First Molecular Evidence of Theileria and Anaplasma Genospecies in Subulo gouazoubira Exhibiting Clinical Symptoms from Entre Ríos Province, Argentina
Abstract
1. Introduction
2. Materials and Methods
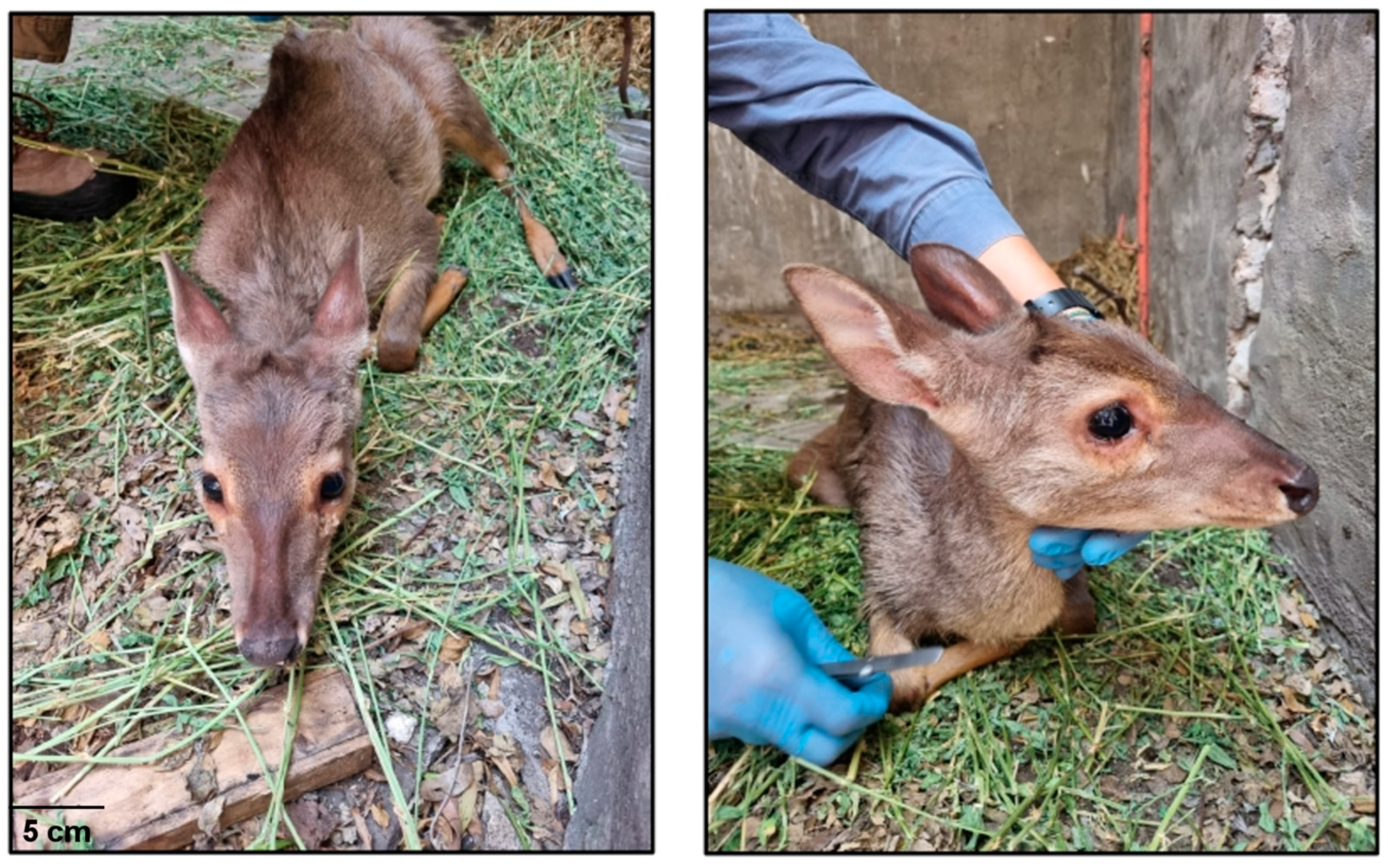
2.1. Case Report
2.2. Sample Collection
2.3. Molecular, Biochemical, Serological and Phylogenetic Analysis
2.4. Ethical Statement
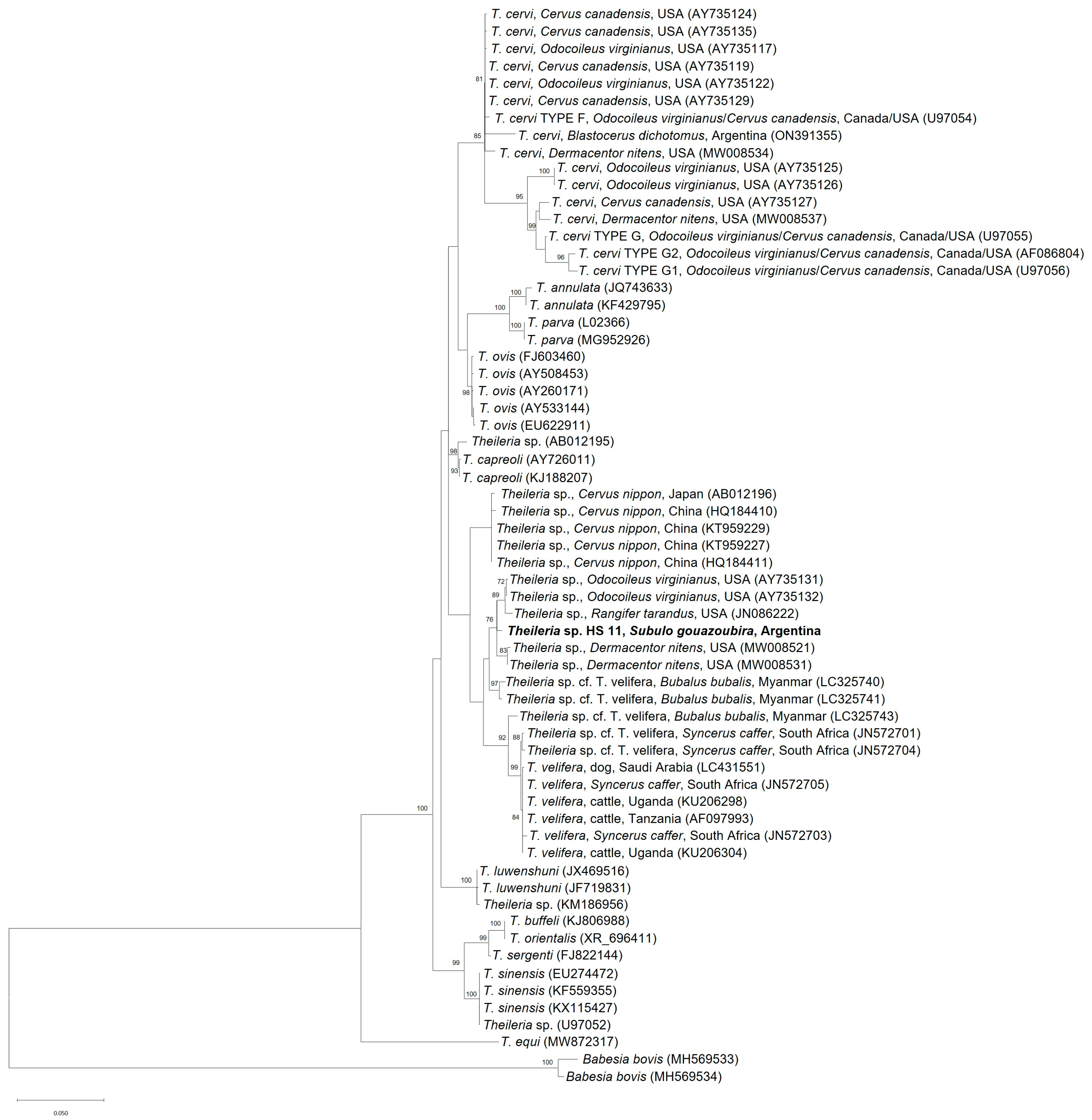
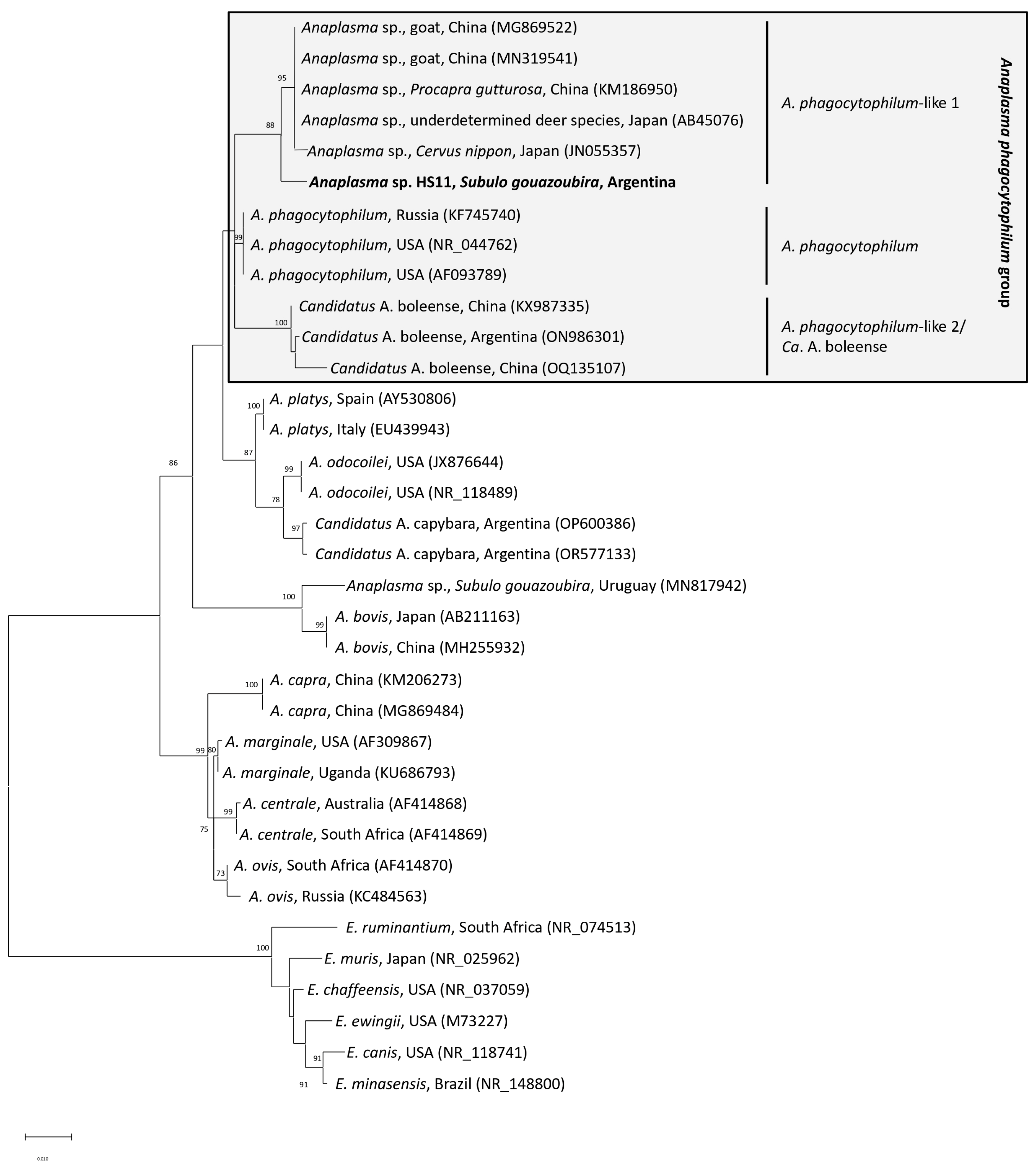
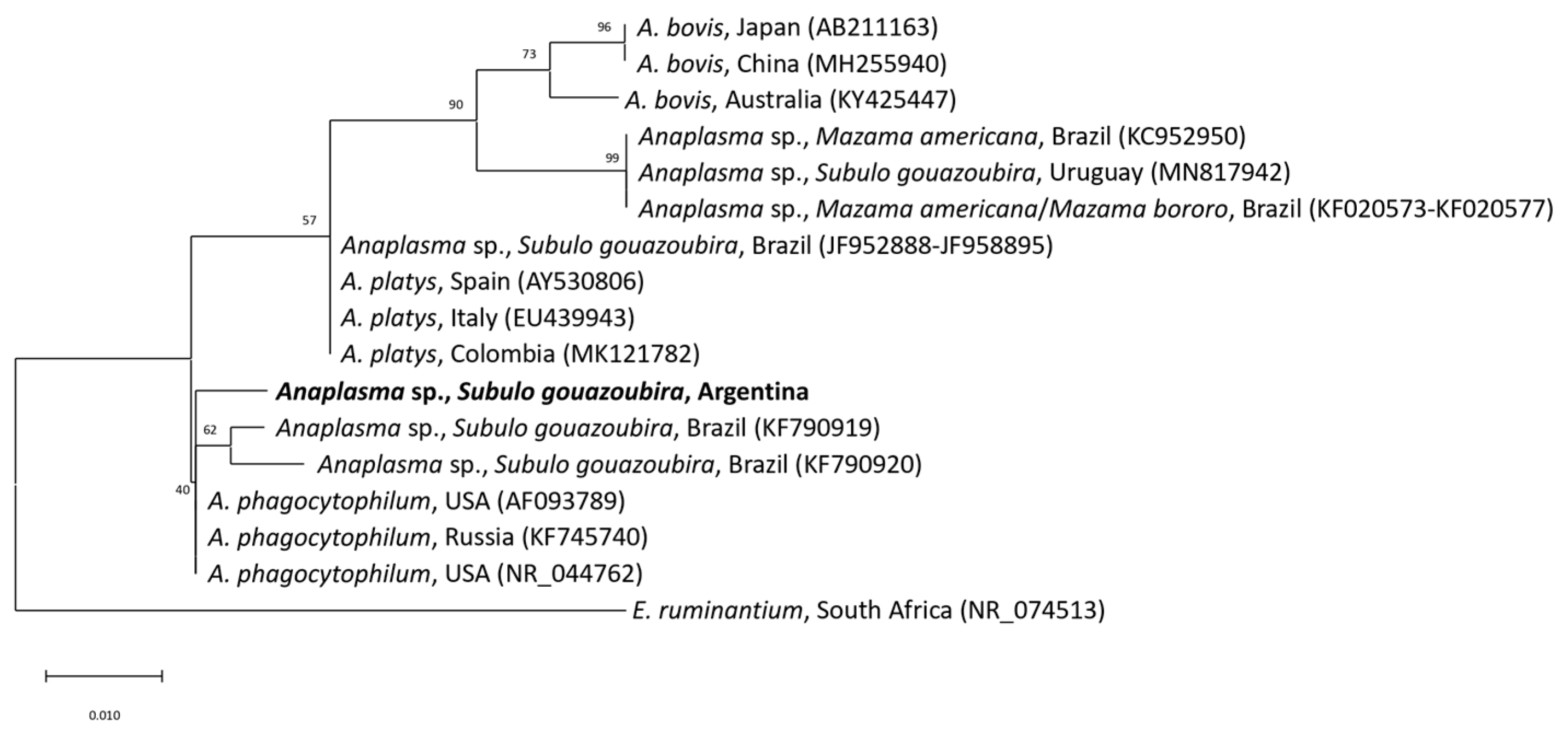
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernegossi, A.M.; Borges, C.H.D.S.; Sandoval, E.D.P.; Cartes, J.L.; Cernohorska, H.; Kubickova, S.; Vozdova, M.; Caparroz, R.; González, S.; Duarte, J.M.B. Resurrection of the genus Subulo for the gray brocket deer, with designation of a neotype. J. Mammal 2022, 104, 619–633. [Google Scholar] [CrossRef]
- Silva-Caballero, A.; Ortega, J. Mazama gouazoubira (Cetartiodactyla: Cervidae). Mamm. Species 2022, 54, seac008. [Google Scholar] [CrossRef]
- Black-Decima, P.A.; Vogliotti, A. 2016. Mazama gouazoubira. The IUCN Red List of Threatened Species. 2016. Available online: https://www.iucnredlist.org/species/29620/22154584 (accessed on 11 May 2025).
- Da Silveira, J.A.G.; Rabelo, É.M.L.; Ribeiro, M.F.B. Detection of Theileria and Babesia in brown brocket deer (Mazama gouazoubira) and marsh deer (Blastocerus dichotomus) in the State of Minas Gerais, Brazil. Vet. Parasitol. 2011, 177, 61–66. [Google Scholar] [CrossRef]
- Félix, M.L.; Armúa-Fernández, M.T.; Parodi, P.; Bazzano, V.; Mangold, A.J.; Venzal, J.M. Detection of a putative novel genotype of Anaplasma in gray-brocket deer (Mazama gouazoubira) from Uruguay. Exp. Appl. Acarol. 2020, 81, 575–583. [Google Scholar] [CrossRef]
- Mongruel, A.C.B.; Benevenute, J.L.; André, M.R.; Carrasco, A.D.O.T.; Machado, R.Z.; Seki, M.C. Molecular Characterization of Anaplasma sp. in Free-Living Gray Brockets (Mazama gouazoubira). Vector-Borne Zoonotic Dis. 2017, 17, 165–171. [Google Scholar] [CrossRef]
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, K.-T.; Kwon, O.-D.; Ock, Y.; Kim, T.; Choi, D.; Kwak, D. Novel Detection of Coxiella spp., Theileria luwenshuni, and T. ovis endosymbionts in Deer Keds (Lipoptena fortisetosa). PLoS ONE 2016, 11, e0156727. [Google Scholar] [CrossRef] [PubMed]
- Tiawsirisup, S.; Yurayart, N.; Thongmeesee, K.; Sri-in, C.; Akarapas, C.; Rittisornthanoo, G.; Bunphungbaramee, N.; Sipraya, N.; Maikaew, U.; Kongmakee, P.; et al. Possible role of Lipoptena fortisetosa (Diptera: Hippoboscidae) as a potential vector for Theileria spp. in captive Eld’s deer in Khao Kheow open zoo, Thailand. Acta Trop. 2023, 237, 106737. [Google Scholar] [CrossRef]
- Montini, M.; Torrents, J.; Nava, S.; Sebastian, P.S. Theileria cervi in Amblyomma neumanni (Acari: Ixodida, Ixodidae) collected on horses from North-western Argentina. J. Parasitic Dis. 2025, 49, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Orozco, M.M.; Argibay, H.D.; Minatel, L.; Guillemi, E.C.; Berra, Y.; Schapira, A.; Di Nucci, D.; Marcos, A.; Lois, F.; Falzone, M.; et al. A participatory surveillance of marsh deer (Blastocerus dichotomus) morbidity and mortality in Argentina: First results. BMC Vet. Res. 2020, 16, 321. [Google Scholar] [CrossRef]
- Sebastian, P.S.; Benitez-Ibalo, A.P.; Flores, F.S.; Debárbora, V.N.; Martinez, E.I.; Thompson, C.S.; Mangold, A.J. Molecular detection and phylogenetic characterization of Theileria equi in horses (Equus caballus) from a peri-urban area of Argentina. Ticks Tick-Borne Dis. 2021, 12, 101810. [Google Scholar] [CrossRef]
- Sebastian, P.S.; Falzone, M.P.; Lois, M.F.; Sartori, R.; Zimmerman, J.; Tarragona, E.L.; Nava, S. Phylogenetic position of Theileria cervi detected in Blastocerus dichotomus (Artiodactyla: Cervidae) with clinical symptoms from Argentina. Emerg. Anim. Species 2022, 5, 100014. [Google Scholar] [CrossRef]
- Benitez-Ibalo, A.P.; Debárbora, V.N.; Mangold, A.J.; Nava, S.; Sebastian, P.S. Molecular genotyping of Theileria spp. detected in horses from Corrientes City, Argentina. Vet. Res. Commun. 2024, 49, 54. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. 2021, 91, 104833. [Google Scholar] [CrossRef] [PubMed]
- Monje, L.D.; Eberhardt, A.T.; Vaschalde, P.J.; Barolin, J.; Costa, F.B.; Labruna, M.B.; Beldomenico, P.M. Natural infection of free-ranging capybaras (Hydrochoerus hydrochaeris) with Anaplasmataecea and Rickettsiaceae bacteria in the Iberá wetlands ecoregion, Argentina. Vet. Res. Commun. 2024, 48, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, P.S.; Panizza, M.N.M.; Ríos, I.J.M.G.; Tarragona, E.L.; Trova, G.B.; Negrette, O.S.; Primo, M.E.; Nava, S. Molecular detection and phylogenetic analysis of Anaplasma platys-like and Candidatus Anaplasma boleense strains from Argentina. Comp. Immunol. Microbiol. Infect. Dis. 2023, 96, 101980. [Google Scholar] [CrossRef]
- Winter, M.; Sebastian, P.S.; Tarragona, E.L.; Flores, F.S.; Abate, S.D.; Nava, S. Tick-borne microorganisms in Amblyomma tigrinum (Acari: Ixodidae) from the Patagonian region of Argentina. Exp. Appl. Acarol. 2024, 92, 151–159. [Google Scholar] [CrossRef]
- Nava, S.; Venzal, J.M.; Martins, T.F.; Guglielmone, A.A.; González Acuña, D. Ticks of the Southern Cone of America: Diagnosis, Distribution and Hosts with Taxonomy, Ecology and Sanitary Importance; Academic Press: London, UK; Elsevier: San Diego, CA, USA, 2017. [Google Scholar]
- Pinheiro, M.F.; Bonato, R.M.; Ikeda, P.; Souza, R.A.M.D.; Carrasco, A.D.O.T.; Seki, M.C. Occurrence of Lipoptena mazama and Lipoptena guimaraesi (Diptera: Hippoboscidae) on gray brocket deer (Mazama gouazoubira) in southern Brazil. Rev. Bras. Parasitol. Vet. 2021, 30, e008621. [Google Scholar] [CrossRef]
- Thompson, C.S.; Mangold, A.J.; Félix, M.L.; Carvalho, L.; Armúa-Fernández, M.T.; Venzal, J.M. Molecular evidence of Babesia species in Procyon cancrivorus (Carnivora, Procyonidae) in Uruguay. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 230–233. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Camargo, C.M.S.; Duarte, J.M.B.; Fagliari, J.J.; Santana, A.M.; Simplício, K.M.M.G.; Santana, A.E. Effect of sex and seasons of the year on hematologic and serum biochemical variables of captive brown brocket deer (Mazama gouazoubira). Pesqui. Vet. Bras. 2013, 33, 1364–1370. [Google Scholar] [CrossRef]
- Garner, B.C.; Holman, P.; Berent, L.M. Theileriosis in a reindeer (Rangifer tarandus tarandus) associated with a potentially novel Theileria sp. Vet. Clin. Pathol. 2012, 41, 497–501. [Google Scholar] [CrossRef]
- Olafson, P.U.; Buckmeier, B.G.; May, M.A.; Thomas, D.B. Molecular screening for rickettsial bacteria and piroplasms in ixodid ticks surveyed from white-tailed deer (Odocoileus virginianus) and nilgai antelope (Boselaphus tragocamelus) in southern Texas. Int. J. Parasitol. Parasites Wildl. 2020, 13, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, M.-J.; Yin, H.; Bai, Q.; Liu, G.; Nijman, I.J.; Jongejan, F. The phylogenetic position of the Theileria buffeli group in relation to other Theileria species. Parasitol. Res. 2002, 88, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Calchi, A.C.; Duarte, J.M.B.; Castro-Santiago, A.C.; Bassini-Silva, R.; Barros-Battesti, D.M.; Machado, R.Z.; André, M.R. Genetic diversity of Theileria spp. in deer (Artiodactyla: Cervidae) from Brazil. Parasitol. Res. 2024, 123, 384. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, A.P.; Matsumoto, K.; Kishimoto, T.; Yokoyama, N.; Inokuma, H. Dual Presence of Anaplasma phagocytophilum and Its Closely Related Anaplasma sp. in Ixodid Ticks in Hokkaido, Japan, and Their Specific Molecular Detection. J. Vet. Med. Sci. 2012, 74, 1551–1560. [Google Scholar] [CrossRef]
- Ybañez, A.P.; Tagawa, M.; Matsumoto, K.; Kishimoto, T.; Yokoyama, N.; Inokuma, H. Specific Molecular Detection of Anaplasma sp. Closely Related to Anaplasma phagocytophilum in Ixodid Ticks and Cattle in a Pastureland in Hokkaido, Japan. Vector-Borne Zoonotic Dis. 2013, 13, 6–11. [Google Scholar] [CrossRef]
- Seo, M.-G.; Ouh, I.-O.; Kwon, O.-D.; Kwak, D. Molecular detection of Anaplasma phagocytophilum-like Anaplasma spp. and pathogenic A. phagocytophilum in cattle from South Korea. Mol. Phylogenet. Evol. 2018, 126, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zobba, R.; Ben Said, M.; Belkahia, H.; Pittau, M.; Cacciotto, C.; Pinna Parpaglia, M.L.; Messadi, L.; Alberti, A. Molecular epidemiology of Anaplasma spp. related to A. phagocytophilum in Mediterranean small ruminants. Acta Trop. 2020, 202, 105286. [Google Scholar] [CrossRef] [PubMed]
- Benedict, B.M.; Barboza, P.S. Adverse effects of Diptera flies on northern ungulates: Rangifer, Alces, and Bison. Mammal Rev. 2022, 52, 425–437. [Google Scholar] [CrossRef]
| Parameter * | Reference Values 1 | |
|---|---|---|
| Hematogram | ||
| Hematocrit (%) | 9.7 | 46.33 ± 4.39 |
| Erythrocytes/mm3 (×106) | 2.59 | 14.63 ± 1.53 |
| Leukocytes/mm3 | 5120 | 4.48 ± 1.14 |
| Hemoglobin (g/dL) | 11.8 | 16.38 ± 1.32 |
| MCV (µm3) | 38 | 32.48 ± 6.47 |
| MCH (%) | 45.6 | 11.49 ± 2.17 2 |
| MCHC (g/dL) | 121.3 | 35.35 ± 1.93 |
| Biochemical blood profile | ||
| Urea (mg/dL) | 50 | 63.25 ± 22.21 |
| Creatinine (mg/dL) | 0.67 | 1.75 ± 0.27 |
| Glucose (g/L) | 0.51 | N/A |
| Total protein (g/dL) | 5.17 | 8.22 ± 0.80 |
| AST/ASA/GOT (IU) | 977 | 97.71 ± 79.70 |
| ALT/ALA/GPT (IU) | 52 | 16.5 ± 5.13 |
| γGT/GGT (IU) | 80 | 62.65 ± 17.89 |
| Alkaline phosphatase (IU) | 73 | N/A |
| Bilirubin (total, mg/dL) | 0.05 | N/A |
| Bilirubin (direct, mg/dL) | 0.03 | N/A |
| Bilirubin (indirect, mg/dL) | 0.02 | N/A |
| CPK (IU) | 6332 | 171 ± 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastian, P.S.; Vaschalde, P.J.; Pelosi, M.C.; Flores, F.S.; Yedro, J.; Tarragona, E.L. First Molecular Evidence of Theileria and Anaplasma Genospecies in Subulo gouazoubira Exhibiting Clinical Symptoms from Entre Ríos Province, Argentina. Wild 2025, 2, 35. https://doi.org/10.3390/wild2030035
Sebastian PS, Vaschalde PJ, Pelosi MC, Flores FS, Yedro J, Tarragona EL. First Molecular Evidence of Theileria and Anaplasma Genospecies in Subulo gouazoubira Exhibiting Clinical Symptoms from Entre Ríos Province, Argentina. Wild. 2025; 2(3):35. https://doi.org/10.3390/wild2030035
Chicago/Turabian StyleSebastian, Patrick S., Paula J. Vaschalde, María C. Pelosi, Fernando S. Flores, Julio Yedro, and Evelina L. Tarragona. 2025. "First Molecular Evidence of Theileria and Anaplasma Genospecies in Subulo gouazoubira Exhibiting Clinical Symptoms from Entre Ríos Province, Argentina" Wild 2, no. 3: 35. https://doi.org/10.3390/wild2030035
APA StyleSebastian, P. S., Vaschalde, P. J., Pelosi, M. C., Flores, F. S., Yedro, J., & Tarragona, E. L. (2025). First Molecular Evidence of Theileria and Anaplasma Genospecies in Subulo gouazoubira Exhibiting Clinical Symptoms from Entre Ríos Province, Argentina. Wild, 2(3), 35. https://doi.org/10.3390/wild2030035






