Ecological Assessment of Riparian Vegetation Along the Banks of the River Panjkora, Hindukush Range
Abstract
1. Introduction
2. Materials and Methods
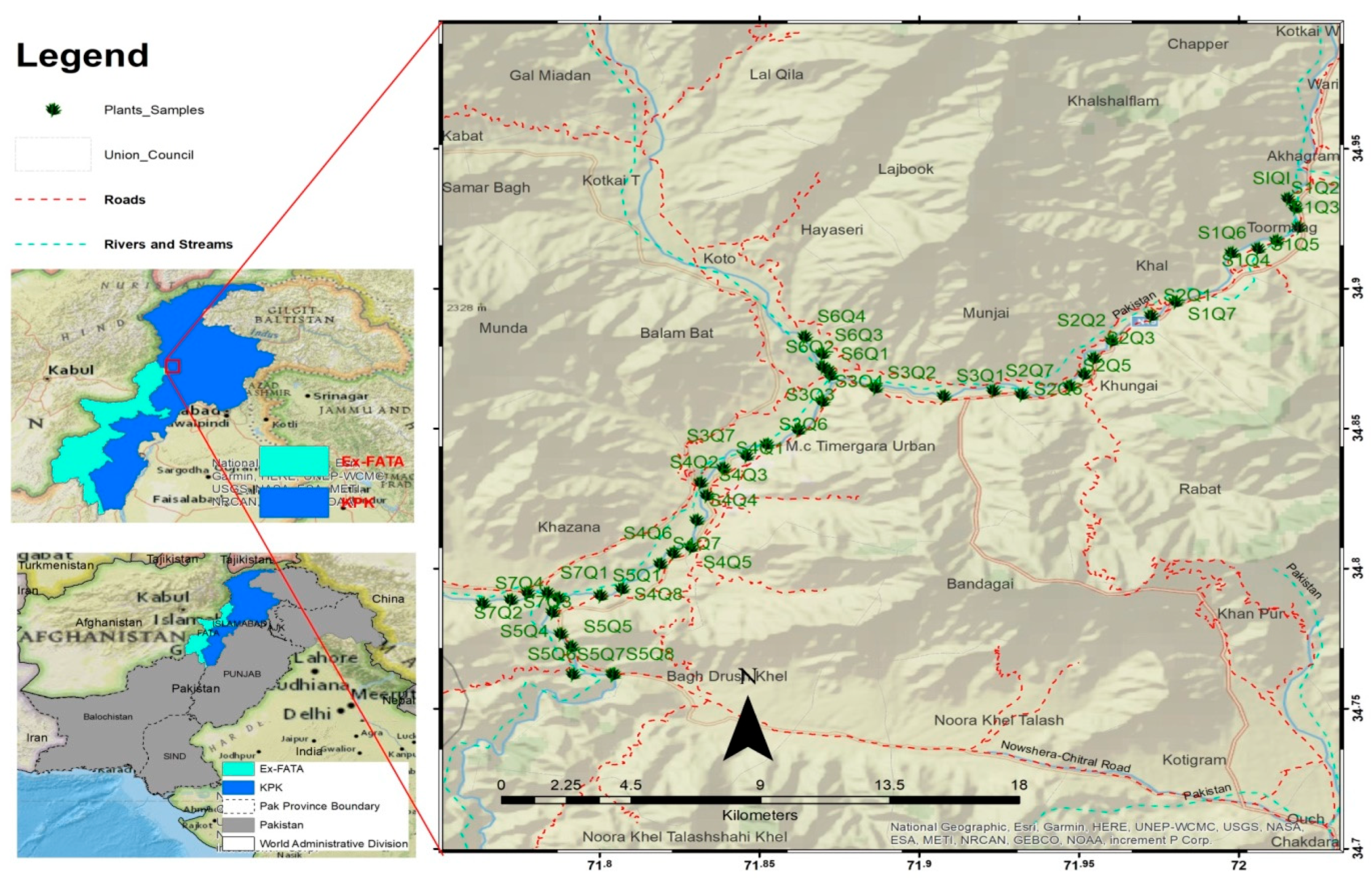
2.1. Study Area
2.2. Collection of Data
2.3. Soil Collection
2.4. Preparation of Soil Samples for Screening of the Atomic Absorption Spectrometry
2.5. Data Analysis
3. Results
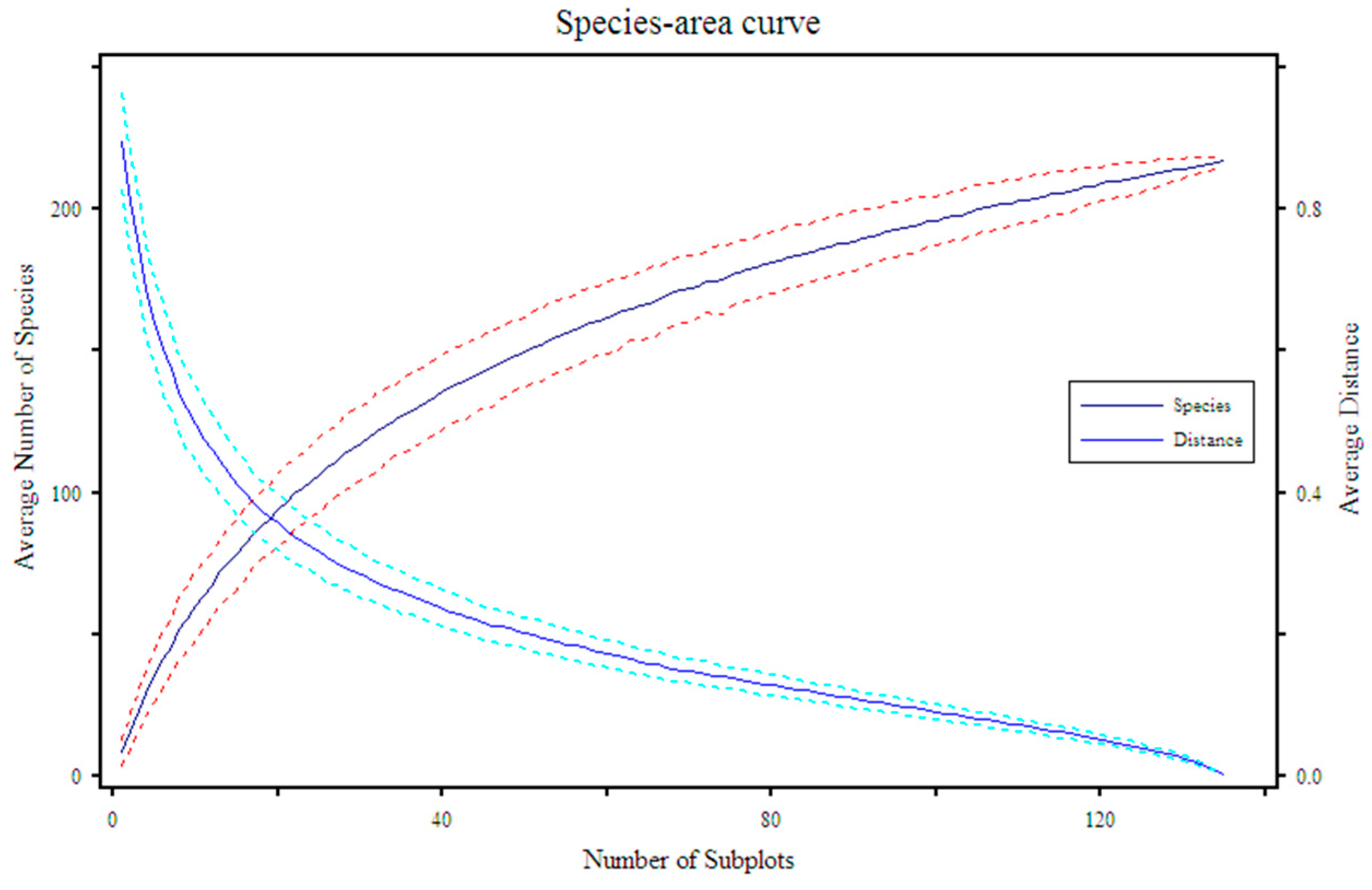
3.1. Species Area Curve
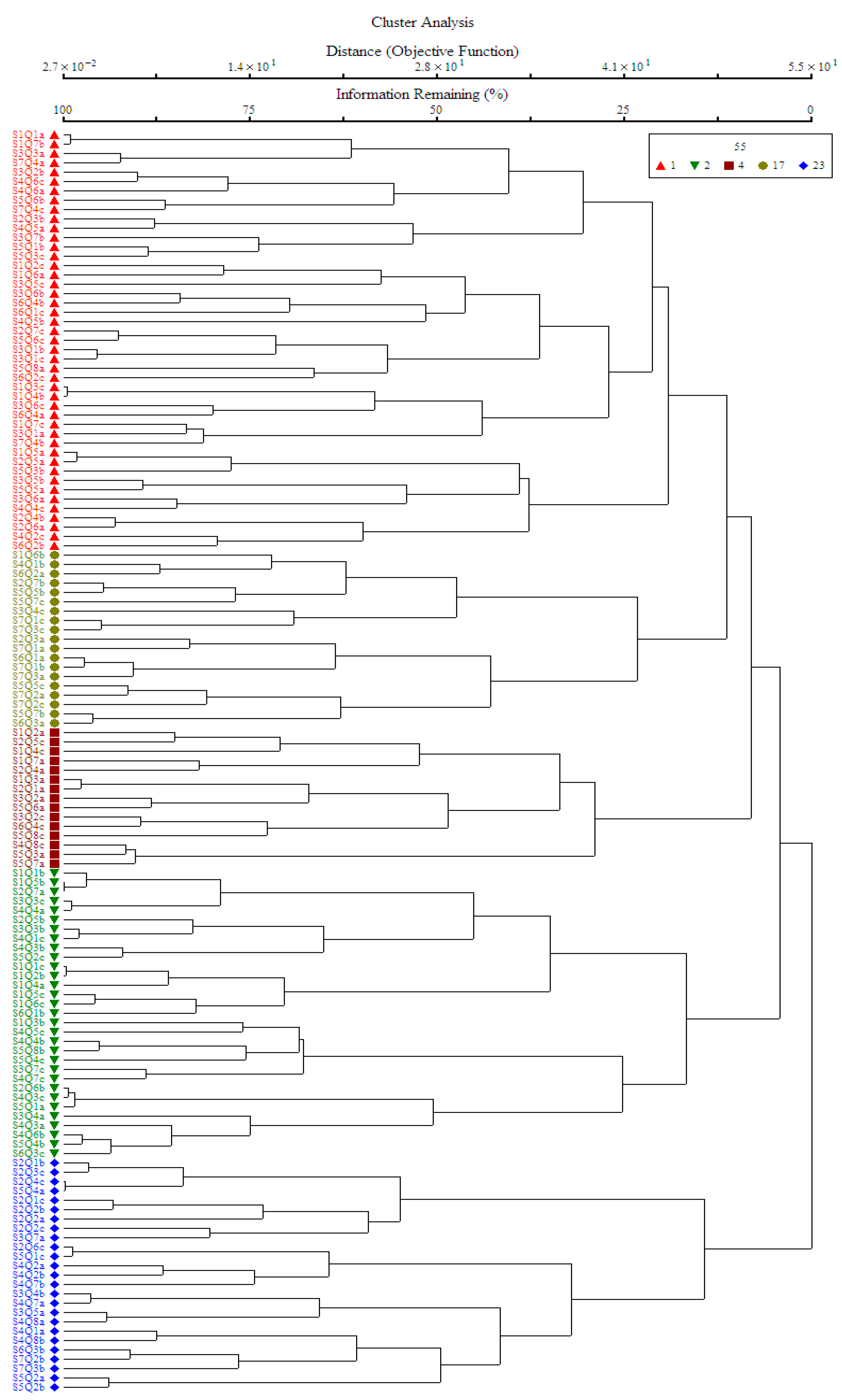
3.2. Cluster Analysis
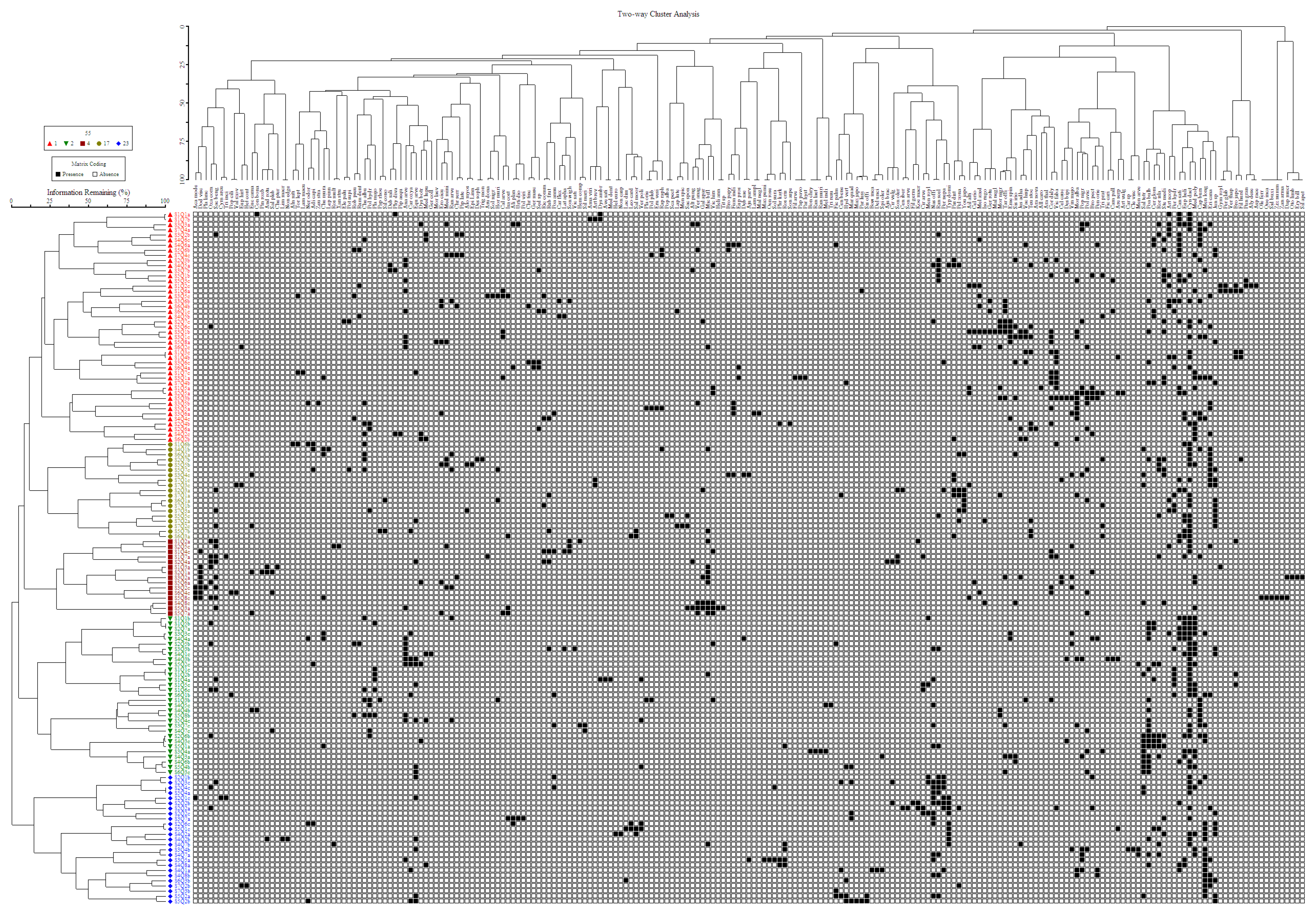
3.3. Two-Way Cluster Analysis
3.4. Classification of Plant Communities
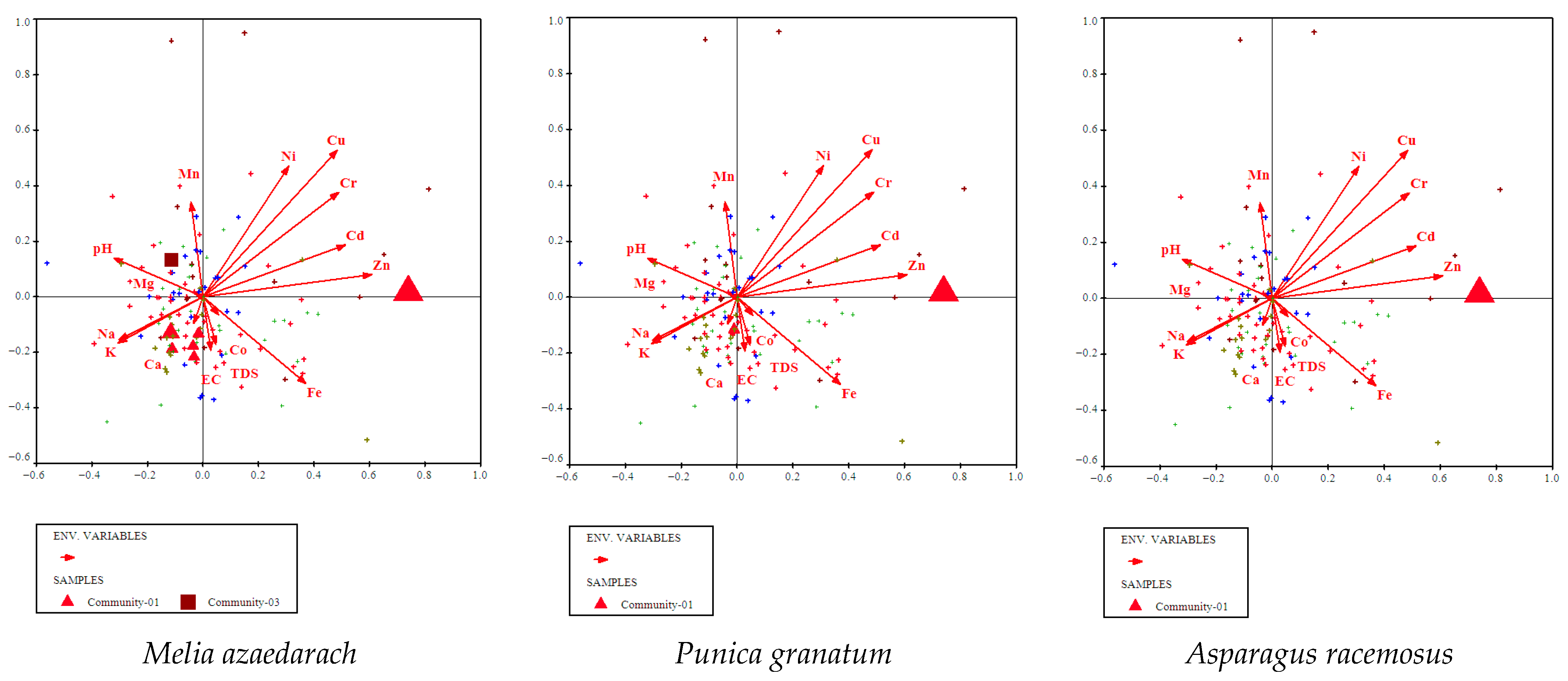
3.4.1. Melia azedarach, Punica granatum, and Asparagus racemosus Community
3.4.2. Populus alba, Debregeasia saeneb, and Youngia japonica, Community
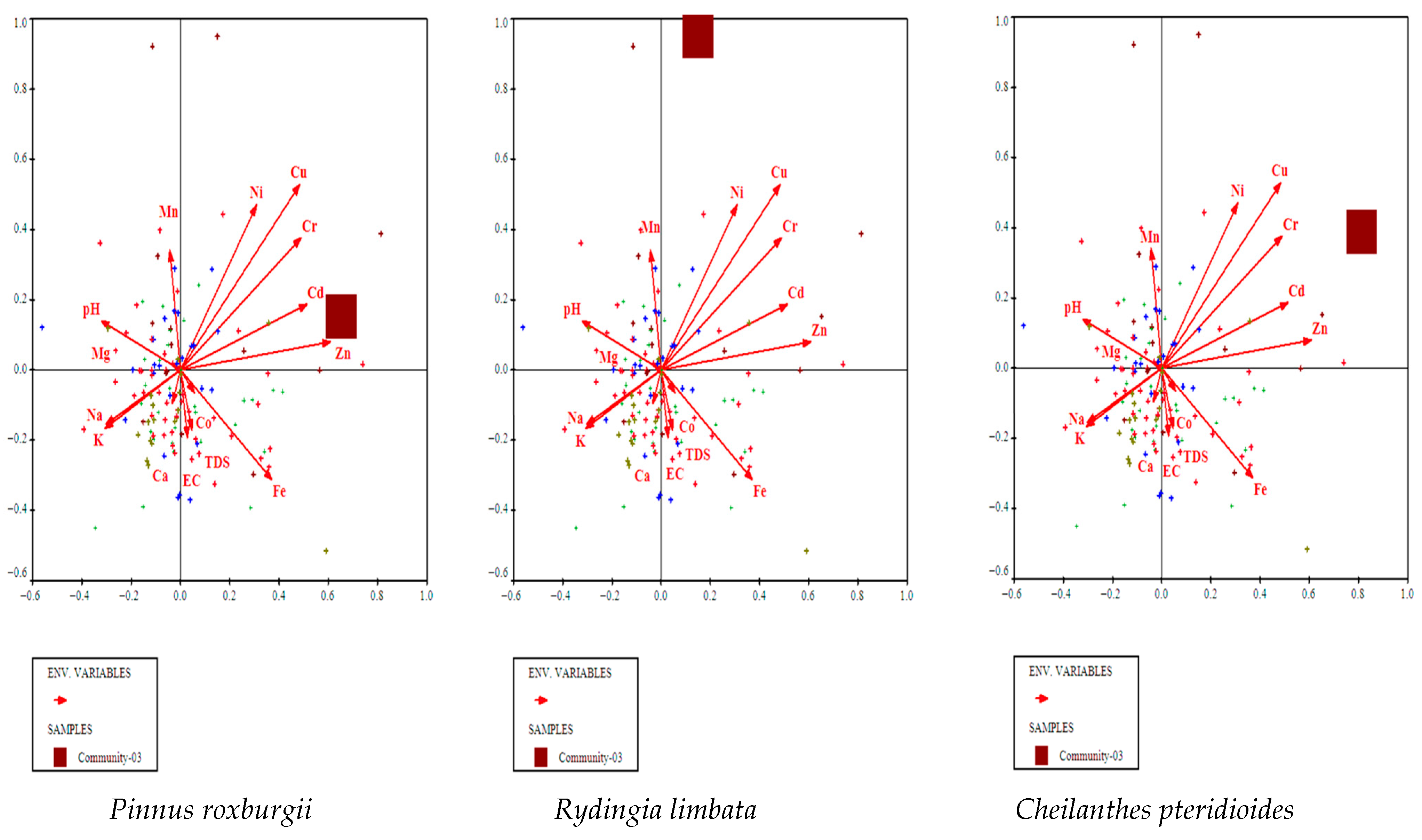
3.4.3. Pinus roxburghii, Rydingia limbata, and Cheilanthes pteridioides Community
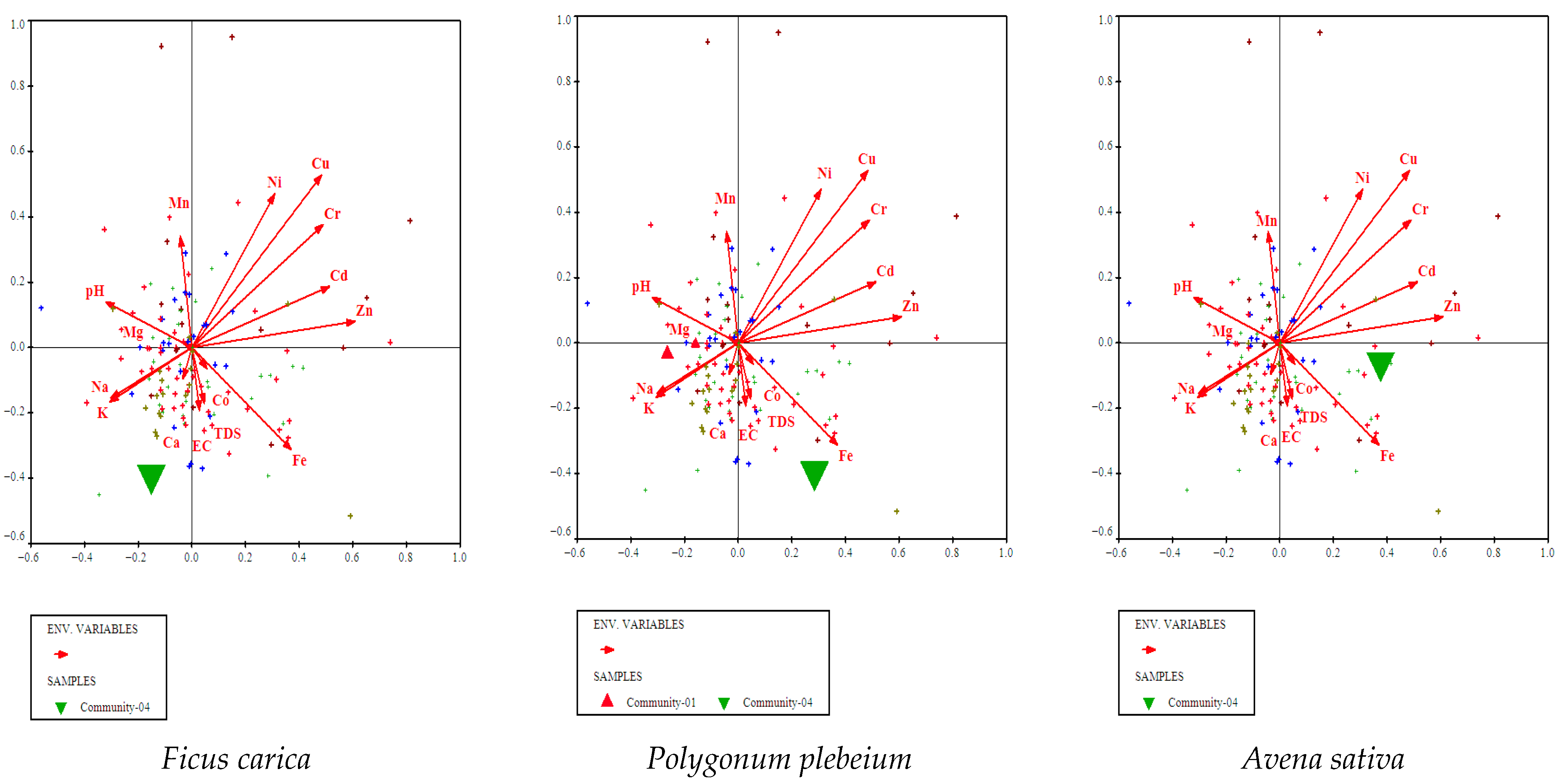
3.4.4. Ficus carica, Polygonum plebeium, and Avena sativa Community
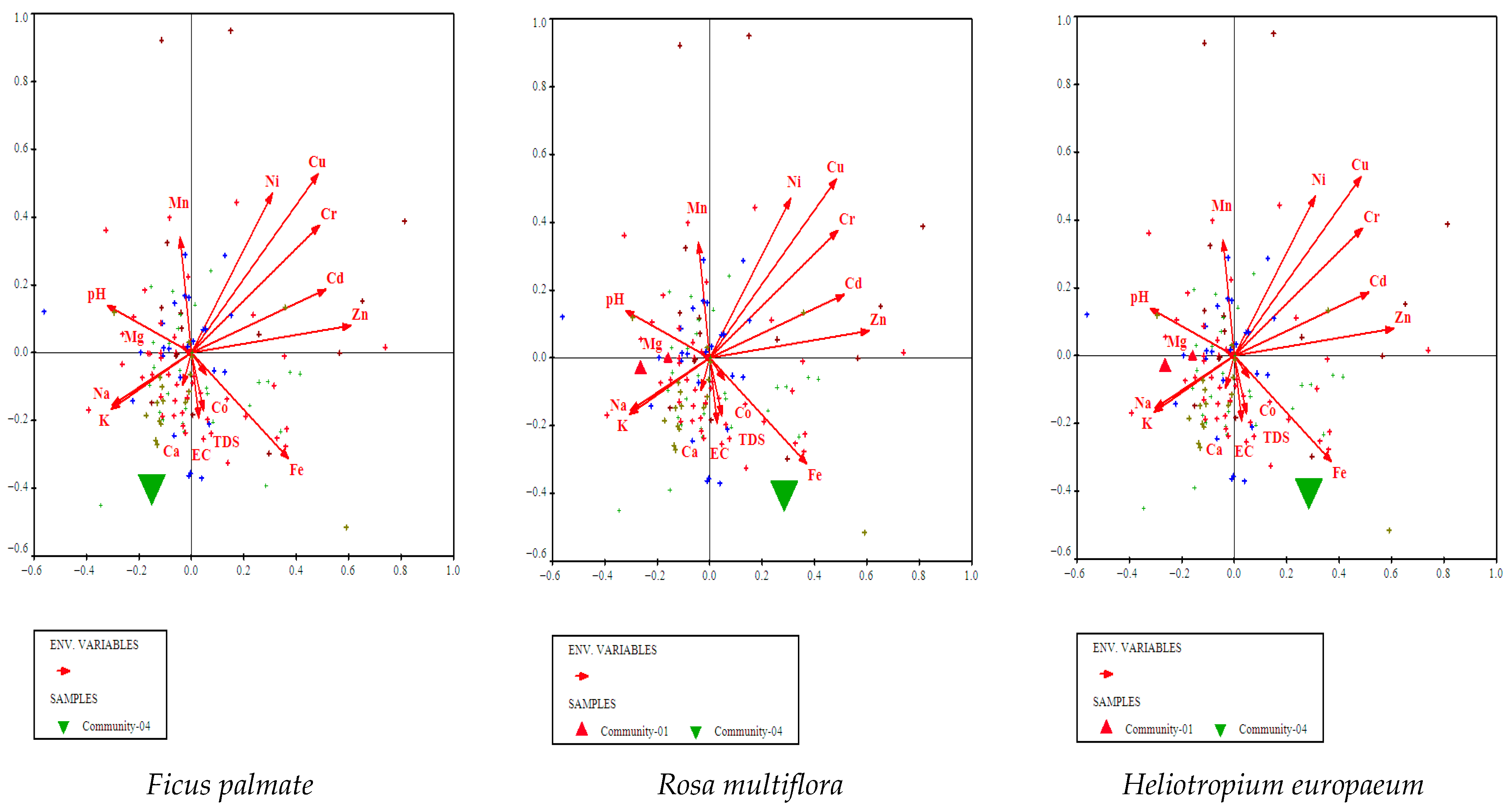
3.4.5. Ficus palmata, Rosa multiflora, and Heliotropium europaeum Community
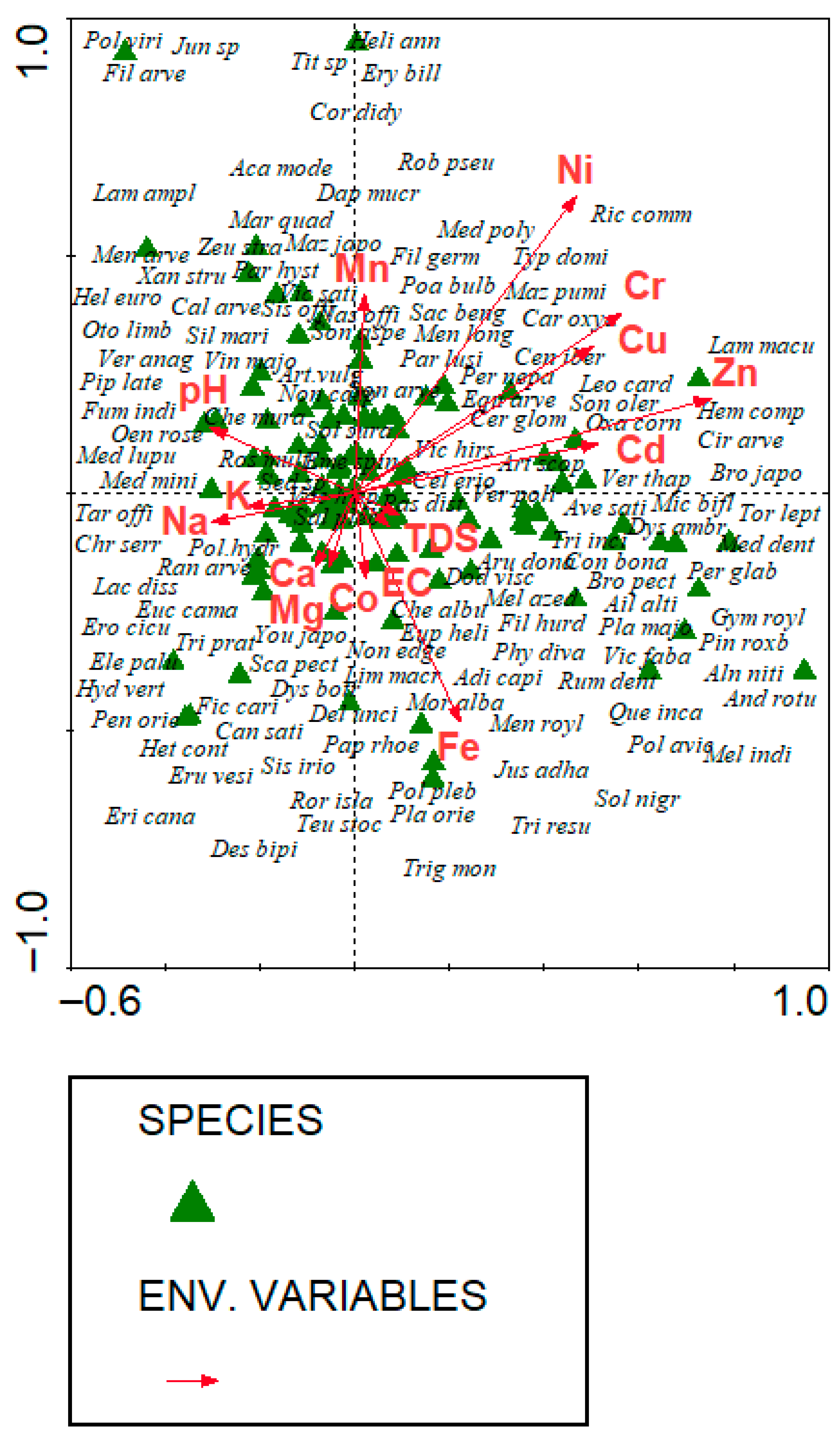
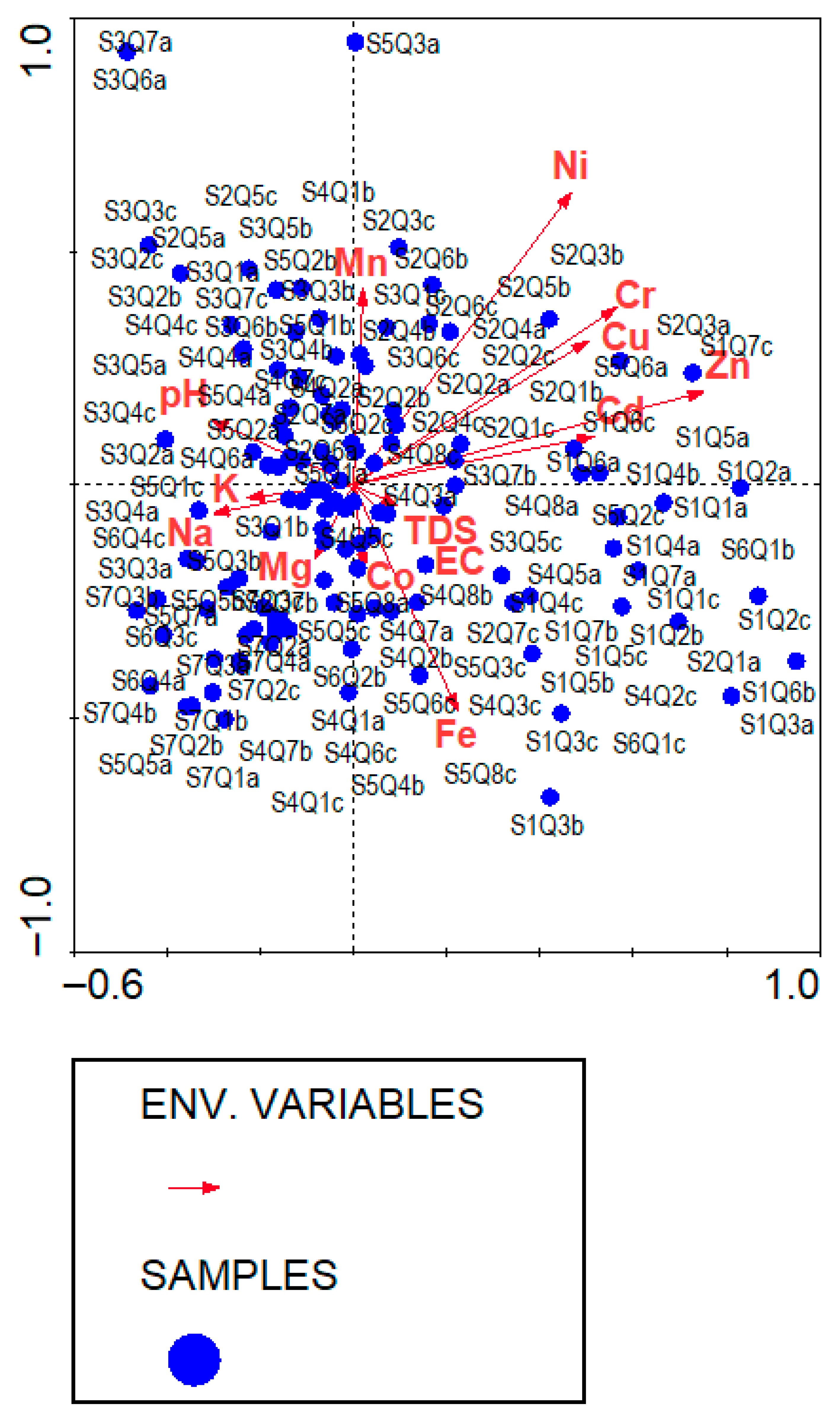
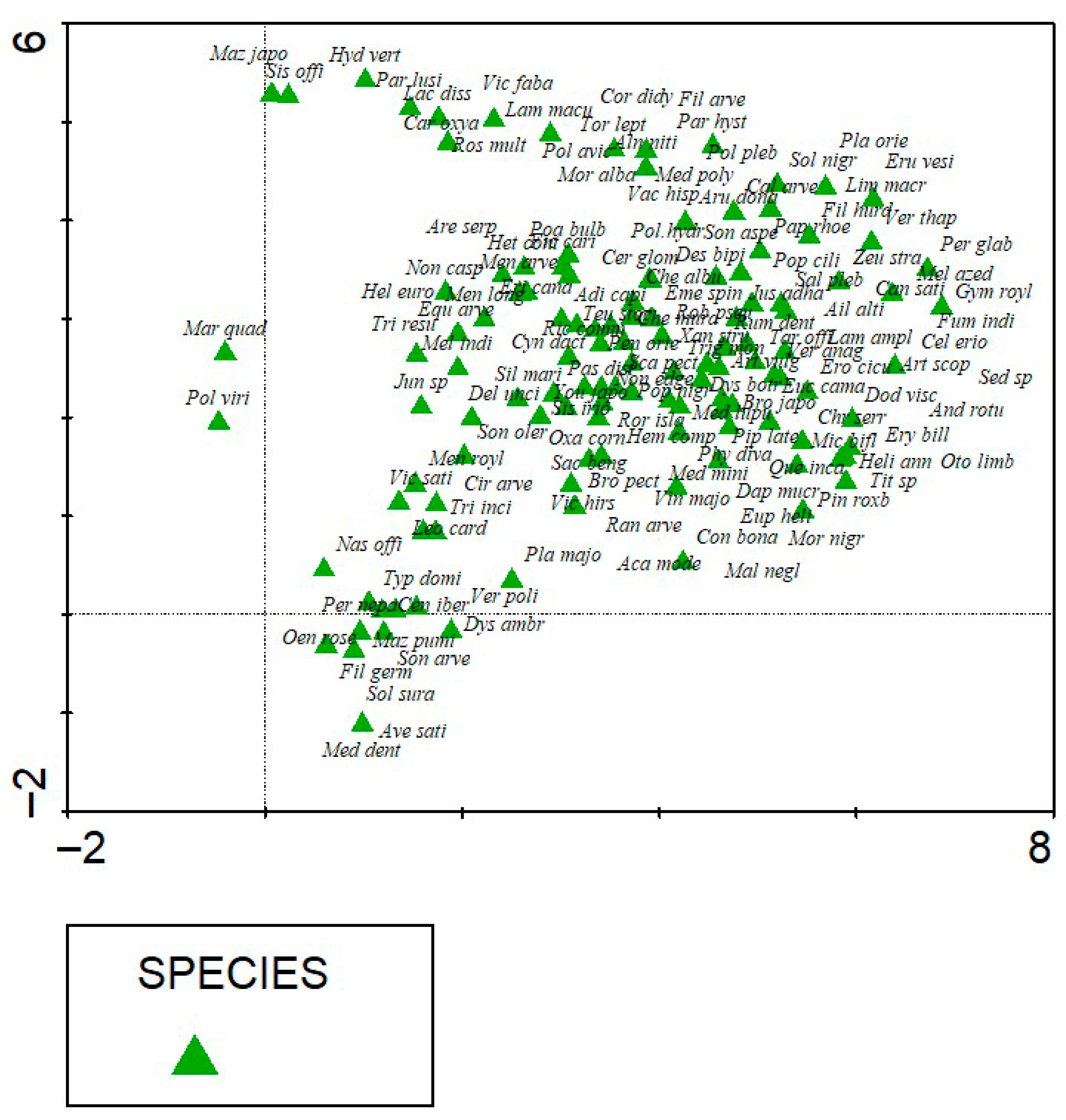
3.5. Environmental Gradients
3.5.1. CCA Identifies Key Soil Drivers of Vegetation Composition
3.5.2. DCA Reveals Species Turnover Across Elevation and Soil Gradients
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Singh, R.; Tiwari, A.; Singh, G. Managing riparian zones for river health improvement: An integrated approach. Landsc. Ecol. Eng. 2021, 17, 195–223. [Google Scholar] [CrossRef]
- Patowary, S.; Debnath, M.; Sarma, A.K. Best Management Practices in Stream: Debris and Runoff Reduction, Riparian Buffers and Plantings, and Stabilizing Stream Banks. In Hydrosystem Restoration Handbook; Elsevier: Amsterdam, The Netherlands, 2025; pp. 73–82. [Google Scholar]
- Beugnon, R.; Le Guyader, N.; Milcu, A.; Lenoir, J.; Puissant, J.; Morin, X.; Hättenschwiler, S. Microclimate modulation: An overlooked mechanism influencing the impact of plant diversity on ecosystem functioning. Glob. Change Biol. 2024, 30, e17214. [Google Scholar] [CrossRef]
- Shuaib, M.; Ali, K.; Ahmed, S.; Hussain, F.; Ilyas, M.; Hassan, N.; Khan, I.; Hussain, F. Impact of rapid urbanization on the floral diversity and agriculture land of district Dir, Pakistan. Acta Ecol. Sin. 2018, 38, 394–400. [Google Scholar] [CrossRef]
- Lindenmayer, D.B. Future directions for biodiversity conservation in managed forests: Indicator species, impact studies and monitoring programs. For. Ecol. Manag. 1999, 115, 277–287. [Google Scholar] [CrossRef]
- Shaheen, H.; Awan, S.N.; Aziz, S. Distribution pattern, conservation status, and associated flora of the genus Juniperus in subalpine pastures of the Kashmir Himalayas. Mt. Res. Dev. 2017, 37, 487–493. [Google Scholar] [CrossRef]
- Paudel, P.K.; Sipos, J.; Brodie, J.F. Threatened species richness along a Himalayan elevational gradient: Quantifying the influences of human population density, range size, and geometric constraints. BMC Ecol. 2018, 18, 6. [Google Scholar] [CrossRef]
- Brown, L.; Bezuidenhout, H. The vegetation of the farms Ingleside and Welgedacht of the Mountain Zebra National Park, Eastern Cape. Koedoe 2005, 48, 23–42. [Google Scholar] [CrossRef]
- Cleaver, G.; Brown, L.; Bredenkamp, G. The phytosociology of the Vermaaks, Marnewicks and Buffelsklip valleys of the Kammanassie Nature Reserve, Western Cape. Koedoe 2023, 48, 1–16. [Google Scholar] [CrossRef]
- Juárez–Fragoso, M.A.; Perroni, Y.; Dáttilo, W.; Gómez–Díaz, J.A.; Guevara, R. The landscape scale of effect on the alpha and beta diversities of woody species in a semideciduous tropical forest. Landsc. Ecol. 2024, 39, 33. [Google Scholar] [CrossRef]
- Tavili, A.; Jafari, M. Interrelations between plants and environmental variables. Int. J. Environ. Res. 2009, 3, 24981578. [Google Scholar]
- Rahman, A.U.; Khan, S.M.; Khan, S.; Hussain, A.; Rahman, I.U.; Iqbal, Z.; Ijaz, F. Ecological assessment of plant communities and associated edaphic and topographic variables in the Peochar Valley of the Hindu Kush mountains. Mt. Res. Dev. 2016, 36, 332–341. [Google Scholar] [CrossRef]
- Parmesan, C.; Burrows, M.T.; Duarte, C.M.; Poloczanska, E.S.; Richardson, A.J.; Schoeman, D.S.; Singer, M.C. Beyond climate change attribution in conservation and ecological research. Ecol. Lett. 2013, 16, 58–71. [Google Scholar] [CrossRef]
- Box, E.O.; Fujiwara, K. Vegetation Types and Their Broad-Scale Distribution. In Vegetation Ecology; Wiley: Hoboken, NJ, USA, 2013; pp. 455–485. [Google Scholar]
- Suding, K.N.; Lavorel, S.; Chapin Iii, F.; Cornelissen, J.H.; DIAz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Change Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Gauthray-Guyénet, V.; Schneider, R.; Paré, D.; Achim, A.; Loi, C.; Sirois, L. Influence of shifts over an 80-year period in forest composition on soil properties. Plant Soil 2018, 433, 111–125. [Google Scholar] [CrossRef]
- Smith, M.E.; Facelli, J.M.; Cavagnaro, T.R. Interactions between soil properties, soil microbes and plants in remnant-grassland and old-field areas: A reciprocal transplant approach. Plant Soil 2018, 433, 127–145. [Google Scholar] [CrossRef]
- Zeb, S.A.; Khan, S.M.; Ahmad, Z.; Abdullah. Phytogeographic elements and vegetation along the river Panjkora-Classification and ordination studies from the Hindu Kush Mountains range. Bot. Rev. 2021, 87, 518–542. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Hu, A.; Wang, J.; Sun, H.; Niu, B.; Si, G.; Wang, J.; Yeh, C.-F.; Zhu, X.; Lu, X.; Zhou, J. Mountain biodiversity and ecosystem functions: Interplay between geology and contemporary environments. ISME J. 2020, 14, 931–944. [Google Scholar] [CrossRef]
- Hussain, J.; Khan, S.M.; Khan, M.S.; Saqib, Z.; Ahmad, Z.; Shah, M.; Mohammad, N.; Batool, Z.; Sohail, A.; Liu, J. Distribution modelling and future prediction of a threatened species-Heracleum candicans Wall. ex DC.; Within the framework of biotic and abiotic interactions. J. Environ. Manag. 2025, 387, 125818. [Google Scholar] [CrossRef]
- Khan, M.S.; Khan, S.M.; Abdullah Liu, J.; Wu, Z.Y.; Hussain, J.; Zeb, S.A.; Mohammad, N.; Batool, Z.; Saqib, Z.; Afza, R. Ecological assessment of Iris hookeriana across subalpine and alpine regions of the Hindu-Himalayas. Front. For. Glob. Change 2025, 8, 1539025. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, S.M.; Afza, R.; Ullah, A.; Zeb, S.A.; Issayeva, K.S.; Bekzatqyzy, I.S. Angiosperms distribution under the influence of microclimatic factors across a polluted ecosystem. J. Hazard. Mater. Adv. 2023, 9, 100223. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Raghib, F.; Dar, M.I.; Khan, F.A.; Hessini, K.; Ahmad, P. Uptake, accumulation and elimination of cadmium in a soil-Faba bean (Vicia faba)-Aphid (Aphis fabae)-Ladybird (Coccinella transversalis) food chain. Chemosphere 2021, 279, 130522. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Page, S.; Ahmad, H.; Harper, D. Identifying plant species and communities across environmental gradients in the Western Himalayas: Method development and conservation use. Ecol. Inform. 2013, 14, 99–103. [Google Scholar] [CrossRef]
- Tu, W.-q.; Lu, W.-x.; Gu, J.-q.; Lou, A.-r. The species diversity and phylogenetic structure patterns of desert plant communities in the Turpan-Hami region, Xinjiang. Glob. Ecol. Conserv. 2024, 55, e03239. [Google Scholar] [CrossRef]
- Xie, P.; Liu, T.; Chen, H.; Su, Z. Community Structure and Soil Mineral Concentration in Relation to Plant Invasion in a Subtropical Urban and Rural Ecotone. Forests 2021, 12, 185. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Bussmann, R.W.; Alsamadany, H.; Calixto, E.S.; Shah, G.M.; Kausar, R.; Shah, M.; Ali, N. Ecological gradients hosting plant communities in Himalayan subalpine pastures: Application of multivariate approaches to identify indicator species. Ecol. Inform. 2020, 60, 101162. [Google Scholar] [CrossRef]
- Mayor, J.R.; Sanders, N.J.; Classen, A.T.; Bardgett, R.D.; Clement, J.-C.; Fajardo, A.; Lavorel, S.; Sundqvist, M.K.; Bahn, M.; Chisholm, C. Elevation alters ecosystem properties across temperate treelines globally. Nature 2017, 542, 91–95. [Google Scholar] [CrossRef]
- Waqas Khan, W.K.; Khan, S.; Habib Ahmad, H.A.; Zeeshan Ahmad, Z.A.; Page, S. Vegetation mapping and multivariate approach to indicator species of a forest ecosystem: A case study from the Thandiani sub Forests Division (TsFD) in the Western Himalayas. Ecol. Indic. 2016, 71, 336–351. [Google Scholar] [CrossRef]
- Rahman, I.; Afzal, A.; Iqbal, Z.; Abd Allah, E.; Alqarawi, A.; Calixto, E.; Ali, N.; Ijaz, F.; Kausar, R.; Alsubeie, M. Role of multivariate approaches in floristic diversity of Manoor Valley (Himalayan Region), Pakistan. Appl. Ecol. Environ. Res 2019, 17, 1475–1498. [Google Scholar] [CrossRef]
- Haq, F.; Ahmad, H.; Iqbal, Z.; Alam, M.; Aksoy, A. Multivariate approach to the classification and ordination of the forest ecosystem of Nandiar valley western Himalayas. Ecol. Indic. 2017, 80, 232–241. [Google Scholar] [CrossRef]
- Bergmeier, E. The vegetation of the high mountains of Crete-a revision and multivariate analysis. Phytocoenologia 2002, 32, 205–249. [Google Scholar] [CrossRef]
- Badshah, L.; Hussain, F.; Sher, Z. Floristic inventory, ecological characteristics and biological spectrum of rangeland, District Tank, Pakistan. Pak. J. Bot 2013, 45, 1159–1168. [Google Scholar]
- Bardgett, R.D.; Manning, P.; Morriën, E.; De Vries, F.T. Hierarchical responses of plant–soil interactions to climate change: Consequences for the global carbon cycle. J. Ecol. 2013, 101, 334–343. [Google Scholar] [CrossRef]
- Yimer, F.; Ledin, S.; Abdelkadir, A. Soil property variations in relation to topographic aspect and vegetation community in the south-eastern highlands of Ethiopia. For. Ecol. Manag. 2006, 232, 90–99. [Google Scholar] [CrossRef]


| Indicator Species | Influencing Factors | IV | p * Value | |
|---|---|---|---|---|
| 1 | Melia azedarach | Cr | 46.2 | 0.01 |
| Fe | 47.5 | 0.01 | ||
| Zn | 20.7 | 0.04 | ||
| 2 | Punica granatum | Cr | 49.5 | 0.01 |
| Fe | 49.7 | 0.008 | ||
| Mg | 26.1 | 0.03 | ||
| 3 | Asparagus racemosus | Cr | 50.0 | 0.03 |
| Fe | 50.0 | 0.01 | ||
| Zn | 20.0 | 0.03 |
| S.no | Indicator Species | Influencing Factors | IV | p * Value |
|---|---|---|---|---|
| 1 | Populus alba | Co | 50.0 | 0.01 |
| 2 | Debregeasia saeneb | pH | 42.3 | 0.03 |
| 3 | Youngia japonica | Cd | 45.5 | 0.04 |
| EC | 36.6 | 0.03 | ||
| TDSs | 25.3 | 0.03 |
| S.no | Indicator Species | Influencing Factor | IV | p * Value |
|---|---|---|---|---|
| 1 | Pinnus roxburgii | Cr | 25.0 | 0.05 |
| 2 | Rydingia limbate | Cu | 50.0 | 0.02 |
| 3 | Cheilanthes pteridioides | Cr | 50.0 | 0.03 |
| Cu | 50.0 | 0.03 | ||
| TDSs | 33.3 | 0.02 | ||
| Zn | 20.0 | 0.03 |
| S.no | Indicator Species | Influencing Factor | IV | p * Value |
|---|---|---|---|---|
| 1 | Ficus carica | Na | 50.0 | 0.01 |
| 2 | Polygonum plebeium | K | 26.0 | 0.05 |
| 3 | Avena sativa | K | 26.0 | 0.03 |
| Fe | 27.0 | 0.04 |
| S.no | Indicator Species | Influencing Factors | IV | p * Value |
|---|---|---|---|---|
| 1 | Ficus palmate | pH | 20.0 | 0.009 |
| 2 | Rosa multiflora | pH | 28.0 | 0.04 |
| 3 | Heliotropium europaeum | Mn | 25.0 | 0.03 |
| Axes | 1 | 2 | 3 | 4 | Total Inertia |
|---|---|---|---|---|---|
| Eigenvalues | 0.404 | 0.362 | 0.358 | 0.296 | 23.765 |
| Species–environmental correlation | 0.854 | 0.854 | 0.809 | 0.843 | |
| Cumulative percentage variance of species | 1.7 | 3.2 | 4.7 | 6.0 | |
| Species–environment relationship | 13.9 | 24.6 | 36.1 | 45.6 | |
| Summary of Monte Carlo test (499 permutations under reduced model) | |||||
| Test of significance of the first canonical axis | Test of significance of all canonical axes | ||||
| Eigenvalue | 0.404 | Trace | 3.113 | ||
| F-ratio | 2.058 | F-ratio | 1.196 | ||
| p-value | 0.0780 | p-value | 0.0160 | ||
| Axes | 1 | 2 | 3 | 4 | Total Inertia |
|---|---|---|---|---|---|
| Eigenvalue | 0.742 | 0.662 | 0.516 | 0.464 | 23.665 |
| Length gradients | 6.443 | 5.222 | 4.053 | 4.791 | |
| Cumulative percentage variance of species data | 3.1 | 5.9 | 8.1 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeb, S.A.; Khan, S.M.; Abdullah, A.; Ahmad, Z.; Zeb, T.A. Ecological Assessment of Riparian Vegetation Along the Banks of the River Panjkora, Hindukush Range. Wild 2025, 2, 37. https://doi.org/10.3390/wild2030037
Zeb SA, Khan SM, Abdullah A, Ahmad Z, Zeb TA. Ecological Assessment of Riparian Vegetation Along the Banks of the River Panjkora, Hindukush Range. Wild. 2025; 2(3):37. https://doi.org/10.3390/wild2030037
Chicago/Turabian StyleZeb, Shakil Ahmad, Shujaul Mulk Khan, Abdullah Abdullah, Zeeshan Ahmad, and Tufail Ahmad Zeb. 2025. "Ecological Assessment of Riparian Vegetation Along the Banks of the River Panjkora, Hindukush Range" Wild 2, no. 3: 37. https://doi.org/10.3390/wild2030037
APA StyleZeb, S. A., Khan, S. M., Abdullah, A., Ahmad, Z., & Zeb, T. A. (2025). Ecological Assessment of Riparian Vegetation Along the Banks of the River Panjkora, Hindukush Range. Wild, 2(3), 37. https://doi.org/10.3390/wild2030037







