Abstract
The field of climate modeling is undergoing a significant transformation, moving away from the traditional General Circulation Models (GCMs) and toward the use of sophisticated artificial intelligence (AI)-based prediction systems. Research has shown that AI has the potential to improve climate modeling’s regional accuracy and computing efficiency. Machine learning downscaling better captures local precipitation extremes than GCMs, while hybrid AI–physics models cut ensemble costs by reducing computational demand without sacrificing accuracy. Nevertheless, these investigations have frequently functioned in discrete settings and oversimplified situations without a thorough connection with basic physical concepts. This drawback emphasizes the necessity of a more comprehensive strategy that can handle the intricacies of climatic variability and guarantee reliable model validation. In order to assess the possibilities and challenges of hybrid models in comparison to conventional GCMs, highlighting that AI complements GCMs in regional downscaling and extremes, while GCMs provide stronger global consistency, this study synthesizes proven climate models, AI methodologies, and their accuracy in climate predictions and analyzes existing climate models to evaluate the potential and limitations of hybrid models compared to traditional GCMs. Integrated AI-driven models show notable improvements in predicting regional variations in climate and accelerating simulation processes, especially when dealing with the growing presence of extreme weather occurrences. However, it is important to have consistent datasets and open evaluation procedures in order to guarantee accuracy and deal with the difficulties that come with model benchmarking. This research highlights how crucial it is to maintain interdisciplinary cooperation in order to properly utilize what AI has to offer in climate modeling. This partnership is essential to creating more accurate and useful climate projections, which will eventually guide successful mitigation and adaptation plans for a changing global environment. In order to have a greater understanding of our climate’s future, we must keep pushing the limits of the existing modeling tools.
1. Introduction
Accurate climate modeling is essential for understanding, forecasting, and reducing the impacts of climate change. Reliable prediction systems are vital for adaptation and resilience planning as global climate threats intensify. Since the mid-20th century, GCMs have been central to simulating the Earth’s climate system by integrating atmospheric, oceanic, terrestrial, and cryospheric processes [1]. They enabled major breakthroughs, including confirming greenhouse gas–driven warming and testing alternative emission scenarios [2]. Yet, GCMs face enduring challenges: computational intensity, coarse spatial resolution, and limited representation of local-scale variability. Downscaling techniques emerged to bridge this gap, with statistical approaches increasingly refined through machine learning (ML), which captures nonlinear climate patterns [3]. AI methods such as deep learning, ensemble modeling, and neural emulation are further transforming forecasting, enabling real-time analysis, feature detection, and improved simulation of processes like convection and cloud dynamics [4]. These advances support faster, more accurate forecasts of extremes and have enhanced hazard mapping and attribution studies [5]. Nevertheless, significant research gaps persist in terms of data availability, model interpretability, and generalizability, particularly within data-scarce regions [4,5]. Few studies integrate physics-based and AI methods into unified frameworks. This work compares GCMs, ML, and hybrids, addressing their strengths, limits, ethical issues, key challenges, and future prospects.
2. Evolution of Climate Prediction
2.1. Evolution of General Circulation Models
GCMs which developed from the early work of Bjerknes and Richardson, simulate the climate of Earth [6]. In 1922, building on this foundation Richardson first attempted full hydrodynamic and thermodynamic equations for numerical weather prediction, while Bjerknes formalized the primitive equations that is the base for modern climate models [7]. GCMs form the physical core of modern climate science, simulating atmospheric and oceanic circulation through fundamental energy and mass balance equations. Early atmospheric models evolved into comprehensive Earth System Models (ESMs) by integrating oceans, land, sea ice, and biogeochemical cycles. Their major strength lies in providing a physics-based framework for attributing observed climate change and projecting future scenarios in global assessments like the IPCC reports [6]. GCMs effectively capture large-scale circulation and global temperature trends but face persistent limitations, chiefly coarse spatial resolution and uncertainty in parameterizing sub-grid processes such as clouds and aerosols. Despite these drawbacks, they remain indispensable as the physical foundation for next-generation models [8].
2.2. Machine Learning & Deep Learning
ML refines traditional statistical methods by capturing nonlinear climate relationships with data-driven models [3]. Early applications (1990s–2000s) focused on downscaling, bias correction, and post-processing using regression, decision trees, and simple neural networks. With the growth of deep learning in the 2010s [9,10]. Both ML and DL models offer speed and strong pattern recognition but face issues of interpretability, data dependence, and physical inconsistency. While unsuitable as standalone climate predictors, they are increasingly vital in hybrid frameworks shown in Figure 1 [11].
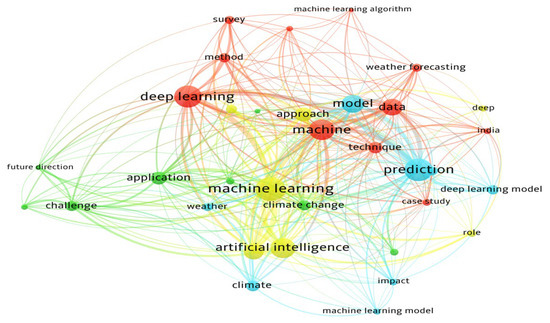
Figure 1.
Co-occurrence network, showing four clusters: (i) deep learning, (ii) forecasting and prediction, (iii) AI in climate applications, and (iv) challenges and future directions, highlighting the shift toward AI-driven hybrid modeling.
2.3. AI-Enhanced Modeling
AI–physics hybrid models show strong promise for advancing climate and hydroclimatic forecasting by reducing biases [12,13,14]. AI in climate modeling has advanced from simple bias correction to hybrid approaches combining physics with data-driven learning. Deep learning now resolves subgrid processes. Still, challenges in data, generalization, and transparency remain [14,15]. Hybrids improve regional accuracy and computational efficiency compared to traditional GCMs and ESMs. However, their reliability still depends on data quality and interpretability, which limits full autonomy. Even so, they are expected to play a major role in future projections [16].
3. Predictive Analytics
3.1. Physics-Based Models
Physics-based models remain the cornerstone of predictive climate analytics. GCMs and Earth System Models simulate large-scale climate [17,18]. Primitive Equations of Atmospheric Motion (core of GCMs/ESMs) the equations are
Here, Momentum: v = wind velocity, f = Coriolis effect, p = pressure, ρ = density, F = external forces. In Continuity: ρ = air density, v = velocity, ensures mass conservation. Energy: T = temperature, Q = heat input, cp = specific heat [19].
3.2. ML-Based Emulation & Surrogate Modeling
ML is increasingly used as a surrogate for traditional models, delivering comparable performance to physics-based systems at much lower computational cost [16,20]. Techniques such as feedforward neural networks and support vector regression (SVR) support predictive and classification tasks in climate and hydrology [21].
Figure 2 shows Workflow of an ML climate model. Raw observations are encoded into latent features by the trained encoder. The model core processes these features, learning nonlinear spatial–temporal dependencies. A trained decoder reconstructs outputs such as temperature, precipitation, or event probabilities. In surrogate modeling, neural networks approximate the outputs of computationally expensive physical parameterizations. A typical feedforward neural network maps climate inputs to outputs ŷ through successive linear transformations and nonlinear activations:
Here, x = climate inputs, ŷ = predicted output, W = weights, b = biases, σ = activation, fθ = neural network [22].
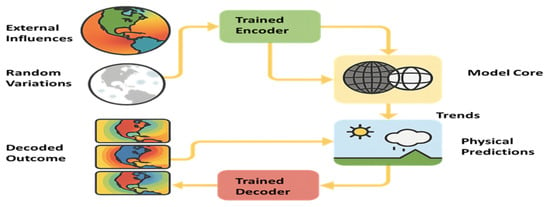
Figure 2.
ML driven Climate Modeling Process.
3.3. AI-Enhanced Hybrid Predictive Analytics
While purely physics-based models provide global consistency and ML models excel at capturing nonlinear regional dynamics, both approaches face limitations when used independently. Work process of AI models is shown in Figure 3 [12,13,14].
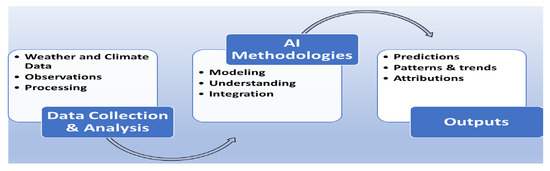
Figure 3.
AI-Driven Climate Modeling Flowchart.
3.3.1. Physics-Informed ML
The incorporation of physics-informed ML has addressed some of the challenges associated with purely data-driven models. While modern AI methods operate as “black boxes” that identify patterns without relying on physical principles, this creates challenges in maintaining interpretability and physical consistency [12,13].
PINN Residual Loss Function
Here, = total loss, data = data error, λ = weight, [uθ] = physical operator applied to ML output, f = forcing [23].
3.3.2. Hybrid Modeling Approaches
Hybrid models and EANN improve prediction accuracy, especially for extreme events and data-scarce setting [21,24]. Their wider adoption requires progress in data assimilation, model optimization, and physical consistency Systems like Adaptive Neuro-Fuzzy Inference, which combine neural learning with fuzzy logic, have shown strong performance in soil temperature forecasting and drought prediction [20].
A common formulation of hybrid modeling is the Additive Hybrid Model, expressed as
where Yphysics(t) is the prediction from a traditional GCM or ESM, and fθ(X(t)) is the ML correction term applied to input variables X(t). This structure preserves the physical model’s global consistency while allowing AI to capture unresolved nonlinear processes [11].
4. Comparative Performance
Comparative studies show each predictive approach has distinct strengths and limits. Physics-based models like GCMs capture global variability but lack resolution for local applications [17]. Statistical and dynamical techniques partly address this gap, though hydrological processes remain uncertain [25]. ML and AI models better capture nonlinear dynamics and local extremes at lower computational cost [16,20]. Hybrid frameworks combine global consistency with stronger regional performance [12,13,14]. Table 1 summarizes these comparative strengths and limitations as documented in recent Studies.

Table 1.
Strengths and Limitations of Different Climate Models.
5. Applications in Predictive Climate Science
5.1. Downscaling
Downscaling remains a critical method for translating global climate information into actionable regional insights. Statistical downscaling, refined through ML, has achieved higher precision by capturing nonlinear correlations in climate patterns [3]. RCMs, with fine-scale resolutions of 1–5 km, provide enhanced depictions of local processes such as land sea contrasts and heavy precipitation [18,28]. Convection-permitting models further improve the simulation of extreme weather but require vast computational resources [26]. Despite these advances, downscaling is still challenged by parameterization uncertainties and the limited availability of high-quality datasets. This highlights the growing importance of AI-driven approaches that can enhance local-scale reliability, especially in regions with sparse observations [24].
5.2. Forecasting
Forecasting capabilities have been significantly transformed by the introduction of AI and deep learning. Neural networks and ensemble techniques now enable real-time data processing and pattern recognition, enhancing prediction accuracy for key variables such as temperature, precipitation, and atmospheric circulation [27]. Deep learning models, including convolutional neural networks and long short-term memory networks, have consistently outperformed traditional approaches in forecasting major climate phenomena such as the El Niño–Southern Oscillation, cyclone intensity, and precipitation nowcasting [29,30]. AI-enhanced forecasting systems also streamline data analysis and automate bias correction, creating more efficient workflows that support disaster preparedness and climate risk assessment. However, the quality and size of available training datasets remain limiting factors, particularly when predicting rare or unprecedented events [11,20].
5.3. Extremes
Predicting extreme events is difficult due to limited resolution and reliance on large ensembles [26]. AI and hybrid models improve accuracy in data-scarce settings, with methods like EANNs, MARS, and deep learning aiding nowcasting of floods, droughts, and cyclones [24,31]. These models face difficulties in maintaining physical consistency and stability, and they carry risks of overfitting when datasets are noisy [15]. Addressing these challenges will require continued development of hybrid modeling strategies.
6. Challenges
6.1. Computational Demand & Scalability
Climate modeling requires immense computing power, especially for kilometer-scale simulations that can take months to complete. Exascale efforts face bottlenecks in memory, I/O, and energy use [32]. Vast data outputs also strain storage, prompting interest in ML-based compression and reconstruction [33]. Emerging solutions include GPUs, hybrid CPU–GPU systems, quantum computing, and ML-accelerated parameterizations [34].
6.2. Uncertainty, Interpretability and Data Quality
Uncertainty arises from multiple sources, including model structure, internal variability, and external forcing assumptions [17,26]. Large ensembles help quantify this, but AI-driven models often operate as “black boxes”, limiting physical interpretability [12,13,14]. Their reliability also depends heavily on data quality. Sparse or noisy datasets reduce accuracy, especially in extremes, while many ML methods require process-level data that are often unavailable [21,24]. Many supervised ML approaches require process-level data, which are often unavailable, forcing reliance on incomplete observations [11,31,35].
6.3. Ethical Concerns
Beyond technical limitations, ethical considerations are emerging as an important dimension of AI-driven climate modeling. The integration of AI into climate modeling raises ethical issues around transparency, equity, and responsibility AI-based models also risk reinforcing global inequities, as they perform better in data-rich regions while vulnerable areas often receive less accurate forecasts. Additional concerns include bias amplification from historical data, misuse of forecasts for political or economic purposes, and unequal access to AI expertise [36]
7. Future Prospects of Climate Modeling
Tackling growing climate risks demands large-scale cooperation among academia, industry, and government [20]. AI will be essential for repeated simulations to assess uncertainty and calibrate unresolved processes. Kilometer-scale simulations remain unfeasible, focusing on 10–50 km resolutions could enhance forecasts [11]. The climate research can better comprehend important processes with the help of CMIP6 [37,38]. Looking ahead, hybrid AI–physics models represent the future of climate modeling, combining accuracy with interpretability. Future research directions emphasize the integration of AI for uncertainty quantification and the emulation of high-resolution processes that exceed current computational capabilities. The application of ensemble learning and multi-model frameworks offers a means to enhance the reliability of extreme event predictions [16]. Despite gains in resolution and ensemble forecasting, unresolved small-scale processes will persist [4,39].
8. Conclusions
ML and AI are developing quickly and have a wide range of applications in climate and environmental prediction. Deep learning now significantly outperforms classical statistical models, especially when larger or more diverse datasets are available [35]. Air temperature and solar radiation are crucial, but other elements, such as precipitation, can occasionally be left out without compromising accuracy [33]. Weather and climate forecasting is changing due to AI-driven modeling [4]. Nevertheless, there are still difficulties in making the switch to these next-generation models: problems with data quality, interpretability, and uncertainty still exist [37]. Ethical concerns around transparency, accountability, and equity are also pressing, as AI often performs best in data-rich regions, risking global disparities [36,40]. Hybrid approaches that combine physics-based and AI methods now improve accuracy and interpretability, supporting more reliable forecasts [4]. Future progress will depend on high-resolution observations, global initiatives such as CMIP6 and SMILEs [27,38,41], and advances in explainable and physics-informed AI [10,11]. Hybrid AI–physics frameworks offer a balanced approach for climate modeling, maintaining GCMs’ physical consistency while exploiting AI’s nonlinear learning and efficiency [12,13,14]. These models improve regional downscaling and extreme event forecasting, reducing computational costs without compromising accuracy, though their potential depends on standardized datasets [20,21,37]. AI-driven and hybrid approaches mark a critical shift in climate science. Advances in data assimilation, ethical AI governance, and interdisciplinary integration will enable more reliable projections [11,16]. By coupling innovation with ethical responsibility and interdisciplinary collaboration, climate science can deliver more transparent, actionable, and equitable projections for adaptation and mitigation in a changing world [4,37].
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bordoni, S.; Kang, S.M.; Shaw, T.A.; Simpson, I.R.; Zanna, L. The Futures of Climate Modeling. Npj Clim. Atmospheric Sci. 2025, 8, 99. [Google Scholar] [CrossRef]
- Shiru, M.S.; Chung, E.-S. Performance Evaluation of CMIP6 Global Climate Models for Selecting Models for Climate Projection over Nigeria. Theor. Appl. Climatol. 2021, 146, 599–615. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T. Advancements in Downscaling Global Climate Model Temperature Data in Southeast Asia: A Machine Learning Approach. Forecasting 2023, 6, 1–17. [Google Scholar] [CrossRef]
- Camps-Valls, G.; Fernández-Torres, M.-Á.; Cohrs, K.-H.; Höhl, A.; Castelletti, A.; Pacal, A.; Robin, C.; Martinuzzi, F.; Papoutsis, I.; Prapas, I.; et al. Artificial Intelligence for Modeling and Understanding Extreme Weather and Climate Events. Nat. Commun. 2025, 16, 1919. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T. Advancements and Challenges of Artificial Intelligence in Climate Modeling for Sustainable Urban Planning. Front. Artif. Intell. 2025, 8, 1517986. [Google Scholar] [CrossRef]
- Edwards, P.N. History of Climate Modeling. WIREs Clim. Change 2011, 2, 128–139. [Google Scholar] [CrossRef]
- Bjerknes, V. The Problem of Weather Prediction, Considered from the Viewpoints of Mechanics and Physics. Meteorol. Z. 2009, 18, 663–667. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) Experimental Design and Organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- De Burgh-Day, C.O.; Leeuwenburg, T. Machine Learning for Numerical Weather and Climate Modelling: A Review. Geosci. Model Dev. 2023, 16, 6433–6477. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Xue, W.; Yang, G.; Zhang, G.J. Stable Climate Simulations Using a Realistic General Circulation Model with Neural Network Parameterizations for Atmospheric Moist Physics and Radiation Processes. Geosci. Model Dev. 2022, 15, 3923–3940. [Google Scholar] [CrossRef]
- Schneider, T.; Lan, S.; Stuart, A.; Teixeira, J. Earth System Modeling 2.0: A Blueprint for Models That Learn From Observations and Targeted High—Resolution Simulations. Geophys. Res. Lett. 2017, 44, 12396–12417. [Google Scholar] [CrossRef]
- Soldatenko, S.A. Artificial Intelligence and Its Application in Numerical Weather Prediction. Russ. Meteorol. Hydrol. 2024, 49, 283–298. [Google Scholar] [CrossRef]
- Slater, L.J.; Arnal, L.; Boucher, M.-A.; Chang, A.Y.-Y.; Moulds, S.; Murphy, C.; Nearing, G.; Shalev, G.; Shen, C.; Speight, L.; et al. Hybrid Forecasting: Blending Climate Predictions with AI Models. Hydrol. Earth Syst. Sci. 2023, 27, 1865–1889. [Google Scholar] [CrossRef]
- Materia, S.; García, L.P.; Van Straaten, C.; Sungmin, O.; Mamalakis, A.; Cavicchia, L.; Coumou, D.; De Luca, P.; Kretschmer, M.; Donat, M. Artificial Intelligence for Climate Prediction of Extremes: State of the Art, Challenges, and Future Perspectives. WIREs Clim. Change 2024, 15, e914. [Google Scholar] [CrossRef]
- Huntingford, C.; Jeffers, E.S.; Bonsall, M.B.; Christensen, H.M.; Lees, T.; Yang, H. Machine Learning and Artificial Intelligence to Aid Climate Change Research and Preparedness. Environ. Res. Lett. 2019, 14, 124007. [Google Scholar] [CrossRef]
- Schneider, T.; Behera, S.; Boccaletti, G.; Deser, C.; Emanuel, K.; Ferrari, R.; Leung, L.R.; Lin, N.; Müller, T.; Navarra, A.; et al. Harnessing AI and Computing to Advance Climate Modelling and Prediction. Nat. Clim. Change 2023, 13, 887–889. [Google Scholar] [CrossRef]
- Raju, K.S.; Kumar, D.N. Review of Approaches for Selection and Ensembling of GCMs. J. Water Clim. Change 2020, 11, 577–599. [Google Scholar] [CrossRef]
- Rummukainen, M. State–of–the–art with Regional Climate Models. WIREs Clim. Change 2010, 1, 82–96. [Google Scholar] [CrossRef]
- Donatelli, D.; Juhász, N. The Primitive Equations of the Polluted Atmosphere as a Weak and Strong Limit of the 3D Navier-Stokes Equations in Downwind-Matching Coordinates. Discrete Contin. Dyn. Syst. 2022, 42, 2859. [Google Scholar] [CrossRef]
- Govett, M.; Bah, B.; Bauer, P.; Berod, D.; Bouchet, V.; Corti, S.; Davis, C.; Duan, Y.; Graham, T.; Honda, Y.; et al. Exascale Computing and Data Handling: Challenges and Opportunities for Weather and Climate Prediction. Bull. Am. Meteorol. Soc. 2024, 105, E2385–E2404. [Google Scholar] [CrossRef]
- Azimi, S.M.E.; Sadatinejad, S.J.; Malekian, A.; Jahangir, M.H. Application of Artificial Intelligence Hybrid Models for Meteorological Drought Prediction. Nat. Hazards 2022, 116, 2565–2589. [Google Scholar] [CrossRef]
- Rasp, S.; Pritchard, M.S.; Gentine, P. Deep Learning to Represent Subgrid Processes in Climate Models. Proc. Natl. Acad. Sci. USA 2018, 115, 9684–9689. [Google Scholar] [CrossRef]
- Raissi, M.; Perdikaris, P.; Karniadakis, G.E. Physics-Informed Neural Networks: A Deep Learning Framework for Solving Forward and Inverse Problems Involving Nonlinear Partial Differential Equations. J. Comput. Phys. 2019, 378, 686–707. [Google Scholar] [CrossRef]
- Reddy, B.S.N.; Pramada, S.K.; Roshni, T. Monthly Surface Runoff Prediction Using Artificial Intelligence: A Study from a Tropical Climate River Basin. J. Earth Syst. Sci. 2021, 130, 35. [Google Scholar] [CrossRef]
- Chokkavarapu, N.; Mandla, V.R. Comparative Study of GCMs, RCMs, Downscaling and Hydrological Models: A Review toward Future Climate Change Impact Estimation. SN Appl. Sci. 2019, 1, 1698. [Google Scholar] [CrossRef]
- Maher, N.; Milinski, S.; Ludwig, R. Large Ensemble Climate Model Simulations: Introduction, Overview, and Future Prospects for Utilising Multiple Types of Large Ensemble. Earth Syst. Dyn. 2021, 12, 401–418. [Google Scholar] [CrossRef]
- Citakoglu, H. Comparison of Artificial Intelligence Techniques for Prediction of Soil Temperatures in Turkey. Theor. Appl. Climatol. 2017, 130, 545–556. [Google Scholar] [CrossRef]
- Rummukainen, M. Added Value in Regional Climate Modeling. WIREs Clim. Change 2016, 7, 145–159. [Google Scholar] [CrossRef]
- McGovern, A.; Elmore, K.L.; Gagne, D.J.; Haupt, S.E.; Karstens, C.D.; Lagerquist, R.; Smith, T.; Williams, J.K. Using Artificial Intelligence to Improve Real-Time Decision-Making for High-Impact Weather. Bull. Am. Meteorol. Soc. 2017, 98, 2073–2090. [Google Scholar] [CrossRef]
- Guo, Q.; He, Z.; Wang, Z. Monthly Climate Prediction Using Deep Convolutional Neural Network and Long Short-Term Memory. Sci. Rep. 2024, 14, 17748. [Google Scholar] [CrossRef]
- Yang, T.; Asanjan, A.A.; Welles, E.; Gao, X.; Sorooshian, S.; Liu, X. Developing Reservoir Monthly Inflow Forecasts Using Artificial Intelligence and Climate Phenomenon Information. Water Resour. Res. 2017, 53, 2786–2812. [Google Scholar] [CrossRef]
- Adamidis, P.; Pfister, E.; Bockelmann, H.; Zobel, D.; Beismann, J.-O.; Jacob, M. The Real Challenges for Climate and Weather Modelling on Its Way to Sustained Exascale Performance: A Case Study Using ICON (v2.6.6). Geosci. Model Dev. 2025, 18, 905–919. [Google Scholar] [CrossRef]
- Imanian, H.; Hiedra Cobo, J.; Payeur, P.; Shirkhani, H.; Mohammadian, A. A Comprehensive Study of Artificial Intelligence Applications for Soil Temperature Prediction in Ordinary Climate Conditions and Extremely Hot Events. Sustainability 2022, 14, 8065. [Google Scholar] [CrossRef]
- Bull, J.M.; Coughtrie, A.; Deeptimahanti, D.; Hedley, M.; Laoide-Kemp, C.; Maynard, C.; Shepherd, H.; Van De Bund, S.; Weiland, M.; Went, B. Performance and Scaling of the LFRic Weather and Climate Model on Different Generations of HPE Cray EX Supercomputers. In Proceedings of the Cray User Group; ACM: Perth, Australia, 2024; pp. 1–11. [Google Scholar]
- Abebe, W.T.; Endalie, D. Artificial Intelligence Models for Prediction of Monthly Rainfall without Climatic Data for Meteorological Stations in Ethiopia. J. Big Data 2023, 10, 2. [Google Scholar] [CrossRef]
- Nordgren, A. Artificial Intelligence and Climate Change: Ethical Issues. J. Inf. Commun. Ethics Soc. 2023, 21, 1–15. [Google Scholar] [CrossRef]
- Eyring, V.; Cox, P.M.; Flato, G.M.; Gleckler, P.J.; Abramowitz, G.; Caldwell, P.; Collins, W.D.; Gier, B.K.; Hall, A.D.; Hoffman, F.M.; et al. Taking Climate Model Evaluation to the next Level. Nat. Clim. Change 2019, 9, 102–110. [Google Scholar] [CrossRef]
- Maraun, D. Bias Correcting Climate Change Simulations—A Critical Review. Curr. Clim. Change Rep. 2016, 2, 211–220. [Google Scholar] [CrossRef]
- Alizadeh, O. Advances and Challenges in Climate Modeling. Clim. Change 2022, 170, 18. [Google Scholar] [CrossRef]
- Joshi, M.N.; Dixit, A.K.; Saxena, S.; Memoria, M.; Choudhury, T.; Sar, A. A Study of the Application of AI & ML to Climate Variation, with Particular Attention to Legal & Ethical Concerns. EAI Endorsed Trans. Internet Things 2024, 10, 1–11. [Google Scholar] [CrossRef]
- Giorgi, F. Thirty Years of Regional Climate Modeling: Where Are We and Where Are We Going Next? J. Geophys. Res. Atmospheres 2019, 124, 5696–5723. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).