Anthropogenic Impacts as a Driver of Sensory Organ Morphology
Simple Summary
Abstract
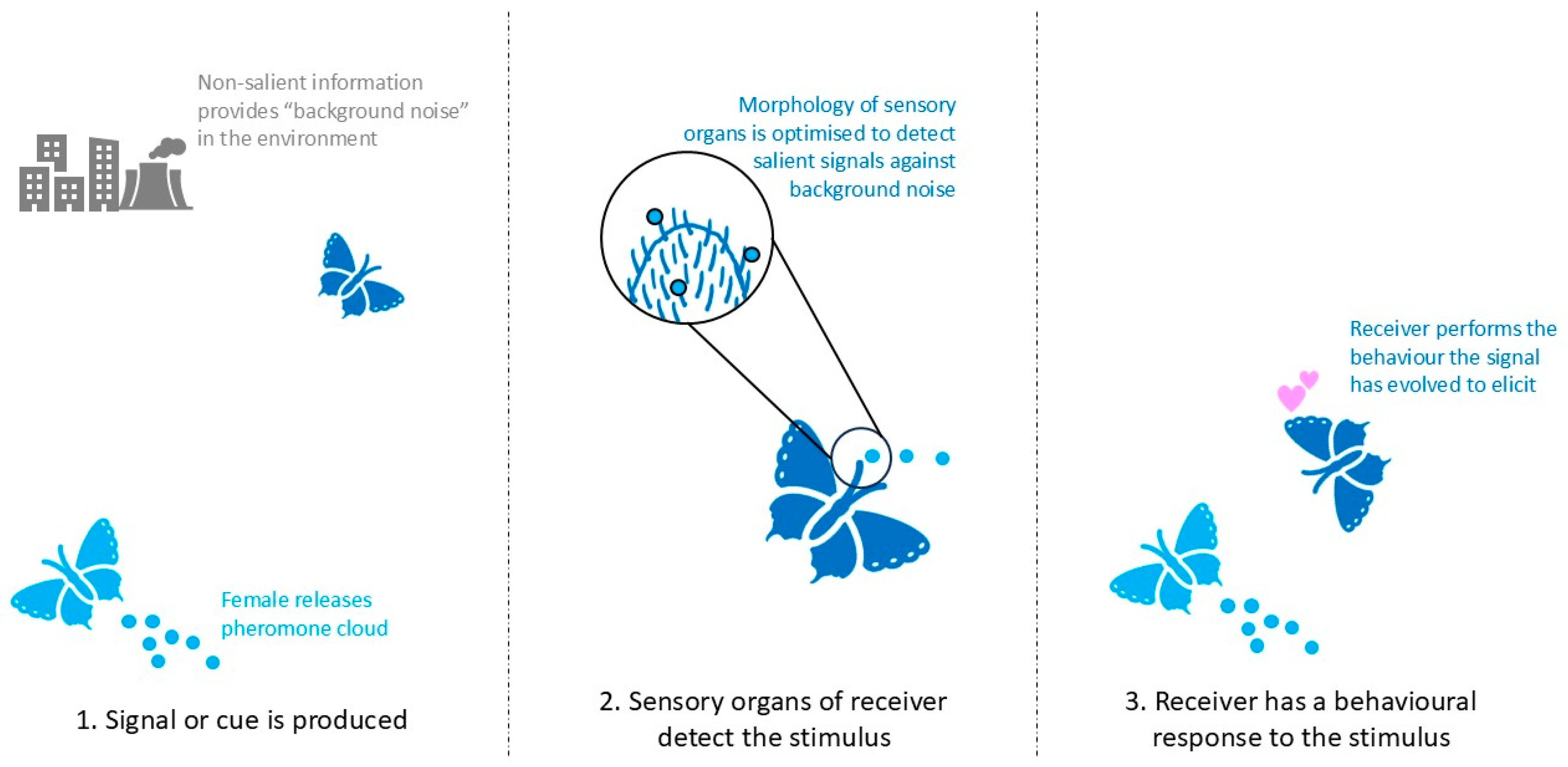
1. Introduction
2. Anthropogenically Generated Sensory Environments: Captivity
3. Anthropogenic Disruption to the Sensory Environment
3.1. Light Pollution
3.2. Noise Pollution
3.3. Chemical Pollution
4. Future Directions
4.1. Timeframe
4.2. Functional Impact
4.3. Mechanism
4.4. Taxonomic Breadth
4.5. Multimodality
5. Conclusions
Funding
Conflicts of Interest
References
- Darwin, C. Chapter VIII. Principles of sexual selection. In The Descent of Man, and Selection in Relation to Sex; John Murray: London, UK, 1871; pp. 253–320. [Google Scholar]
- Endler, J.A. Signals, Signal Conditions, and the Direction of Evolution. Am. Nat. 1992, 139, S125–S153. [Google Scholar] [CrossRef]
- Davies, N.B.; Krebs, J.R.; West, S.A. Communication and Signals. In An Introduction to Behavioural Ecology, 4th ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 394–423. [Google Scholar]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human Domination of Earth’s Ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Halfwerk, W.; Slabbekoorn, H. Pollution going multimodal: The complex impact of the human-altered sensory environment on animal perception and performance. Biol. Lett. 2015, 11, 20141051. [Google Scholar] [CrossRef] [PubMed]
- Brumm, H.; Slabbekoorn, H. Acoustic Communication in Noise. In Advances in the Study of Behavior; Academic Press: Cambridge, MA, USA, 2005; Volume 35, pp. 151–209. [Google Scholar]
- Dominoni, D.M.; Halfwerk, W.; Baird, E.; Buxton, R.T.; Fernández-Juricic, E.; Fristrup, K.M.; McKenna, M.F.; Mennitt, D.J.; Perkin, E.K.; Seymoure, B.M.; et al. Why conservation biology can benefit from sensory ecology. Nat. Ecol. Evol. 2020, 4, 502–511. [Google Scholar] [CrossRef]
- Endler, J.A. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vis. Res. 1991, 31, 587–608. [Google Scholar] [CrossRef]
- Niven, J.E.; Laughlin, S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008, 211, 1792–1804. [Google Scholar] [CrossRef]
- Kelley, J.L.; Chapuis, L.; Davies, W.I.L.; Collin, S.P. Sensory System Responses to Human-Induced Environmental Change. Front. Ecol. Evol. 2018, 6, 95. [Google Scholar] [CrossRef]
- Freelance, C.B.; Magrath, M.J.L.; Elgar, M.A.; Wong, B.B.M. Long-term captivity is associated with changes to sensory organ morphology in a critically endangered insect. J. Appl. Ecol. 2022, 59, 504–513. [Google Scholar] [CrossRef]
- Tosetto, L.; Ryan, L.A.; Hart, N.S. Conservation is in the eye of the beholder: Taking a sensory approach to animal management and conservation in Australia. Aust. Zool. 2024, 43, 652–662. [Google Scholar] [CrossRef]
- Lim, M.L.M.; Sodhi, N.S.; Endler, J.A. Conservation with Sense. Science 2008, 319, 281. [Google Scholar] [CrossRef]
- Elmer, L.K.; Madliger, C.L.; Blumstein, D.T.; Elvidge, C.K.; Fernández-Juricic, E.; Horodysky, A.Z.; Johnson, N.S.; McGuire, L.P.; Swaisgood, R.R.; Cooke, S.J. Exploiting common senses: Sensory ecology meets wildlife conservation and management. Conserv. Physiol. 2021, 9, coab002. [Google Scholar] [CrossRef] [PubMed]
- Patricelli, G.L.; Blickley, J.L. Avian Communication in Urban Noise: Causes and Consequences of Vocal Adjustment. Auk 2006, 123, 639–649. [Google Scholar] [CrossRef]
- Brown, A.D.; Sisneros, J.A.; Jurasin, T.; Nguyen, C.; Coffin, A.B. Differences in Lateral Line Morphology between Hatchery- and Wild-Origin Steelhead. PLoS ONE 2013, 8, e59162. [Google Scholar] [CrossRef] [PubMed]
- Nakae, M.; Hasegawa, K.; Miyamoto, K. Domestication of captive-bred masu salmon Oncorhynchus masou masou (Salmonidae) leads to a significant decrease in numbers of lateral line organs. Sci. Rep. 2022, 12, 16780. [Google Scholar] [CrossRef]
- Keinath, S.; Hölker, F.; Müller, J.; Rödel, M.-O. Impact of light pollution on moth morphology–A 137-year study in Germany. Basic Appl. Ecol. 2021, 56, 1–10. [Google Scholar] [CrossRef]
- Jones, T.M.; Llamas, A.P.; Phillips, J.N. Phenotypic signatures of urbanization? Resident, but not migratory, songbird eye size varies with urban-associated light pollution levels. Glob. Change Biol. 2023, 29, 6635–6646. [Google Scholar] [CrossRef]
- Williams, S.E.; Hoffman, E.A. Minimizing genetic adaptation in captive breeding programs: A review. Biol. Conserv. 2009, 142, 2388–2400. [Google Scholar] [CrossRef]
- Lacy, R.C. Loss of Genetic Diversity from Managed Populations: Interacting Effects of Drift, Mutation, Immigration, Selection, and Population Subdivision. Conserv. Biol. 1987, 1, 143–158. [Google Scholar] [CrossRef]
- Lewis, O.T.; Thomas, C.D. Adaptations to Captivity in the Butterfly Pieris brassicae (L.) and the Implications for Ex situ Conservation. J. Insect Conserv. 2001, 5, 55–63. [Google Scholar] [CrossRef]
- Sutherland, W.J. Managing habitats and species. In Conservation Science and Action; Sutherland, W.J., Ed.; Blackwell Science: Hoboken, NJ, USA, 1998; pp. 202–219. [Google Scholar]
- Kraaijeveld-Smit, F.J.L.; Griffiths, R.A.; Moore, R.D.; Beebee, T.J.C. Captive breeding and the fitness of reintroduced species: A test of the responses to predators in a threatened amphibian. J. Appl. Ecol. 2006, 43, 360–365. [Google Scholar] [CrossRef]
- Aoyama, T.; Omori, H.; Iijima, A.; Murakami, Y.; Izawa, T.; Urabe, H.; Miyakoshi, Y. Comparison of adults return rates of hatchery-reared smolts originating from captive-brood and wild masu salmon. In Scientific Reports of the Hokkaido Fish Hatchery; Food and Agriculture Organization: Rome, Italy, 2010; pp. 1–6. [Google Scholar]
- Jakob-Hoff, R.; Harley, D.; Magrath, M.; Lancaster, M.; Kuchling, G. Advances in the contribution of zoos to reintroduction programs. In Advances in Reintroduction Biology of Australian and New Zealand Fauna; Armstrong, D.P., Hayward, M.W., Moro, D., Seddon, P.J., Eds.; CSIRO Publishing: Clayton, VIC, Australia, 2015; pp. 201–216. [Google Scholar]
- Priddel, D.; Carlile, N.; Humphrey, M.; Fellenberg, S.; Hiscox, D. Rediscovery of the ‘extinct’ Lord Howe Island stick-insect (Dryococelus australis (Montrouzier)) (Phasmatodea) and recommendations for its conservation. Biodivers. Conserv. 2003, 12, 1391–1403. [Google Scholar] [CrossRef]
- Land, M.F.; Nilsson, D.-E. Animal Eyes; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Spaethe, J.; Brockmann, A.; Halbig, C.; Tautz, J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 2007, 94, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.P.; van Wilgenburg, E.; Macmillan, D.L.; Elgar, M.A. Density of antennal sensilla influences efficacy of communication in a social insect. Am Nat 2013, 182, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Kyba, C.C.M.; Kuester, T.; Sánchez de Miguel, A.; Baugh, K.; Jechow, A.; Hölker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3, e1701528. [Google Scholar] [CrossRef]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef]
- Bennie, J.; Duffy, J.P.; Davies, T.W.; Correa-Cano, M.E.; Gaston, K.J. Global Trends in Exposure to Light Pollution in Natural Terrestrial Ecosystems. Remote Sens. 2015, 7, 2715–2730. [Google Scholar] [CrossRef]
- Tuxbury, S.M.; Salmon, M. Competitive interactions between artificial lighting and natural cues during seafinding by hatchling marine turtles. Biol. Conserv. 2005, 121, 311–316. [Google Scholar] [CrossRef]
- Da Silva, A.; Samplonius, J.M.; Schlicht, E.; Valcu, M.; Kempenaers, B. Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav. Ecol. 2014, 25, 1037–1047. [Google Scholar] [CrossRef]
- Kempenaers, B.; Borgström, P.; Loës, P.; Schlicht, E.; Valcu, M. Artificial Night Lighting Affects Dawn Song, Extra-Pair Siring Success, and Lay Date in Songbirds. Curr. Biol. 2010, 20, 1735–1739. [Google Scholar] [CrossRef]
- Da Silva, A.; Kempenaers, B. Singing from North to South: Latitudinal variation in timing of dawn singing under natural and artificial light conditions. J. Anim. Ecol. 2017, 86, 1286–1297. [Google Scholar] [CrossRef]
- Baker, B.J.; Richardson, J.M.L. The effect of artificial light on male breeding-season behaviour in green frogs, Rana clamitans melanota. Can. J. Zool. 2006, 84, 1528–1532. [Google Scholar] [CrossRef]
- Dias, K.S.; Dosso, E.S.; Hall, A.S.; Schuch, A.P.; Tozetti, A.M. Ecological light pollution affects anuran calling season, daily calling period, and sensitivity to light in natural Brazilian wetlands. Sci. Nat. 2019, 106, 46. [Google Scholar] [CrossRef]
- Bird, S.; Parker, J. Low levels of light pollution may block the ability of male glow-worms (Lampyris noctiluca L.) to locate females. J. Insect Conserv. 2014, 18, 737–743. [Google Scholar] [CrossRef]
- Moubarak, E.M.; David Fernandes, A.S.; Stewart, A.J.A.; Niven, J.E. Artificial light impairs local attraction to females in male glow-worms. J. Exp. Biol. 2023, 226, jeb245760. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.R.; Gaston, K.J.; Visser, M.E.; Elgar, M.A.; Jones, T.M. Artificial light at night as a driver of evolution across urban–rural landscapes. Front. Ecol. Environ. 2018, 16, 472–479. [Google Scholar] [CrossRef]
- Dowling, T.E.; Martasian, D.P.; Jeffery, W.R. Evidence for Multiple Genetic Forms with Similar Eyeless Phenotypes in the Blind Cavefish, Astyanax mexicanus. Mol. Biol. Evol. 2002, 19, 446–455. [Google Scholar] [CrossRef]
- Lavoie, K.H.; Helf, K.L.; Poulson, T.L. The biology and ecology of North American cave crickets. J. Cave Karst Stud. 2007, 69, 114–134. [Google Scholar]
- Parimuchová, A.; Žurovcová, M.; Papáč, V.; Kováč, Ľ. Subterranean Deuteraphorura Absolon, 1901, (Hexapoda, Collembola) of the Western Carpathians—Troglomorphy at the northern distributional limit in Europe. PLoS ONE 2020, 15, e0226966. [Google Scholar] [CrossRef]
- Tierney, S.M.; Langille, B.; Humphreys, W.F.; Austin, A.D.; Cooper, S.J.B. Massive Parallel Regression: A Précis of Genetic Mechanisms for Vision Loss in Diving Beetles. Integr. Comp. Biol. 2018, 58, 465–479. [Google Scholar] [CrossRef]
- Freelance, C.B.; Tierney, S.M.; Rodriguez, J.; Stuart-Fox, D.M.; Wong, B.B.M.; Elgar, M.A. The eyes have it: Dim-light activity is associated with the morphology of eyes but not antennae across insect orders. Biol. J. Linn. Soc. 2021, 134, 303–315. [Google Scholar] [CrossRef]
- Hall, M.I.; Ross, C.F. Eye shape and activity pattern in birds. J. Zool. 2007, 271, 437–444. [Google Scholar] [CrossRef]
- Sordello, R.; Ratel, O.; Flamerie De Lachapelle, F.; Leger, C.; Dambry, A.; Vanpeene, S. Evidence of the impact of noise pollution on biodiversity: A systematic map. Environ. Evid. 2020, 9, 20. [Google Scholar] [CrossRef]
- Velilla, E.; Halfwerk, W. Adjustments to Facilitate Communication in Noisy Environments. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Academic Press: Oxford, UK, 2019; pp. 598–605. [Google Scholar]
- Classen-Rodríguez, L.; Tinghitella, R.; Fowler-Finn, K. Anthropogenic noise affects insect and arachnid behavior, thus changing interactions within and between species. Curr. Opin. Insect Sci. 2021, 47, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Greif, S.; Nemeth, E.; Brumm, H. Airport noise predicts song timing of European birds. Ecol. Evol. 2016, 6, 6151–6159. [Google Scholar] [CrossRef]
- de Framond, L.; Brumm, H. Long-term effects of noise pollution on the avian dawn chorus: A natural experiment facilitated by the closure of an international airport. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220906. [Google Scholar] [CrossRef]
- Fuller, R.A.; Warren, P.H.; Gaston, K.J. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 2007, 3, 368–370. [Google Scholar] [CrossRef]
- Bent, A.M.; Ings, T.C.; Mowles, S.L. Anthropogenic noise disrupts mate searching in Gryllus bimaculatus. Behav. Ecol. 2018, 29, 1271–1277. [Google Scholar] [CrossRef]
- Orci, K.M.; Petróczki, K.; Barta, Z. Instantaneous song modification in response to fluctuating traffic noise in the tree cricket Oecanthus pellucens. Anim. Behav. 2016, 112, 187–194. [Google Scholar] [CrossRef]
- Bee, M.A.; Swanson, E.M. Auditory masking of anuran advertisement calls by road traffic noise. Anim. Behav. 2007, 74, 1765–1776. [Google Scholar] [CrossRef]
- Zaffaroni-Caorsi, V.; Both, C.; Márquez, R.; Llusia, D.; Narins, P.; Debon, M.; Borges-Martins, M. Effects of anthropogenic noise on anuran amphibians. Bioacoustics 2023, 32, 90–120. [Google Scholar] [CrossRef]
- Caorsi, V.Z.; Both, C.; Cechin, S.; Antunes, R.; Borges-Martins, M. Effects of traffic noise on the calling behavior of two Neotropical hylid frogs. PLoS ONE 2017, 12, e0183342. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.S.; Young, R.J.; Davies, W.J.; Waddington, D.; Wood, M.D. A Systematic Review of Anthropogenic Noise Impact on Avian Species. Curr. Pollut. Rep. 2024, 10, 684–709. [Google Scholar] [CrossRef]
- Raboin, M.; Elias, D.O. Anthropogenic noise and the bioacoustics of terrestrial invertebrates. J. Exp. Biol. 2019, 222, jeb178749. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.D.; Uetz, G.W. Environmental interference: Impact of acoustic noise on seismic communication and mating success. Behav. Ecol. 2012, 23, 707–714. [Google Scholar] [CrossRef]
- Wu, C.-H.; Elias, D.O. Vibratory noise in anthropogenic habitats and its effect on prey detection in a web-building spider. Anim. Behav. 2014, 90, 47–56. [Google Scholar] [CrossRef]
- Shier, D.M.; Lea, A.J.; Owen, M.A. Beyond masking: Endangered Stephen’s kangaroo rats respond to traffic noise with footdrumming. Biol. Conserv. 2012, 150, 53–58. [Google Scholar] [CrossRef]
- Phillips, M.E.; Chio, G.; Hall, C.L.; ter Hofstede, H.M.; Howard, D.R. Seismic noise influences brood size dynamics in a subterranean insect with biparental care. Anim. Behav. 2020, 161, 15–22. [Google Scholar] [CrossRef]
- Caorsi, V.; Guerra, V.; Furtado, R.; Llusia, D.; Miron, L.R.; Borges-Martins, M.; Both, C.; Narins, P.M.; Meenderink, S.W.F.; Márquez, R. Anthropogenic substrate-borne vibrations impact anuran calling. Sci. Rep. 2019, 9, 19456. [Google Scholar] [CrossRef]
- Roberts, L.; Cheesman, S.; Elliott, M.; Breithaupt, T. Sensitivity of Pagurus bernhardus (L.) to substrate-borne vibration and anthropogenic noise. J. Exp. Mar. Biol. Ecol. 2016, 474, 185–194. [Google Scholar] [CrossRef]
- Roberts, L.; Howard, D.R. Substrate-Borne Vibrational Noise in the Anthropocene: From Land to Sea. In Biotremology: Physiology, Ecology, and Evolution; Hill, P.S.M., Mazzoni, V., Stritih-Peljhan, N., Virant-Doberlet, M., Wessel, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 123–155. [Google Scholar]
- Saaristo, M.; Johnstone, C.P.; Xu, K.; Allinson, M.; Wong, B.B.M. The endocrine disruptor, 17α-ethinyl estradiol, alters male mate choice in a freshwater fish. Aquat. Toxicol. 2019, 208, 118–125. [Google Scholar] [CrossRef]
- Wiles, S.C.; Bertram, M.G.; Martin, J.M.; Tan, H.; Lehtonen, T.K.; Wong, B.B.M. Long-Term Pharmaceutical Contamination and Temperature Stress Disrupt Fish Behavior. Environ. Sci. Technol. 2020, 54, 8072–8082. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.; Nagarajan-Radha, V.; Tan, H.; Bertram, M.G.; Brand, J.A.; Saaristo, M.; Dowling, D.K.; Wong, B.B.M. Antidepressant exposure causes a nonmonotonic reduction in anxiety-related behaviour in female mosquitofish. J. Hazard. Mater. Lett. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Lagesson, A.; Saaristo, M.; Brodin, T.; Fick, J.; Klaminder, J.; Martin, J.M.; Wong, B.B.M. Fish on steroids: Temperature-dependent effects of 17β-trenbolone on predator escape, boldness, and exploratory behaviors. Environ. Pollut. 2019, 245, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, G.; Yan, L.; Xu, W.; Hilton, D.J.; Liu, X.; Pei, W.; Li, X.; Wu, J.; Zhao, H.; et al. Short-term particulate matter contamination severely compromises insect antennal olfactory perception. Nat. Commun. 2023, 14, 4112. [Google Scholar] [CrossRef]
- Cripps, I.L.; Munday, P.L.; McCormick, M.I. Ocean Acidification Affects Prey Detection by a Predatory Reef Fish. PLoS ONE 2011, 6, e22736. [Google Scholar] [CrossRef]
- Munday, P.L.; Dixson, D.L.; Donelson, J.M.; Jones, G.P.; Pratchett, M.S.; Devitsina, G.V.; Døving, K.B. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. USA 2009, 106, 1848–1852. [Google Scholar] [CrossRef]
- Dixson, D.L.; Munday, P.L.; Jones, G.P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 2010, 13, 68–75. [Google Scholar] [CrossRef]
- Williams, C.R.; Dittman, A.H.; McElhany, P.; Busch, D.S.; Maher, M.T.; Bammler, T.K.; MacDonald, J.W.; Gallagher, E.P. Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob. Change Biol. 2019, 25, 963–977. [Google Scholar] [CrossRef]
- Welch, M.J.; Munday, P.L. Heritability of behavioural tolerance to high CO2 in a coral reef fish is masked by nonadaptive phenotypic plasticity. Evol. Appl. 2017, 10, 682–693. [Google Scholar] [CrossRef]
- Schunter, C.; Welch, M.J.; Nilsson, G.E.; Rummer, J.L.; Munday, P.L.; Ravasi, T. An interplay between plasticity and parental phenotype determines impacts of ocean acidification on a reef fish. Nat. Ecol. Evol. 2018, 2, 334–342. [Google Scholar] [CrossRef]
- Elgar, M.A.; Zhang, D.; Wang, Q.; Wittwer, B.; Thi Pham, H.; Johnson, T.L.; Freelance, C.B.; Coquilleau, M. Insect Antennal Morphology: The Evolution of Diverse Solutions to Odorant Perception. Yale J. Biol. Med. 2018, 91, 457–469. [Google Scholar] [PubMed]
- Suli, A.; Watson, G.M.; Rubel, E.W.; Raible, D.W. Rheotaxis in Larval Zebrafish Is Mediated by Lateral Line Mechanosensory Hair Cells. PLoS ONE 2012, 7, e29727. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.P.; Farrugia, T.J.; Kinnison, M.T. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 2008, 17, 20–29. [Google Scholar] [CrossRef]
- Sanderson, S.; Beausoleil, M.-O.; O’Dea, R.E.; Wood, Z.T.; Correa, C.; Frankel, V.; Gorné, L.D.; Haines, G.E.; Kinnison, M.T.; Oke, K.B.; et al. The pace of modern life, revisited. Mol. Ecol. 2022, 31, 1028–1043. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.E.; Farquharson, K.A.; Bruford, M.W.; Coates, D.J.; Elliott, C.P.; Mergeay, J.; Ottewell, K.M.; Segelbacher, G.; Hoban, S.; Hvilsom, C.; et al. Global meta-analysis shows action is needed to halt genetic diversity loss. Nature 2025, 638, 704–710. [Google Scholar] [CrossRef]
- Alaasam, V.J.; Kernbach, M.E.; Miller, C.R.; Ferguson, S.M. The Diversity of Photosensitivity and its Implications for Light Pollution. Integr. Comp. Biol. 2021, 61, 1170–1181. [Google Scholar] [CrossRef]
- Partan, S.; Marler, P. Communication Goes Multimodal. Science 1999, 283, 1272–1273. [Google Scholar] [CrossRef]
- Gomes, D.G.E.; Page, R.A.; Geipel, I.; Taylor, R.C.; Ryan, M.J.; Halfwerk, W. Bats perceptually weight prey cues across sensory systems when hunting in noise. Science 2016, 353, 1277–1280. [Google Scholar] [CrossRef]
- Heuschele, J.; Mannerla, M.; Gienapp, P.; Candolin, U. Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 2009, 20, 1223–1227. [Google Scholar] [CrossRef]
| Environment | Context | Species | Sense Affected | Morphological Changes Observed | References |
|---|---|---|---|---|---|
| Artificial/captive | Conservation breeding | Lord Howe Island stick insect (Dryococelus australis) | Vision | Smaller compound eye | Freelance, Magrath, Elgar and Wong [11] |
| Olfaction | Lower density of olfactory antennal sensilla | ||||
| Aquaculture | Steelhead salmon (Oncorhynchus mykiss) | Mechanoreception, hearing | Fewer lateral line organs | Brown, et al. [16] | |
| Masu salmon (O. mykiss) | Mechanoreception, hearing | Fewer lateral line organs | Nakae, et al. [17] | ||
| Natural | Artificial light at night | Heart and dart moth (Agrotis exclamationis) | Vision | Smaller compound eye (females) | Keinath, et al. [18] |
| Carolina wren (Thryothorus ludovicianus) | Vision | Smaller eye size | Jones, et al. [19] | ||
| Northern cardinal (Cardinalis cardinalis) | Vision | Smaller eye size | Jones, Llamas and Phillips [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freelance, C.B. Anthropogenic Impacts as a Driver of Sensory Organ Morphology. Wild 2025, 2, 17. https://doi.org/10.3390/wild2020017
Freelance CB. Anthropogenic Impacts as a Driver of Sensory Organ Morphology. Wild. 2025; 2(2):17. https://doi.org/10.3390/wild2020017
Chicago/Turabian StyleFreelance, Christopher B. 2025. "Anthropogenic Impacts as a Driver of Sensory Organ Morphology" Wild 2, no. 2: 17. https://doi.org/10.3390/wild2020017
APA StyleFreelance, C. B. (2025). Anthropogenic Impacts as a Driver of Sensory Organ Morphology. Wild, 2(2), 17. https://doi.org/10.3390/wild2020017






