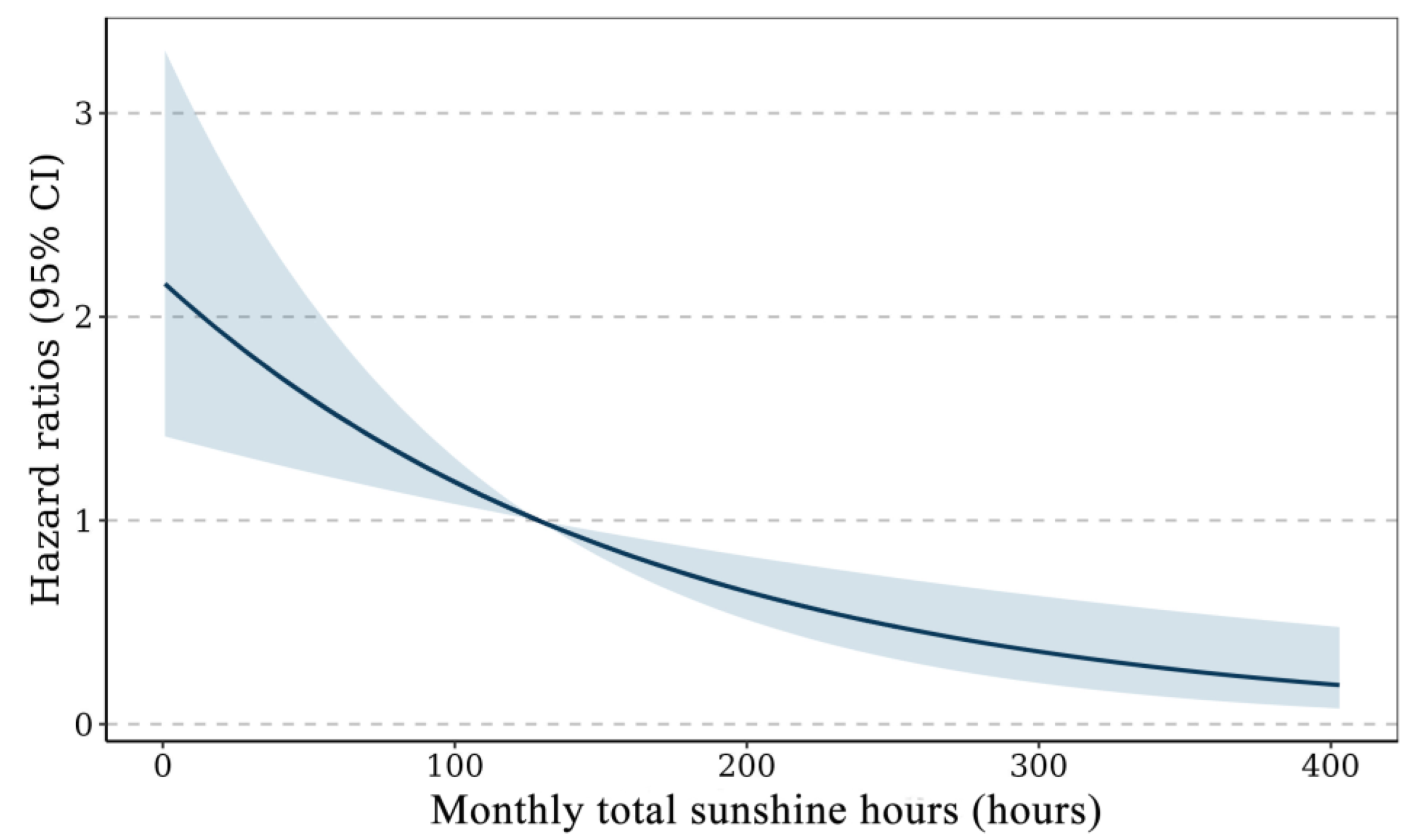
3.2. Survival Analysis on the Association Between Sunshine Duration and Depression
3.3. Stratification and Interaction Analysis
3.4. Mediation Analyses
3.4.1. Biochemical Markers
3.4.2. Sleep Pattern
3.5. Parallel Mediation Analysis
3.6. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Carreira-Míguez, M.; Navarro-Jiménez, E.; Clemente-Suárez, V.J. Behavioral Patterns of Depression Patients and Control Population. Int. J. Environ. Res. Public Health 2022, 19, 9506. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.; Jones, L.; Atun, R.; Thornicroft, G. The true global disease burden of mental illness: Still elusive. Lancet Psychiatry 2022, 9, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Abbas, M. Seasonal Depressive Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lindskov, F.O.; Iversen, H.K.; West, A.S. Clinical outcomes of light therapy in hospitalized patients—A systematic review. Chronobiol. Int. 2022, 39, 299–310. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, C.; Xu, G.; Song, J.; Su, H.; Wang, H. Effects of sunshine duration on daily outpatient visits for depression in Suzhou, Anhui Province, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 2075–2085. [Google Scholar] [CrossRef]
- Huibers, M.J.; de Graaf, L.E.; Peeters, F.P.; Arntz, A. Does the weather make us sad? Meteorological determinants of mood and depression in the general population. Psychiatry Res. 2010, 180, 143–146. [Google Scholar] [CrossRef]
- Kim, S.Y.; Bang, M.; Wee, J.H.; Min, C.; Yoo, D.M.; Han, S.M.; Kim, S.; Choi, H.G. Short- and long-term exposure to air pollution and lack of sunlight are associated with an increased risk of depression: A nested case-control study using meteorological data and national sample cohort data. Sci. Total Environ. 2021, 757, 143960. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef]
- Chan, C.B.; Ryan, D.A. Assessing the effects of weather conditions on physical activity participation using objective measures. Int. J. Environ. Res. Public Health 2009, 6, 2639–2654. [Google Scholar] [CrossRef]
- Ronaldson, A.; Arias de la Torre, J.; Gaughran, F.; Bakolis, I.; Hatch, S.L.; Hotopf, M.; Dregan, A. Prospective associations between vitamin D and depression in middle-aged adults: Findings from the UK Biobank cohort. Psychol. Med. 2022, 52, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Hollis, D.; McCarthy, M.; Kendon, M.; Legg, T.; Simpson, I. HadUK-Grid—A new UK dataset of gridded climate observations. Geosci. Data J. 2019, 6, 151–159. [Google Scholar] [CrossRef]
- Perry, M.; Hollis, D. The development of a new set of long-term climate averages for the UK. Int. J. Climatol. 2005, 25, 1023–1039. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Qian, S.E.; Cai, M.; Li, H.; Wang, C.; Zou, H.; Chen, L.; Vaughn, M.G.; McMillin, S.E.; et al. Ambient air pollution associated with incidence and dynamic progression of type 2 diabetes: A trajectory analysis of a population-based cohort. BMC Med. 2022, 20, 375. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, H.; Shi, H.; Zheng, D.; Wang, X.; Ai, B.; Tian, F.; Lin, H. Effect modification of dietary diversity on the association of air pollution with incidence, complications, and mortality of type 2 diabetes: Results from a large prospective cohort study. Sci. Total Environ. 2024, 908, 168314. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, B.; Yang, C.; Zhou, Y.; Yao, S.; Qian, X.; Wang, C.; Wu, B.; Wu, J. Extended time series (2000–2018) of global NPP-VIIRS-like nighttime light data from a cross-sensor calibration. Earth Syst. Sci. Data 2021, 13, 889–906. [Google Scholar] [CrossRef]
- Shi, H.; Schweren, L.J.S.; Ter Horst, R.; Bloemendaal, M.; van Rooij, D.; Vasquez, A.A.; Hartman, C.A.; Buitelaar, J.K. Low-grade inflammation as mediator between diet and behavioral disinhibition: A UK Biobank study. Brain Behav. Immun. 2022, 106, 100–110. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. A score of low-grade inflammation and risk of mortality: Prospective findings from the Moli-sani study. Haematologica 2016, 101, 1434–1441. [Google Scholar] [CrossRef]
- Gan, X.; He, P.; Ye, Z.; Zhou, C.; Liu, M.; Yang, S.; Zhang, Y.; Zhang, Y.; Qin, X. Adherence to a healthy sleep pattern and new-onset acute kidney injury. Sleep. Health 2023, 9, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cai, M.; Qian, Z.M.; Wang, X.; Zhang, Z.; Wang, C.; Wang, Y.; Arnold, L.D.; Howard, S.W.; Li, H.; et al. Ambient air pollution, lifestyle, and genetic predisposition associated with type 2 diabetes: Findings from a national prospective cohort study. Sci. Total Environ. 2022, 849, 157838. [Google Scholar] [CrossRef]
- Tennant, P.W.G.; Murray, E.J.; Arnold, K.F.; Berrie, L.; Fox, M.P.; Gadd, S.C.; Harrison, W.J.; Keeble, C.; Ranker, L.R.; Textor, J.; et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: Review and recommendations. Int. J. Epidemiol. 2021, 50, 620–632. [Google Scholar] [CrossRef]
- Wu, X.; Braun, D.; Schwartz, J.; Kioumourtzoglou, M.A.; Dominici, F. Evaluating the impact of long-term exposure to fine particulate matter on mortality among the elderly. Sci. Adv. 2020, 6, eaba5692. [Google Scholar] [CrossRef]
- Imbens, G.W. The role of the propensity score in estimating dose-response functions. Biometrika 2000, 87, 706–710. [Google Scholar] [CrossRef]
- Hirano, K.; Imbens, G.W. The propensity score with continuous treatments. Appl. Bayesian Model. Causal Inference Incomplete-Data Perspectives 2004, 226164, 73–84. [Google Scholar]
- Kim, E.; Lee, W.; Lee, J.Y.; Kim, Y.; Lee, J.H.; Hong, Y.C.; Park, H.S.; Kim, Y.; Ha, M.; Kim, Y.J.; et al. The effect of residential greenness during pregnancy on infant neurodevelopment using propensity score weighting: A prospective mother-infant paired cohort study. Sci. Total Environ. 2023, 894, 164888. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cai, M.; Qian, Z.M.; Zhang, S.; Yang, Y.; McMillin, S.E.; Chen, G.; Hua, J.; Tabet, M.; Wang, C.; et al. The effects of long-term exposure to air pollution on incident mental disorders among patients with prediabetes and diabetes: Findings from a large prospective cohort. Sci. Total Environ. 2023, 897, 165235. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Assessing covariate balance when using the generalized propensity score with quantitative or continuous exposures. Stat Methods Med Res. 2019, 28, 1365–1377. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Liang, J.; Sun, H.; Wang, T. High-dimensional generalized propensity score with application to omics data. Brief Bioinform. 2021, 22, bbab331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, M.; Liu, P.; Shi, L.; Yu, Z.; Abu Awad, Y.; Zanobetti, A.; Schwartz, J. Doubly Robust Additive Hazards Models to Estimate Effects of a Continuous Exposure on Survival. Epidemiology 2017, 28, 771–779. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.-K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- Verdiesen, R.M.G.; Onland-Moret, N.C.; van Gils, C.H.; van der Schouw, Y.T. Circulating anti-Müllerian hormone levels and markers of subclinical cardiovascular disease in middle-aged and older men. Maturitas 2022, 163, 38–45. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Luo, L. Decomposition of the total effect for two mediators: A natural mediated interaction effect framework. J. Causal Inference 2022, 10, 18–44. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, X.; Tan, K.; Guo, Y.; Zhao, X.; Chen, G.; Guo, B.; Li, S.; Feng, S.; Pan, Q.; et al. Mediation of metabolic syndrome in the association between long-term exposure to particulate matter and incident cardiovascular disease: Evidence from a population-based cohort in Chengdu. Ecotoxicol. Environ. Saf. 2023, 269, 115827. [Google Scholar] [CrossRef]
- He, L.; Ma, T.; Wang, X.; Cheng, X.; Bai, Y. Association between longitudinal change of sleep patterns and the risk of cardiovascular diseases. Sleep 2024, 47, zsae084. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Tan, Y.J.; Blum, M.R. Limitations and Misinterpretations of E-Values for Sensitivity Analyses of Observational Studies. Ann. Intern. Med. 2019, 170, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Yang, H.I. Causal Mediation Analysis of Survival Outcome with Multiple Mediators. Epidemiology 2017, 28, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, H.E.; White, R.A.; Sylvén, S.M.; Papadopoulos, F.C.; Skalkidou, A. Meteorological parameters and air pollen count in association with self-reported peripartum depressive symptoms. Eur. Psychiatry 2018, 54, 10–18. [Google Scholar] [CrossRef]
- Lin, J.; Yang, H.; Zhang, Y.; Cao, Z.; Li, D.; Sun, L.; Zhang, X.; Wang, Y. Association of time spent in outdoor light and genetic risk with the incidence of depression. Transl. Psychiatry 2023, 13, 40. [Google Scholar] [CrossRef]
- Son, J.; Shin, J. Bimodal effects of sunlight on major depressive disorder. Compr. Psychiatry 2021, 108, 152232. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Lazaretti-Castro, M.; Lips, P.; Marcocci, C.; Bilezikian, J.P. Vitamin D in the older population: A consensus statement. Endocrine 2023, 79, 31–44. [Google Scholar] [CrossRef]
- Ventura-Cots, M.; Watts, A.E.; Cruz-Lemini, M.; Shah, N.D.; Ndugga, N.; McCann, P.; Barritt, A.S.t.; Jain, A.; Ravi, S.; Fernandez-Carrillo, C.; et al. Colder Weather and Fewer Sunlight Hours Increase Alcohol Consumption and Alcoholic Cirrhosis Worldwide. Hepatology 2019, 69, 1916–1930. [Google Scholar] [CrossRef]
- Chauhan, K.; Shahrokhi, M.; Huecker, M.R. Vitamin D. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gospodarska, E.; Dastidar, R.G.; Jaroslawska, J.; Rybiński, M.; Raczyk, M.; Tokarczyk-Malesa, K.; Romaszko, J.; Carlberg, C. Transcriptomic profiling of immune modulation induced by vitamin D(3) in the VitDPAS and VitDHiD cohort studies. Sci. Rep. 2025, 15, 17334. [Google Scholar] [CrossRef] [PubMed]
- Wassif, G.A.; Alrehely, M.S.; Alharbi, D.M.; Aljohani, A.A. The Impact of Vitamin D on Neuropsychiatric Disorders. Cureus 2023, 15, e47716. [Google Scholar] [CrossRef]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The effect of vitamin D supplementation on depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 11784–11801. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Fuzi, S.F.; Mushtaq, S. Vitamin D3 supplementation for 8 weeks leads to improved haematological status following the consumption of an iron-fortified breakfast cereal: A double-blind randomised controlled trial in iron-deficient women. Br. J. Nutr. 2019, 121, 1146–1157. [Google Scholar] [CrossRef]
- Fleury, N.; Geldenhuys, S.; Gorman, S. Sun Exposure and Its Effects on Human Health: Mechanisms through Which Sun Exposure Could Reduce the Risk of Developing Obesity and Cardiometabolic Dysfunction. Int. J. Environ. Res. Public Health 2016, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Tähkämö, L.; Partonen, T.; Pesonen, A.K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019, 36, 151–170. [Google Scholar] [CrossRef]
- Burns, A.C.; Saxena, R.; Vetter, C.; Phillips, A.J.K.; Lane, J.M.; Cain, S.W. Time spent in outdoor light is associated with mood, sleep, and circadian rhythm-related outcomes: A cross-sectional and longitudinal study in over 400,000 UK Biobank participants. J. Affect. Disord. 2021, 295, 347–352. [Google Scholar] [CrossRef]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock—Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef]
- Mead, M.N. Benefits of sunlight: A bright spot for human health. Environ. Health Perspect. 2008, 116, A160–A167. [Google Scholar] [CrossRef]
- Stewart, D.; Albrecht, U. Beyond vision: Effects of light on the circadian clock and mood-related behaviours. npj Biol. Timing Sleep 2025, 2, 12. [Google Scholar] [CrossRef]
- Klimek, M.; Peter, R.S.; Denkinger, M.; Dallmeier, D.; Rapp, K.; Rothenbacher, D.; Klenk, J. The relationship of weather with daily physical activity and the time spent out of home in older adults from Germany—The ActiFE study. Eur. Rev. Aging Phys. Act. 2022, 19, 6. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef] [PubMed]
- Gominak, S.C. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Participants | None-Cases | Incident Depression | p |
|---|---|---|---|---|
| Number of participants | 336,805 | 322,943 | 13,862 | |
| Sex [n (%)] | ||||
| Female | 175,215 (52) | 167,030 (52) | 8185 (59) | <0.001 |
| Male | 161,590 (48) | 155,913 (48) | 5677 (41) | |
| Age [year (mean ± SD)] | 56.9 ± 8.1 | 56.9 ± 8.0 | 56.5 ± 8.4 | <0.001 |
| Household income [n (%)] | ||||
| Less than 18,000 £ | 59,350 (18) | 55,581 (17) | 3769 (27) | <0.001 |
| 18,000 to 30,999 £ | 74,214 (22) | 71,005 (22) | 3209 (23) | |
| 31,000 £ to 51,999 £ | 78,479 (23) | 75,667 (23) | 2812 (20) | |
| Greater than 52,000 £ | 81,309 (24) | 79,209 (25) | 2100 (15) | |
| Unknown | 43,453 (13) | 41,481 (13) | 1972 (14) | |
| Residential area [n (%)] | ||||
| Urban | 284,619 (85) | 272,551 (84) | 12,068 (87) | <0.001 |
| Rural | 52,186 (16) | 50,392 (16) | 1794 (13) | |
| Physical activity [n (%)] | ||||
| Low | 49,875 (15) | 47,535 (15) | 2340 (17) | <0.001 |
| Moderate | 115,947 (34) | 111,550 (35) | 4397 (32) | |
| High | 118,823 (35) | 114,208 (35) | 4615 (33) | |
| Unknown | 52,160 (16) | 49,650 (15) | 2510 (18) | |
| Smoking status [n (%)] | ||||
| Never | 183,528 (55) | 177,101 (55) | 6427 (46) | <0.001 |
| Former | 121,071 (36) | 115,900 (36) | 5171 (37) | |
| Current | 32,206 (10) | 29,942 (9) | 2264 (16) | |
| Polypharmacy [n (%)] | ||||
| 0 | 102,505 (30) | 99,772 (31) | 2733 (20) | <0.001 |
| 1–2 | 119,654 (36) | 115,085 (36) | 4569 (33) | |
| ≥3 | 114,646 (34) | 108,086 (34) | 6560 (47) | |
| Time spent outdoors [n (%)] | ||||
| Low | 108,104 (32) | 103,932 (32) | 4172 (30) | <0.001 |
| Moderate | 82,971 (25) | 79,620 (25) | 3351 (24) | |
| High | 145,730 (43) | 139,391 (43) | 6339 (46) | |
| Townsend deprivation index [n (%)] | ||||
| Low | 115,626 (34) | 111,786 (35) | 3840 (28) | <0.001 |
| Moderate | 113,897 (34) | 109,522 (34) | 4375 (32) | |
| High | 107,282 (32) | 101,635 (32) | 5647 (41) | |
| Greenspace [n (%)] | ||||
| Low | 107,259 (32) | 102,854 (32) | 4405 (32) | <0.001 |
| Moderate | 110,809 (33) | 105,883 (33) | 4926 (36) | |
| High | 118,737 (35) | 114,206 (35) | 4531 (33) | |
| Ambient temperature [n (%)] | ||||
| Low | 113,081 (34) | 108,382 (34) | 4699 (34) | 0.001 |
| Moderate | 114,461 (34) | 109,595 (34) | 4866 (35) | |
| High | 109,263 (32) | 104,966 (33) | 4297 (31) | |
| PM2.5 [n (%)] | ||||
| Low | 111,142 (33) | 106,441 (33) | 4701 (34) | <0.001 |
| Moderate | 111,148 (33) | 106,329 (33) | 4819 (35) | |
| High | 114,515 (34) | 110,173 (34) | 4342 (31) | |
| Average monthly sunshine duration over the three years prior to recruitment (mean (SD)) | 129 ± 8 | 129 ± 8 | 128 ± 8 | <0.001 |
| Model | Analysis 1 | Analysis 2 | ||
|---|---|---|---|---|
| HR (95% CI) | p | β (95% CI) | p | |
| Total population | ||||
| Model 1 | 0.91 (0.90, 0.92) | <0.001 | −0.13 (−0.20, −0.06) | <0.001 |
| Model 2 | 0.92 (0.89, 0.95) | <0.001 | −0.11 (−0.18, −0.04) | 0.002 |
| Model 3 | 0.96 (0.92, 0.97) | <0.001 | −0.10 (−0.17, −0.03) | 0.003 |
| Model 4 | 0.92 (0.89, 0.95) | <0.001 | −0.08 (−0.15, −0.01) | 0.03 |
| Low genetic risk | ||||
| Model 1 | 0.89 (0.83, 0.95) | <0.001 | −0.18 (−0.33, −0.03) | 0.02 |
| Model 2 | 0.92 (0.85, 0.98) | 0.01 | −0.16 (−0.32, −0.01) | 0.04 |
| Model 3 | 0.91 (0.85, 0.98) | 0.01 | −0.16 (−0.31, −0.01) | 0.04 |
| Model 4 | 0.90 (0.85, 0.96) | 0.001 | −0.14 (−0.29, −0.01) | 0.03 |
| Medium genetic risk | ||||
| Model 1 | 0.90 (0.86, 0.93) | <0.001 | −0.13 (−0.22, −0.04) | 0.005 |
| Model 2 | 0.92 (0.89, 0.96) | <0.001 | −0.11 (−0.20, −0.02) | 0.02 |
| Model 3 | 0.92 (0.87, 0.96) | <0.001 | −0.11 (−0.20, −0.02) | 0.02 |
| Model 4 | 0.92 (0.87, 0.96) | <0.001 | −0.08 (−0.17, −0.01) | 0.04 |
| High genetic risk | ||||
| Model 1 | 0.89 (0.84, 0.94) | <0.001 | −0.11 (−0.22, 0.01) | 0.23 |
| Model 2 | 0.91 (0.87, 0.97) | 0.002 | −0.08 (−0.15, −0.02) | 0.02 |
| Model 3 | 0.92 (0.87, 0.97) | 0.002 | −0.04 (−0.08, −0.01) | 0.04 |
| Model 4 | 0.92 (0.87, 0.97) | 0.003 | −0.04 (−0.07, −0.01) | 0.01 |
| Group | Hazard Ratio (95% C) | p for Interaction |
|---|---|---|
| Gender | ||
| Female | 0.94 (0.91, 0.96) | Ref |
| Male | 0.95 (0.91, 0.98) | 0.31 |
| Age | ||
| Young | 0.97 (0.94, 0.99) | Ref |
| Old | 0.94 (0.92, 0.96) | 0.02 |
| Household income | ||
| Less than 18,000 £ | 1.02 (0.98, 1.07) | Ref |
| 18,000 to 30,999 £ | 0.95 (0.91, 0.99) | 0.58 |
| 31,000 £ to 51,999 £ | 0.90 (0.86, 0.94) | 0.08 |
| Greater than 52,000 £ | 0.84 (0.79, 0.89) | 0.25 |
| Alcohol intake | ||
| Never | 1.03 (0.96, 1.11) | Ref |
| Occasional | 0.97 (0.93, 1.02) | 0.14 |
| Moderate | 0.90 (0.87, 0.93) | <0.001 |
| Heavy | 0.94 (0.90, 0.99) | 0.02 |
| Smoking status | ||
| Never smoked | 0.94 (0.91, 0.97) | Ref |
| Previous smoked | 0.94 (0.90, 0.97) | 0.94 |
| Current smoked | 0.93 (0.88, 0.99) | 0.87 |
| Time spent outdoors | ||
| Low | 0.92 (0.88, 0.95) | Ref |
| Moderate | 0.95 (0.91, 0.99) | 0.02 |
| High | 0.97 (0.96, 0.98) | 0.001 |
| Physical activity | ||
| Low | 0.92 (0.87, 0.96) | Ref |
| Moderate | 0.97 (0.94, 0.99) | 0.03 |
| High | 0.92 (0.88, 0.95) | 0.82 |
| Residential area | ||
| Rural | 0.94 (0.92, 0.96) | Ref |
| Urban | 0.94 (0.88, 0.99) | 0.70 |
| Artificial light at night | ||
| Low | 0.94 (0.91, 0.98) | Ref |
| Medium | 0.90 (0.87, 0.94) | 0.08 |
| High | 0.96 (0.94, 0.99) | 0.19 |
| PRS | ||
| Low | 0.90 (0.85, 0.96) | Ref |
| Medium | 0.92 (0.87, 0.96) | 0.19 |
| High | 0.92 (0.87, 0.97) | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Tian, F.; Zhang, J.; Huang, Z.; Chen, G.; Qian, Z.; Wang, Y.; Stamatakis, K.A.; Howard, S.W.; Zheng, G.; et al. Sunshine Duration, Genetic Predisposition, and Incident Depression: Findings from a Prospective Cohort. Green Health 2025, 1, 13. https://doi.org/10.3390/greenhealth1020013
Feng J, Tian F, Zhang J, Huang Z, Chen G, Qian Z, Wang Y, Stamatakis KA, Howard SW, Zheng G, et al. Sunshine Duration, Genetic Predisposition, and Incident Depression: Findings from a Prospective Cohort. Green Health. 2025; 1(2):13. https://doi.org/10.3390/greenhealth1020013
Chicago/Turabian StyleFeng, Jin, Fei Tian, Jingyi Zhang, Zhenhe Huang, Ge Chen, Zhengmin (Min) Qian, Yuhua Wang, Katherine A. Stamatakis, Steven W. Howard, Guzhengyue Zheng, and et al. 2025. "Sunshine Duration, Genetic Predisposition, and Incident Depression: Findings from a Prospective Cohort" Green Health 1, no. 2: 13. https://doi.org/10.3390/greenhealth1020013
APA StyleFeng, J., Tian, F., Zhang, J., Huang, Z., Chen, G., Qian, Z., Wang, Y., Stamatakis, K. A., Howard, S. W., Zheng, G., Wang, C., & Lin, H. (2025). Sunshine Duration, Genetic Predisposition, and Incident Depression: Findings from a Prospective Cohort. Green Health, 1(2), 13. https://doi.org/10.3390/greenhealth1020013






