Independent Associations Between Urinary Bisphenols and Vitamin D Deficiency: Findings from NHANES Study
Abstract
1. Introduction
2. Materials and Methods
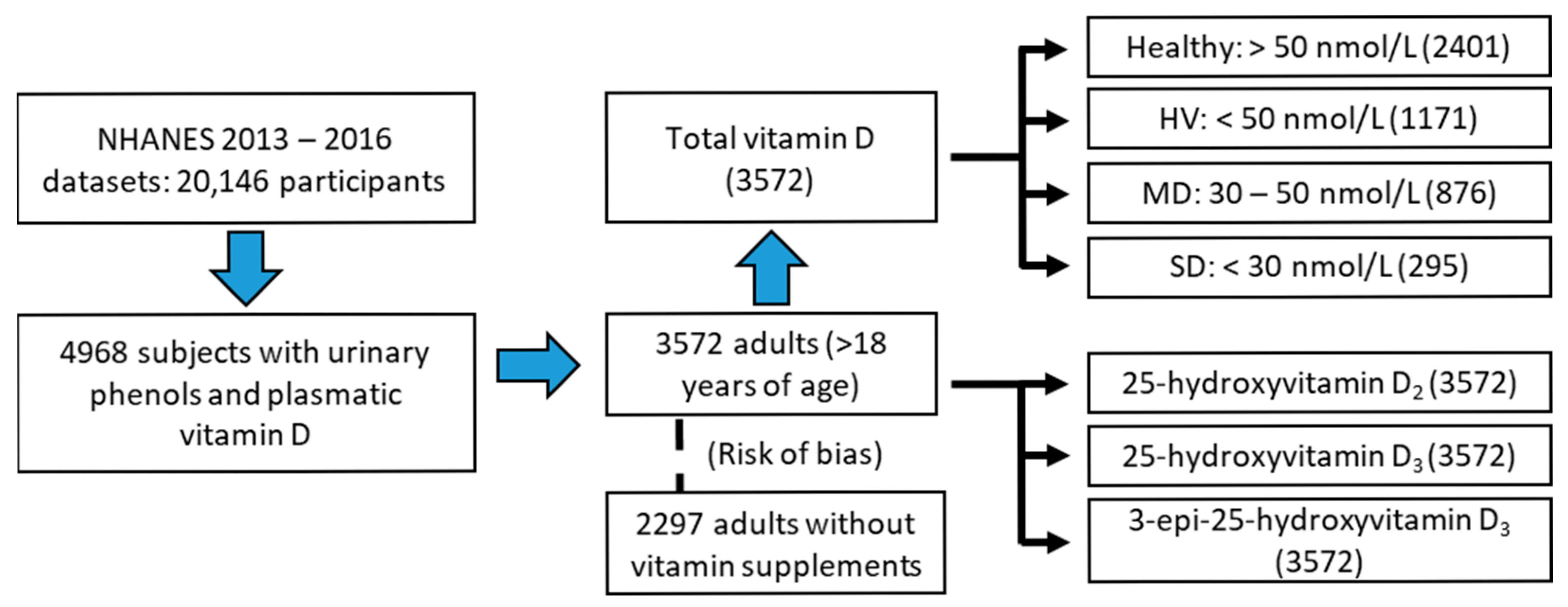
2.1. Study Population (NHANES 13–16)
- Dichotomous classification: subdivided into healthy (total vitamin D values greater than 50 nmol/L) and hypovitaminosis (HV, subjects with values less than 50 nmol/L) groups.
- Classification according to the degree of vitamin D deficiency: subdivided into healthy, moderate deficiency (MD, vitamin D values between 30 and 50 nmol/L), and severe deficiency (SD, <30 nmol/L) groups.
- Risk of bias: The variables were reanalyzed in a new subgroup in which all those individuals who took any vitamin D supplement were eliminated. For this, the files related to the intake of dietary supplements (NHANES Dietary Data [52]) were used.
2.2. Covariates and Corrections
- Diabetes (dichotomous variable [0, healthy; 1, diseased]): Diabetics were all those individuals diagnosed by a doctor, those taking blood glucose medication (NHANES questionnaires [53]), and all subjects with values of fasting glucose ≥ 126 mg/dL or hemoglobin A1c ≥ 6.5%.
- Chronic kidney disease (CKD): Firstly, the estimated glomerular filtration rate (eGFR) was calculated using the two usual formulas for clinical use (Chronic Kidney Disease Epidemiology Collaboration, CKD-EPI, and Modification of Diet in Renal Disease, MDRD-4) [54,55,56]. Subsequently, all those individuals with eGFR less than 60 mL/min/1.73 m2 were included [57,58].
- Albuminuria: All albumin-to-creatinine ratio (ACR) values greater than 30 mg albumin/g creatinine were considered albuminuria.
- Hypertension: Patients diagnosed by their doctor, those taking medication for hypertension, and individuals with systolic pressure ≥ 140 mmHg or systolic ≥ 90 mmHg were considered hypertensive.
- Dyslipidemia: Patients with diagnosed cholesterol disorders, with prescribed medication or fasting total cholesterol ≥ 240 mg/dL.
- Smoking: All individuals who answered affirmatively to the question “have you smoked more than 100 cigarettes in your life?” or individuals with serum cotinine levels > 10 mg/dL [59] were included.
2.3. Statistical Analysis
3. Results
3.1. Analysis of Total Vitamin D (Dichotomous and Multinomial Vitamin D Status)
3.1.1. Descriptive Statistics
3.1.2. Binomial and Multinomial Logistic Regression
3.2. Supplementary Analysis of Vitamin D Metabolites
3.3. Risk of Bias: Exclusion of Vitamin D Supplements
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baralla, E.; Pasciu, V.; Varoni, M.V.; Nieddu, M.; Demuro, R.; Demontis, M.P. Bisphenols’ occurrence in bivalves as sentinel of environmental contamination. Sci. Total Environ. 2021, 785, 147263. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, T.; Harner, T. Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels. Sci. Total Environ. 2021, 789, 148013. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.R.R.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Freire, C.; Molina-Molina, J.M.J.-M.; Iribarne-Duran, L.M.; Jimenez-Diaz, I.; Vela-Soria, F.; Mustieles, V.; Arrebola, J.P.; Fernández, M.F.; Artacho-Cordón, F.; Olea, N. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone-like activities. Environ. Int. 2019, 127, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; Sánchez-Esteban, S.; Cook, A.; Saura, M.; Bosch, R.J. Critical Analysis of Human Exposure to Bisphenol a and its Novel Implications on Renal, Cardiovascular and Hypertensive Diseases. In Hot Topics in Endocrinology and Metabolism, 1st ed.; Heshmati, H.M., Ed.; IntechOpen: London, UK, 2021; pp. 1–20. [Google Scholar]
- Xu, L.C.; Sun, H.; Chen, J.F.; Bian, Q.; Qian, J.; Song, L.; Wang, X.R. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology 2005, 216, 197–203. [Google Scholar] [CrossRef]
- Teng, C.; Goodwin, B.; Shockley, K.; Xia, M.; Huang, R.; Norris, J.; Merrick, B.A.; Jetten, A.M.; Austin, C.P.; Tice, R.R. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem. Biol. Interact. 2013, 203, 556–564. [Google Scholar] [CrossRef]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; Vélez-Vélez, E.; Coll, E.; Quiroga, B.; Bover, J.; Bosch, R.J. Bisphenol A exposure and kidney diseases: Systematic review, meta-analysis and NHANES 03–16 study. Biomolecules 2021, 11, 1046. [Google Scholar] [CrossRef]
- Saura, M.; Marquez, S.; Reventun, P.; Olea-Herrero, N.; Isabel Arenas, M.; Moreno-Gomez-Toledano, R.; Gómez-Parrizas, M.; Muñóz-Moreno, C.; González-Santander, M.; Zaragoza, C.; et al. Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014, 28, 4719–4728. [Google Scholar] [CrossRef]
- Nunez, P.; Fernandez, T.; Garcia-Arevalo, M.; Alonso-Magdalena, P.; Nadal, A.; Perillan, C.; Arguelles, J. Effects of bisphenol A treatment during pregnancy on kidney development in mice: A stereological and histopathological study. J. Dev. Orig. Health Dis. 2018, 9, 208–214. [Google Scholar] [CrossRef]
- Bano, U.; Memon, S.; Shahani, M.Y.; Shaikh, P.; Gul, S. Epigenetic effects of in utero bisphenol A administration: Diabetogenic and atherogenic changes in mice offspring. Iran. J. Basic Med. Sci. 2019, 22, 521–528. [Google Scholar]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Skledar, D.G.; Schmidt, J.; Fic, A.; Klopčič, I.; Trontelj, J.; Dolenc, M.S.; Finel, M.; Mašič, L.P. Influence of metabolism on endocrine activities of bisphenol S. Chemosphere 2016, 157, 152–159. [Google Scholar] [CrossRef]
- Conroy-Ben, O.; Garcia, I.; Teske, S.S. In silico binding of 4,4′-bisphenols predicts in vitro estrogenic and antiandrogenic activity. Environ. Toxicol. 2018, 33, 569–578. [Google Scholar] [CrossRef]
- Marroqui, L.; Martinez-Pinna, J.; Castellano-Muñoz, M.; dos Santos, R.S.; Medina-Gali, R.M.; Soriano, S.; Quesada, I.; Gustafsson, J.A.; Encinar, J.A.; Nadal, A. Bisphenol-S and Bisphenol-F alter mouse pancreatic β-cell ion channel expression and activity and insulin release through an estrogen receptor ERβ mediated pathway. Chemosphere 2021, 265, 129051. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Shao, H.; Lei, P.; Zheng, C.; Qiu, C.; Yang, M.; Zheng, Y. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere 2018, 194, 1–8. [Google Scholar] [CrossRef]
- Ramskov Tetzlaff, C.N.; Svingen, T.; Vinggaard, A.M.; Rosenmai, A.K.; Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 2020, 35, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, I.; Czerniecki, J.; Jarzabek, K.; Zbucka-Kretowska, M.; Wolczynski, S. Cellular, transcriptomic and methylome effects of individual and combined exposure to BPA, BPF, BPS on mouse spermatocyte GC-2 cell line. Toxicol. Appl. Pharmacol. 2018, 359, 1–11. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.; Ivantsova, E.; Guzman, A.P.; Martyniuk, C.J. Critical review of the toxicity mechanisms of bisphenol F in zebrafish (Danio rerio): Knowledge gaps and future directions. Chemosphere 2022, 297, 134132. [Google Scholar] [CrossRef]
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef] [PubMed]
- Lehmler, H.-J.J.; Liu, B.Y.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013-2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef]
- Sol, C.M.; Van Zwol-Janssens, C.; Philips, E.M.; Asimakopoulos, A.G.; Martinez-Moral, M.P.; Kannan, K.; Jaddoe, V.W.V.; Trasande, L.; Santos, S. Maternal bisphenol urine concentrations, fetal growth and adverse birth outcomes: A population-based prospective cohort. Environ. Health 2021, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Liu, C.; Shen, Y.; Wang, Q.; Pan, A.; Yang, P.; Chen, Y.J.; Deng, Y.L.; Lu, Q.; Cheng, L.M.; et al. Urinary levels of bisphenol A, F and S and markers of oxidative stress among healthy adult men: Variability and association analysis. Environ. Int. 2019, 123, 301–309. [Google Scholar] [CrossRef]
- Jin, H.B.; Xie, J.H.; Mao, L.L.; Zhao, M.R.; Bai, X.X.; Wen, J.; Shen, T.; Wu, P. Bisphenol analogue concentrations in human breast milk and their associations with postnatal infant growth. Environ. Pollut. 2020, 259, 113779. [Google Scholar] [CrossRef]
- Li, A.J.; Zhuang, T.F.; Shi, W.; Liang, Y.; Liao, C.Y.; Song, M.Y.; Jiang, G. Serum concentration of bisphenol analogues in pregnant women in China. Sci. Total Environ. 2020, 707, 136100. [Google Scholar] [CrossRef]
- Ross, C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium Vitamin, D. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Cesari, M.; Incalzi, R.A.; Zamboni, V.; Pahor, M. Vitamin D hormone: A multitude of actions potentially influencing the physical function decline in older persons. Geriatr. Gerontol. Int. 2011, 11, 133–142. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhäuser-Berthold, M.; et al. Dietary reference values for vitamin D. EFSA J. 2016, 14, 4547. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.C.Y.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.E.; et al. Influence of Vitamin D supplementation by sunlight or oral D3 on exercise performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; tepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current Vitamin D status in European and Middle East countries and strategies to prevent Vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- Tao, S.; Yuan, Q.; Mao, L.; Chen, F.L.; Ji, F.; Cui, Z.H. Vitamin D deficiency causes insulin resistance by provoking oxidative stress in hepatocytes. Oncotarget 2017, 8, 67605–67613. [Google Scholar] [CrossRef] [PubMed]
- Fiamenghi VI, Mello ED de. Vitamin D deficiency in children and adolescents with obesity: A meta-analysis. J. Pediatr. 2021, 97, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [PubMed]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.E.; Ferguson, K.K.; Meeker, J.D. Relationships Between Urinary Phthalate Metabolite and Bisphenol A Concentrations and Vitamin D Levels in U.S. Adults: National Health and Nutrition Examination Survey (NHANES), 2005–2010. J. Clin. Endocrinol. Metab. 2016, 101, 4062–4069. [Google Scholar] [CrossRef]
- Brandi, M.L.; Bandinelli, S.; Iantomasi, T.; Giusti, F.; Talluri, E.; Sini, G.; Nannipieri, F.; Battaglia, S.; Giusti, R.; Egan, C.G.; et al. Association between vitamin D and bisphenol A levels in an elderly Italian population: Results from the InCHIANTI study. Endocr. Connect. 2022, 11, e210571. [Google Scholar] [CrossRef]
- Erden, E.S.; Genc, S.; Motor, S.; Ustun, I.; Ulutas, K.T.; Bilgic, H.K.; Oktar, S.; Sungur, S.; Erem, C.; Gokce, C. Investigation of serum bisphenol A, vitamin D, and parathyroid hormone levels in patients with obstructive sleep apnea syndrome. Endocrine 2014, 45, 311–318. [Google Scholar] [CrossRef]
- Johns, L.E.; Ferguson, K.K.; Cantonwine, D.E.; McElrath, T.F.; Mukherjee, B.; Meeker, J.D. Urinary BPA and phthalate metabolite concentrations and plasma vitamin D levels in pregnant women: A repeated measures analysis. Environ. Health Perspect. 2017, 125, 87026. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Data. National Center for Health Statistics (NCHS). 2016. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013 (accessed on 15 January 2022).
- Centers for Disease Control and Prevention (CDC). NHANES Dietary Data. National Center for Health Statistics (NCHS). 2016. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary&Cycle=2013-2014 (accessed on 15 January 2022).
- Centers for Disease Control and Prevention (CDC). NHANES Questionnaire Data. National Center for Health Statistics (NCHS). 2016. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire&Cycle=2013-2014 (accessed on 15 January 2022).
- Michels, W.M.; Grootendorst, D.C.; Verduijn, M.; Elliott, E.G.; Dekker, F.W.; Krediet, R.T. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin. J. Am. Soc. Nephrol. 2010, 5, 1003–1009. [Google Scholar] [CrossRef]
- Poggio, E.D.; Wang, X.; Greene, T.; Van Lente, F.; Hall, P.M. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 459–466. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Vega, G.L.; Wang, J.; Grundy, S.M. Chronic kidney disease and statin eligibility. J. Clin. Lipidol. 2021, 15, 173–180. [Google Scholar] [CrossRef]
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.R.; Jhee, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Dietary zinc intake and incident chronic kidney disease. Clin. Nutr. 2021, 40, 1039–1045. [Google Scholar] [CrossRef]
- Pirkle, J.L. Exposure of the US Population to Environmental Tobacco Smoke. JAMA 1996, 275, 1233. [Google Scholar] [CrossRef] [PubMed]
- Danzl, E.; Sei, K.; Soda, S.; Ike, M.; Fujita, M. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int. J. Environ. Res. Public Health 2009, 6, 1472–1484. [Google Scholar] [CrossRef]
- Gayrard, V.; Lacroix, M.Z.; Grandin, F.C.; Collet, S.H.; Mila, H.; Viguie, C.; Gély, C.A.; Rabozzi, B.; Bouchard, M.; Léandri, R.; et al. Oral systemic bioavailability of bisphenol A and bisphenol S in pigs. Environ. Health Perspect. 2019, 127, 77005. [Google Scholar] [CrossRef] [PubMed]
- Grandin, F.; Picard-Hagen, N.; Gayrard, V.; Puel, S.; Viguie, C.; Toutain, P.-L.; Debrauwer, L.; Lacroix, M.Z. Development of an on-line solid phase extraction ultra-high-performance liquid chromatography technique coupled to tandem mass spectrometry for quantification of bisphenol S and bisphenol S glucuronide: Applicability to toxicokinetic investigations. J. Chromatogr. A 2017, 1526, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hercog, K.; Maisanaba, S.; Filipič, M.; Sollner-Dolenc, M.; Kač, L.; Žegura, B. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci. Total Environ. 2019, 687, 267–276. [Google Scholar] [CrossRef]
- Kaimal, A.; Al Mansi, M.H.; Dagher, J.B.; Pope, C.; Varghese, M.G.; Rudi, T.B.; Almond, A.E.; Cagle, L.A.; Beyene, H.K.; Bradford, W.T.; et al. Prenatal exposure to bisphenols affects pregnancy outcomes and offspring development in rats. Chemosphere 2021, 276, 130118. [Google Scholar] [CrossRef]
- Mas, S.; Ruiz-Priego, A.; Abaigar, P.; Santos, J.; Camarero, V.; Egido, J.; Ortiz, A.; Gonzalez-Parra, E. Bisphenol S is a haemodialysis-associated xenobiotic that is less toxic than bisphenol A. Clin. Kidney J. 2021, 14, 1147–1155. [Google Scholar] [CrossRef]
- Ji, G.; Gu, J.; Guo, M.; Zhou, L.; Wang, Z.; Shi, L.; Gu, A. A systematic comparison of the developmental vascular toxicity of bisphenol A and its alternatives in vivo and in vitro. Chemosphere 2022, 291, 132936. [Google Scholar] [CrossRef] [PubMed]
- Frenzilli, G.; Martorell-Ribera, J.; Bernardeschi, M.; Scarcelli, V.; Jonsson, E.; Diano, N.; Moggio, M.; Guidi, P.; Sturve, J.; Asker, N. Bisphenol A and Bisphenol S Induce Endocrine and Chromosomal Alterations in Brown Trout. Front. Endocrinol. 2021, 12, 645519. [Google Scholar] [CrossRef]
- Olea-Herrero, N.; Arenas, M.I.; Munoz-Moreno, C.; Moreno-Gómez-Toledano, R.; Gonzalez-Santander, M.; Arribas, I.; Bosch, R.J. Bisphenol-A induces podocytopathy with proteinuria in mice. J. Cell. Physiol. 2014, 229, 2057–2066. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; González-Martínez, C.; Olea-Herrero, N.; Reventún, P.; Di Nunzio, M.; Sánchez-Esteban, S.; Arilla-Ferreiro, E.; Saura, M.; Bosch, R.J. Bisphenol A impaired cell adhesion by altering the expression of adhesion and cytoskeleton proteins on human podocytes. Sci. Rep. 2020, 10, 16638. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; Muñoz-Moreno, C.; Olea-Herrero, N.; Reventun, P.; Izquierdo-Lahuerta, A.; Antón-Cornejo, A.; González-Santander, M.; Zaragoza, C.; Saura, M.; et al. Comparison of the renal effects of bisphenol A in mice with and without experimental diabetes. Role of sexual dimorphism. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166296. [Google Scholar] [CrossRef]
- Reventun, P.; Sanchez-Esteban, S.; Cook, A.; Cuadrado, I.; Roza, C.; Moreno-Gómez-Toledano, R.; Muñoz, C.; Zaragoza, C.; Bosch, R.J.; Saura, M. Bisphenol A induces coronary endothelial cell necroptosis by activating RIP3/CamKII dependent pathway. Sci. Rep. 2020, 10, 4190. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Sanchez-Esteban, S.; Cook, A.; Mínguez-Moratinos, M.; Ramírez-Carracedo, R.; Reventun, P.; Delgado-Marín, M.; Bosch, R.J.; Saura, M. Bisphenol A induces accelerated cell aging in murine endothelium. Biomolecules 2021, 11, 1429. [Google Scholar] [CrossRef]
- Cantero-Navarro, E.; Fernández-Fernández, B.; Ramos, A.M.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Sánchez-Niño, M.D.; Sanz, A.B.; Ruiz-Ortega, M.; Ortiz, A. Renin-angiotensin system and inflammation update. Mol. Cell. Endocrinol. 2021, 529, 111254. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Sugimoto, M.; Ikeda, S.; Kume, S. Effects of bisphenol A administration to pregnant mice on serum Ca and intestinal Ca absorption. Anim. Sci. J. 2012, 83, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; An, B.-S.S.; Yang, H.; Jeung, E.-B.B. Effects of octylphenol and bisphenol A on the expression of calcium transport genes in the mouse duodenum and kidney during pregnancy. Toxicology 2013, 303, 99–106. [Google Scholar] [CrossRef]
- Moreno-Gomez-Toledano, R.; Velez-Velez, E.; Arenas, I.M.; Saura, M.; Bosch, R.J. Association between urinary concentrations of bisphenol A substitutes and diabetes in adults. World J. Diabetes 2022, 13, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gómez-Toledano, R.; Delgado-Marín, M.; Cook-Calvete, A.; González-Cucharero, C.; Alcharani, N.; Jiménez-Guirado, B.; Hernandez, I.; Ramirez-Carracedo, R.; Tesoro, L.; Botana, L.; et al. New environmental factors related to diabetes risk in humans: Emerging bisphenols used in synthesis of plastics. World J. Diabetes 2023, 14, 1301–1313. Available online: https://pubmed.ncbi.nlm.nih.gov/37664470/ (accessed on 19 December 2023). [CrossRef] [PubMed]
- Moreno-Gómez-Toledano, R.; Delgado-Marín, M.; Sánchez-Esteban, S.; Cook-Calvete, A.; Ortiz, S.; Bosch, R.J.; Saura, M. Combination of Bisphenol A and Its Emergent Substitute Molecules Is Related to Heart Disease and Exerts a Differential Effect on Vascular Endothelium. Int. J. Mol. Sci. 2023, 24, 12188. Available online: https://pubmed.ncbi.nlm.nih.gov/37569562/ (accessed on 31 July 2025). [CrossRef]
- Moreno-Gómez-Toledano, R. Relationship between emergent BPA-substitutes and renal and cardiovascular diseases in adult population. Environ. Pollut. 2022, 313, 120106. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Reis, E.; Sepúlveda-Fragoso, V.; Diniz, L.G.; Soares, D.J.S.; de Souza Carvalho-Laureano, T.; de Paula Alves, A.P.; de Luca, B.G.; Alexandre-Santos, B.; Stockler-Pinto, M.B.; de Carvalho, D.P.; et al. Chronic exposure to bisphenol S impairs hepatic mitochondrial dynamics, induces endoplasmic reticulum stress, and worsens metabolism in high-fat diet fed mice. Environ. Pollut. 2025, 382, 126780. Available online: https://pubmed.ncbi.nlm.nih.gov/40623585/ (accessed on 31 July 2025). [CrossRef]
- Mornagui, B.; Rezg, R.; Repond, C.; Pellerin, L. Bisphenol S favors hepatic steatosis development via an upregulation of liver MCT1 expression and an impairment of the mitochondrial respiratory system. J. Cell. Physiol. 2022, 237, 3057–3068. [Google Scholar] [CrossRef]
- Liang, J.; Xu, C.; Xu, J.; Yang, C.; Kong, W.; Xiao, Z.; Chen, X.; Liu, Q.; Weng, Z.; Wang, J.; et al. PPARα Senses Bisphenol S to Trigger EP300-Mediated Autophagy Blockage and Hepatic Steatosis. Environ. Sci. Technol. 2023, 57, 21581–21592. [Google Scholar] [CrossRef]
- Teumer, A.; Gambaro, G.; Corre, T.; Bochud, M.; Vollenweider, P.; Guessous, I.; Kleber, M.E.; Delgado, G.E.; Pilz, S.; März, W.; et al. Negative effect of vitamin D on kidney function: A Mendelian randomization study. Nephrol. Dial. Transplant. 2018, 33, 2139–2145. [Google Scholar] [CrossRef]
- Geng, J.; Qiu, Y.; Li, Y.; Li, J.; Liao, R.; Du, H.; Jiang, L.; Wang, L.; Qin, Z.; Yang, Q.; et al. Associations Between 25-Hydroxyvitamin D, Kidney Function, and Insulin Resistance Among Adults in the United States of America. Front. Nutr. 2022, 8, 1334. [Google Scholar] [CrossRef]
- Moore, L.W.; Suki, W.N.; Lunsford, K.E.; Sabek, O.M.; Knight, R.J.; Gaber, A.O. Cross-sectional evaluation of the relationship between vitamin D status and supplement use across levels of kidney function in adults. BMJ Open 2019, 9, 22471. [Google Scholar] [CrossRef]
- Priyadarshini, G.; Parameswaran, S.; Sahoo, J.; Selvarajan, S.; Rajappa, M. Vitamin D deficiency in chronic kidney disease: Myth or reality? Clin. Chim. Acta 2021, 523, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Rabe, T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150. Available online: https://pubmed.ncbi.nlm.nih.gov/24717915/ (accessed on 31 July 2025). [CrossRef]
- Yang, L.; Zhao, H.; Liu, K.; Wang, Y.; Liu, Q.; Sun, T.; Chen, S.; Ren, L. Smoking behavior and circulating vitamin D levels in adults: A meta-analysis. Food Sci. Nutr. 2021, 9, 5820–5832. Available online: https://pubmed.ncbi.nlm.nih.gov/34646549/ (accessed on 31 July 2025). [CrossRef]
- Brennan, E.; Butler, A.E.; Nandakumar, M.; Thompson, K.; Sathyapalan, T.; Atkin, S.L. Relationship between endocrine disrupting chemicals (phthalate metabolites, triclosan and bisphenols) and vitamin D in female subjects: An exploratory pilot study. Chemosphere 2024, 349, 140894. Available online: https://pubmed.ncbi.nlm.nih.gov/38070612/ (accessed on 31 July 2025). [CrossRef]
- Duan, Y.; Yao, Y.; Wang, B.; Han, L.; Wang, L.; Sun, H.; Chen, L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: A case-control study. Environ. Pollut. 2018, 243, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gómez-Toledano, R.; Vélez-Vélez, E.; Arenas, M.I.; Saura, M.; Bosch, R.J. Association between urinary concentrations of bisphenol A substitutes and diabetes in adults. World J. Diabetes 2022, 13, 521–531. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.J.; Sun, Y.; Xu, G.; Sun, Q.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association of bisphenol A and its substitutes, bisphenol F and bisphenol S, with obesity in united states children and adolescents. Diabetes Metab. J. 2019, 43, 59–75. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol A substitutes and obesity in US adults: Analysis of a population-based, cross-sectional study. Lancet Planet. Health 2017, 1, e114–e122. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.H.; Woodward, M.; Bao, W.; Liu, B.; Trasande, L. Urinary bisphenols and obesity prevalence among U.S. children and adolescents. J. Endocr. Soc. 2019, 3, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.L.; Liu, H.M.; Zhou, S.; Zhang, X.; Peng, C.; Zhou, H.; Tong, Y.; Lu, Q. Association of bisphenol A and its alternatives bisphenol S and F exposure with hypertension and blood pressure: A cross-sectional study in China. Environ. Pollut. 2020, 257, 113639. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.Y.; Luo, J.; Sun, J.; Ge, H.H.; Wang, Z.G. Associations of urinary bisphenol A and its alternatives bisphenol S and F concentrations with depressive symptoms among adults. Chemosphere 2021, 279, 130573. [Google Scholar] [CrossRef] [PubMed]
| Healthy | HV | MD | SD | |
|---|---|---|---|---|
| N | 2401 (67.22%) | 1171 (32.78%) | 876 (24.52%) | 295 (8.26%) |
| Age | 46.27 (45.49–47.06) | 39.45 (38.53–40.4) d | 39.4 (38.32–40.51) d | 39.61 (37.84–41.47) d |
| Gender, % of men | 47.5 | 46.7 | 47.4 | 44.7 |
| BMI, kg/m2 | 27.92 (27.68–28.16) | 29.54 (29.12–29.96) d | 29.33 (28.85–29.81) d | 30.18 (29.29–31.1) d |
| CKD, % | 10.9 | 4.9 | 4.2 | 6.8 |
| DM, % | 17.8 | 17.6 | 16.4 | 21 |
| Dyslipidemia, % | 45.8 | 31.7 | 32.9 | 28.1 |
| Hypertension, % | 44.6 | 38.7 | 36.9 | 44.1 |
| Smokers, % | 44.1 | 43.2 | 40.4 | 51.5 |
| Albuminuria, % | 11.6 | 13.2 | 11.6 | 18 |
| ACR, mg/g | 9.65 (9.23–10.1) | 10.37 (9.67–11.13) | 9.63 (8.93–10.39) | 12.92 (10.99–15.2) b,# |
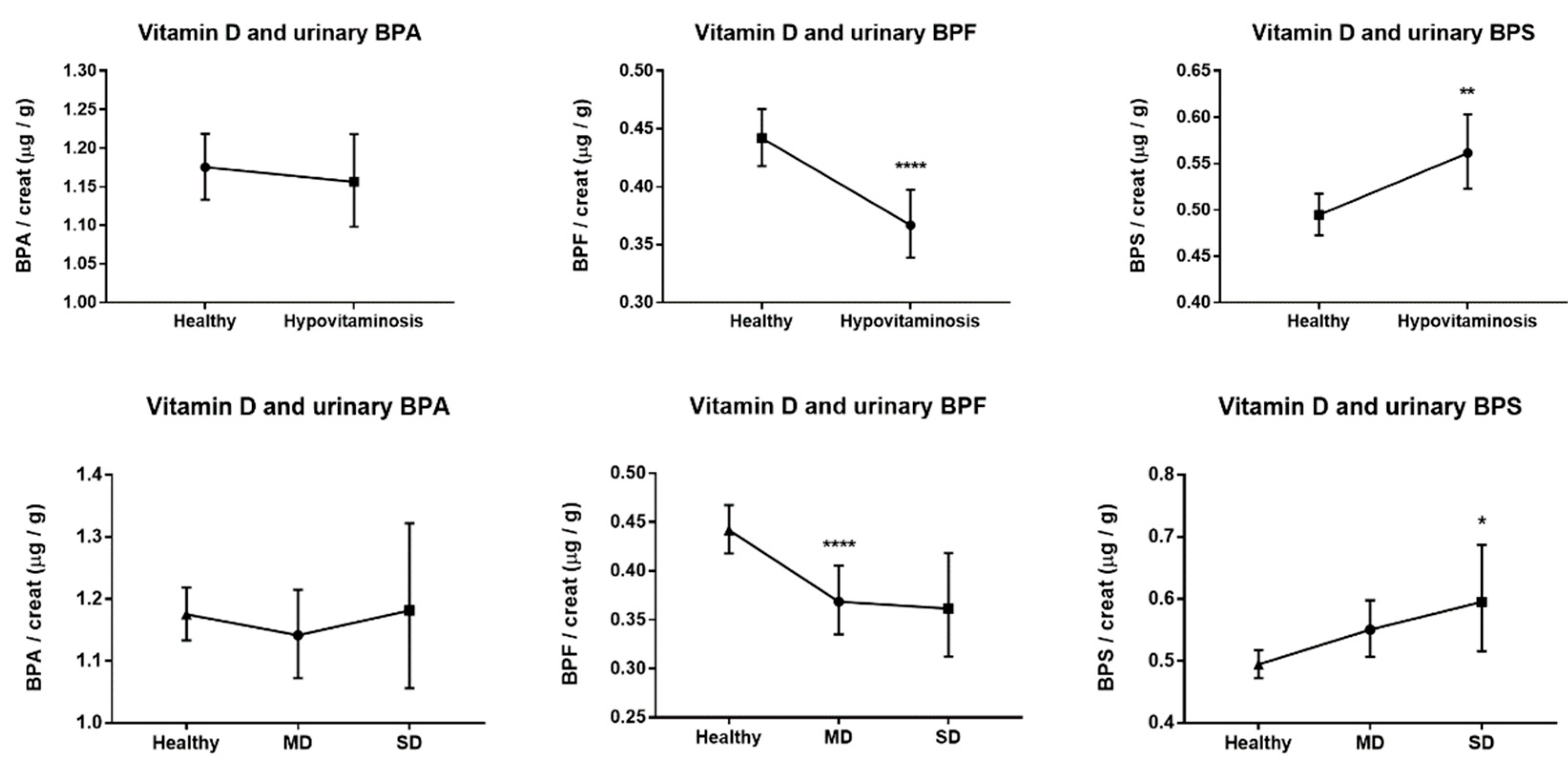
| BPA, µg/g creat. | 1.17 (1.13–1.22) | 1.16 (1.1–1.22) | 1.15 (1.08–1.22) | 1.18 (1.06–1.32) |
| BPF, µg/g creat. | 0.44 (0.42–0.47) | 0.37 (0.34–0.4) d | 0.37 (0.33–0.4) d | 0.36 (0.31–0.42) |
| BPS, µg/g creat. | 0.49 (0.47–0.52) | 0.56 (0.52–0.6) b | 0.55 (0.51–0.6) | 0.59 (0.52–0.69) a |
| Analysis | OR (95% CI) | p-Value | |
|---|---|---|---|
| BPA, µg/g creat. 1 | 1 | 0.98 (0.91–1.06) | 0.602 |
| 2 | 0.99 (0.91–1.07) | 0.747 | |
| 3 | 0.98 (0.91–1.07) | 0.699 | |
| BPF, µg/g creat. 1 | 1 | 0.91 (0.86–0.95) | 0.000 |
| 2 | 0.92 (0.87–0.97) | 0.001 | |
| 3 | 0.92 (0.87–0.97) | 0.001 | |
| BPS, µg/g creat. 1 | 1 | 1.09 (1.03–1.16) | 0.002 |
| 2 | 1.11 (1.05–1.18) | 0.001 | |
| 3 | 1.10 (1.04–1.17) | 0.002 |
| Analysis | Healthy | Moderate Deficiency | Severe Deficiency | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| BPA, µg/g creat. 1 | 1 | REF | - | 0.97 (0.89–1.06) | 0.498 | 1.01 (0.88–1.15) | 0.928 |
| 2 | REF | - | 0.98 (0.90–1.07) | 0.645 | 1.01 (0.88–1.16) | 0.895 | |
| 3 | REF | - | 0.98 (0.90–1.08) | 0.719 | 0.99 (0.86–1.13) | 0.839 | |
| BPF, µg/g creat. 1 | 1 | REF | - | 0.91 (0.86–0.96) | 0.001 | 0.90 (0.82–0.98) | 0.021 |
| 2 | REF | - | 0.92 (0.87–0.98) | 0.005 | 0.91 (0.83–0.99) | 0.039 | |
| 3 | REF | - | 0.92 (0.87–0.98) | 0.009 | 0.89 (0.81–0.98) | 0.015 | |
| BPS, µg/g creat. 1 | 1 | REF | - | 1.08 (1.01–1.15) | 0.020 | 1.14 (1.03–1.26) | 0.010 |
| 2 | REF | - | 1.10 (1.03–1.18) | 0.006 | 1.15 (1.04–1.28) | 0.006 | |
| 3 | REF | - | 1.09 (1.02–1.17) | 0.009 | 1.13 (1.02–1.26) | 0.019 | |
| BPA, µg/g Creat. 1 | BPF, µg/g Creat. 1 | BPS, µg/g Creat. 1 | ||||
|---|---|---|---|---|---|---|
| Dependent Variable | β | p-Value | β | p-Value | β | p-Value |
| Total (25(OH)D2 + 25(OH)D3), nmol/L | 0.000 | 0.978 | 0.060 | 0.000 | −0.051 | 0.000 |
| 25(OH)D2, nmol/L | 0.007 | 0.683 | 0.009 | 0.610 | 0.006 | 0.730 |
| 25(OH)D3, nmol/L | −0.004 | 0.809 | 0.057 | 0.001 | −0.055 | 0.001 |
| epi-25(OH)D3, nmol/L | 0.005 | 0.778 | 0.030 | 0.076 | −0.038 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Gómez-Toledano, R. Independent Associations Between Urinary Bisphenols and Vitamin D Deficiency: Findings from NHANES Study. Green Health 2025, 1, 10. https://doi.org/10.3390/greenhealth1020010
Moreno-Gómez-Toledano R. Independent Associations Between Urinary Bisphenols and Vitamin D Deficiency: Findings from NHANES Study. Green Health. 2025; 1(2):10. https://doi.org/10.3390/greenhealth1020010
Chicago/Turabian StyleMoreno-Gómez-Toledano, Rafael. 2025. "Independent Associations Between Urinary Bisphenols and Vitamin D Deficiency: Findings from NHANES Study" Green Health 1, no. 2: 10. https://doi.org/10.3390/greenhealth1020010
APA StyleMoreno-Gómez-Toledano, R. (2025). Independent Associations Between Urinary Bisphenols and Vitamin D Deficiency: Findings from NHANES Study. Green Health, 1(2), 10. https://doi.org/10.3390/greenhealth1020010






