Abstract
Hematopoietic stem cell (HSC) therapy remains essential in treating blood disorders, autoimmune diseases, neurodegenerative conditions, and cancers. Despite its potential, challenges arise from the inherent heterogeneity of HSCs and the complexity of their regulatory niche. Recent advancements in single-cell RNA sequencing and chromatin accessibility sequencing have provided deeper insights into HSC markers and chromatin dynamics, highlighting the intricate balance between intrinsic and extrinsic regulatory mechanisms. Zebrafish models have emerged as valuable tools in HSC research, particularly through live imaging and cellular barcoding techniques. These models have allowed us to describe critical interactions between HSCs and embryonic macrophages, involving reactive oxygen species and calreticulin signaling. These are essential for ensuring HSC quality and proper differentiation, with implications for improving HSC transplant outcomes. Furthermore, the review examines clonal hematopoiesis, with a focus on mutations in epigenetic regulators such as DNMT3A, TET2, and ASXL1, which elevate the risk of myelodysplastic syndromes and acute myeloid leukemia. Emerging technologies, including in vivo cellular barcoding and CRISPR-Cas9 gene editing, are being investigated to enhance clonal diversity and target specific mutations, offering potential strategies to mitigate these risks. Additionally, macrophages play a pivotal role in maintaining HSC clonality and ensuring niche localization. Interactions mediated by factors such as VCAM-1 and CXCL12/CXCR4 signaling are crucial for HSC homing and the stress response, opening new therapeutic avenues for enhancing HSC transplantation success and addressing clonal hematopoiesis. This review synthesizes findings from zebrafish models, cutting-edge sequencing technologies, and novel therapeutic strategies, offering a comprehensive framework for advancing HSC biology and improving clinical outcomes in stem cell therapy and the treatment of hematologic diseases.
1. Introduction
Hematopoietic stem cells (HSCs) have become a cornerstone in the treatment of various blood disorders, autoimmune diseases, neurodegenerative conditions, and cancers. These multipotent cells are characterized by their ability to self-renew and differentiate into various blood lineages, which is facilitated by niche cells, including endothelial and stromal cells. However, the complex regulatory networks governing HSCs, involving transcription factors, microRNAs, cytokines, and growth factors, present significant challenges for therapy development. This review aims to explore the advancements in HSC research, particularly focusing on zebrafish models for HSC quality assessment, the implications of clonal hematopoiesis in stem cell behavior, and emerging therapeutic strategies. Each section contributes to a holistic understanding of HSC regulation, highlighting the latest technologies and research findings that inform future therapeutic applications.
Research on HSCs focuses on their formation within the bone marrow, the primary site of blood cell production [1]. HSCs are distinguished by their capacity for self-renewal and their ability to differentiate into a variety of blood cell types, processes facilitated by niche cells, such as endothelial and stromal cells. Hematopoiesis, the formation of blood cells, is a tightly regulated process influenced by a network of transcription factors (TFs), microRNAs (miRNAs), epigenetic modifications, cytokines, and growth factors. These regulatory elements maintain a delicate balance between self-renewal and differentiation to sustain a stable hematopoietic pool. Misregulation of these processes can lead to various hematologic malignancies, including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), multiple myeloma, and lymphoma. External factors, such as ionization, obesity, and oxidative stress, also impair the hematopoietic niche, affecting HSC longevity and functionality [1].
Technological advancements like single-cell RNA sequencing, multiphoton intravital microscopy, and zebrafish models have significantly enhanced our understanding of hematopoietic development [2,3]. Zebrafish models, in particular, offer unique insights into HSC regulation through live imaging and genetic manipulation. The use of these models in research has shed light on the interactions between HSCs and their microenvironment, providing valuable knowledge about stem cell quality and differentiation.
Bone marrow (BM) tissues are distributed throughout the body, occupying bone cavities that vary in developmental origin, morphology, and the proportion of cortical and trabecular bone. In cortical regions, such as femoral shafts, marrow fills elongated cavities, whereas in trabecular bone, it is interspersed among bony trabeculae [2]. Despite these structural differences, the hematopoietic and immune content across various bones and regions remains remarkably homogeneous, indicating finely tuned systemic regulatory mechanisms that maintain hematopoietic tissue composition [3]. HSCs, although present in low frequencies, along with progenitor cells, generate billions of mature cells daily. The BM serves as a major reservoir for immune regulation, housing mature cells of both the innate and adaptive immune systems, including dendritic cells, macrophages, neutrophils, and various lymphoid cells [2]. The dynamic hematopoietic landscape is organized around a stromal compartment composed of mesenchymal, endothelial, and neural cells. These stromal cells provide structural support and regulate hematopoiesis and bone metabolism [2]. An original theory of niches conceptualized them as discrete anatomical domains within the bone marrow (BM) tissue ecosystem, consisting of highly specialized, interacting cell types that nurture HSCs [3,4]. These niches act as deterministic regulators of cell fate, where stem cell identity is maintained through cellular anchorage, while detachment leads to differentiation or loss of stemness. This framework implies that the number of HSCs in the BM is limited by the availability of niches [4,5]. Advances in immunohistological techniques, 3D microscopy, genetic labeling, and single-cell technologies have revealed the complexity of BM stromal cell biology, highlighting their critical role in hematopoietic regulation [6,7].
In a clinical context, the regulation and application of HSCs have undergone recent advancements, mainly focusing on engineered HSCs, ex vivo expansion, and extracellular vesicles (EVs) [8]. Engineered HSCs aim to achieve sustained self-renewal and controlled differentiation for therapeutic purposes. Efforts include modulating heterochronic genes and utilizing transcription factor (TF) regulation, with promising avenues like LIN28/let-7 miRNA families and small molecules as cost-effective alternatives to cytokines [8]. Zebrafish models offer insights into hematopoiesis, highlighting chromatin modifiers such as Bmi-1 and Ezh1, crucial for HSC maintenance. Despite gene therapy’s success in treating immunodeficiencies like X-SCID and ADA-SCID, the risk of mutagenesis underscores the need for stable, clinically viable HSCs [8]. Ex vivo HSC expansion faces challenges in mimicking the in vivo environment, with Notch signaling and hypoxic conditions improving long-term engraftment [9,10]. Notably, AhR antagonists like SR1 and UM171 show the potential to enhance HSC numbers and maintain a primitive phenotype [10]. Epigenetic modifiers and control of mitochondrial activity further support HSC quiescence and self-renewal. EVs, integral to the hematopoietic niche, contribute to homeostasis and offer biomarkers for diseases like chronic lymphoid leukemia, AML, and myelodysplastic syndromes. Autologous EVs from MSCs, T, and NK cells exhibit therapeutic potential, particularly in immunomodulation and inflammatory disease management. However, further research is needed to optimize organ-specific EV loading and ensure effective clinical application. Overall, a deeper understanding of HSC biology and EV interactions is essential for advancing these therapeutic strategies [8]. Engineered HSCs and controlled ex vivo expansion using EVs could create non-immunogenic HSCs, suitable for therapeutic purposes across histocompatibility barriers without rejection risk. Further comprehension of inflammation-upregulated HSCs and extracellular vesicle (EV) trafficking could reveal new therapies for organ-specific HSC homing.
In recent years, extracellular vesicles (EVs) derived from mesenchymal stromal cells (MSCs) have emerged as promising candidates for cell-free regenerative therapies, especially in the domain of hematopoietic support and immune modulation. These vesicles, which include exosomes and microvesicles, carry a rich cargo of signaling molecules such as proteins, cytokines, mRNAs, and microRNAs, many of which reflect the regenerative and immunoregulatory profile of their parent MSCs [11,12]. Accumulating evidence suggests that MSC-EVs play an essential paracrine role in hematopoietic stem and progenitor cell (HSPC) maintenance, influencing survival, proliferation, and differentiation [11]. In preclinical models, they have been shown to mitigate hematopoietic injury following high-dose irradiation, enhance bone marrow recovery, and reduce inflammatory responses, making them an attractive alternative to whole-cell transplantation, which carries risks such as graft-versus-host disease and ectopic tissue formation [13]. Simultaneously, the zebrafish model system has provided invaluable insights into the real-time behavior of HSPCs within their native niche. Pioneering work by Dr. Leonard Zon and colleagues has demonstrated how embryonic hematopoietic stem cells emerge from the ventral wall of the dorsal aorta and migrate to secondary hematopoietic organs such as the caudal hematopoietic tissue, which is an analog of the fetal liver in humans, before seeding permanent niches in the kidney marrow (Figure 1) [14]. Live imaging and cellular barcoding techniques have elucidated the interactions between HSPCs and neighboring niche cells, such as endothelial cells, stromal fibroblasts, and embryonic macrophages, which regulate stem cell quality and expansion [14]. Given the conservation of hematopoietic signaling pathways between zebrafish and humans, this model is particularly well suited for studying how EVs may be integrated into or influence these microenvironments.
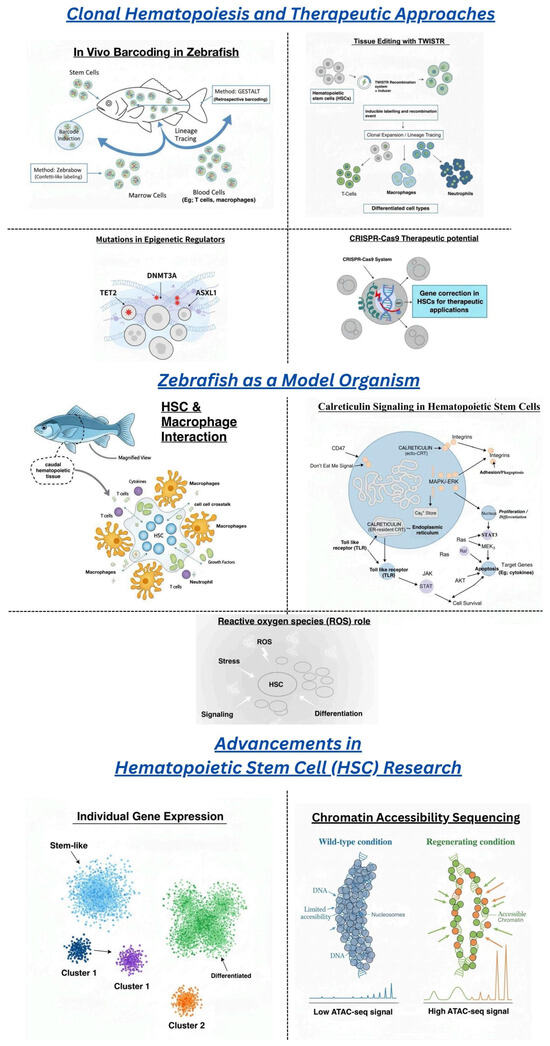
Figure 1.
The figure highlights zebrafish as a model for hematopoietic stem cell (HSC) therapy, showcasing key advancements like single-cell RNA sequencing and chromatin accessibility (ATAC-seq). It emphasizes zebrafish’s roles in HSC-macrophage interactions, reactive oxygen species (ROS), and calreticulin signaling. The figure also explores therapeutic approaches, including epigenetic mutations, stem cell tagging (TWISTR), in vivo cellular barcoding, and CRISPR-Cas9 potential.
The combination of zebrafish in vivo imaging with the therapeutic potential of MSC-EVs opens a novel translational window. MSC-EVs may be further engineered to enhance their regenerative efficacy, for example, by enriching their cargo with antioxidant enzymes to regulate reactive oxygen species (ROS) levels or by targeting niche cells to facilitate HSPC homing and engraftment (Figure 1) [12,13]. This strategy offers a refined approach to hematologic regeneration: one that leverages the molecular specificity of vesicles without the complexity and risk profile of live cell infusion. As the field progresses, integrating these two platforms, zebrafish-based mechanistic studies and MSC-EV-based therapies, may pave the way for optimized treatments in bone marrow failure syndromes, post-transplant recovery, and immune-related hematologic disorders.
Stem cell therapy offers potential for treating various conditions, including blood diseases, autoimmune disorders, neurodegeneration, and cancer [15]. Despite over 30 years of clinical use, HSC transplants remain highly risky. Recent single-cell RNA sequencing (scRNA-Seq) efforts aim to identify novel markers to enrich functional HSCs, but consensus on optimal marker combinations remains elusive due to HSC heterogeneity [16]. Both intrinsic and extrinsic factors regulate HSC function. The stem cell niche, an extrinsic regulator, anchors HSCs and balances self-renewal and differentiation. Soluble signals from the niche, such as interferons, prostaglandins, and growth factors like SCF and G-CSF, influence HSC function during homeostasis and injury (Figure 1) [17]. However, the specificity and heterogeneity of HSC responses to these stimuli are not well understood. Intrinsic regulation also plays a role, with chromatin state being crucial for cell identity and behavior. Hematopoietic differentiation involves significant epigenetic remodeling. Despite knowledge of HSC fate regulators, studies on chromatin states in purified, in vivo-derived HSCs are limited due to technical challenges. Advances in single-cell chromatin accessibility sequencing (scATAC-Seq) now offer a framework to study the diversity and uniqueness of HSC chromatin features at homeostasis and upon external stimulation [17].
2. Zebrafish as a Model for Hematopoietic Stem Cell Research and Drug Discovery
Hematopoietic stem cells [HSCs] formed during embryogenesis are crucial for lifelong tissue homeostasis. Despite their importance, mechanisms for ensuring the quality of these newly formed stem cells are not well documented. To investigate this, researchers studied zebrafish embryonic blood development [1,18]. The zebrafish model is particularly valuable for studying these processes due to its transparent embryos and rapid blood production, with circulating blood forming within 23 h. Transgenic zebrafish, engineered to display every blood cell in different colors, provide an excellent genetic system [1]. Each female zebrafish produces between 100 and 200 offspring weekly, facilitating genetic screens and gene knockdown experiments. Chemicals added to the water can induce phenotypic changes, and the blood program is highly conserved across species. In vertebrates, blood stem cells originate in the dorsal aorta, the largest blood vessel [1,18]. An endothelial cell undergoes a fate change, becoming a blood stem cell. This cell then enters circulation and travels to the tail region of the zebrafish, similar to the fetal liver in humans, where it transmigrates into the niche, doubling the stem cell population. Some stem cells circulate to colonize the marrow, located in the kidney in zebrafish and the bones in humans [1]. Occasionally, these cells bypass the kidney and reach the thymus, initiating the immune system and ensuring lifelong blood production. Within these niches, HSPCs interact with various cell types, including vascular endothelial cells, mesenchymal stromal cells, and macrophages. Using live imaging and cellular barcoding, researchers observed specific interactions between HSCs and embryonic macrophages [1]. These macrophages vet the quality of newly formed HSPCs through prolonged physical contact, leading to either expansion or engulfment. This process is mediated by cell surface calreticulin, which is associated with increased reactive oxygen species (ROS). Elevated ROS levels, while promoting stem cell emergence in the VDA, need to be finely regulated for normal hematopoiesis. Healthy HSPCs with low-to-moderate ROS and calreticulin avoid complete engulfment and respond to pro-proliferative interleukin-1 beta, aiding in marrow colonization [1].
Although previous studies indicated a role for macrophages in HSPC homing and mobilization, some studies found that macrophages in the CHT specifically remove clones with high calreticulin and ROS [19,20]. Recent research suggests that quality assurance mechanisms for stress levels during development impact the clones contributing to blood formation in adulthood [1]. Calreticulin functions as an “eat-me” signal, initiating macrophage–HSPC interaction and leading to programmed cell clearance or stem cell expansion. Interestingly, the orthologs of “don’t-eat-me” signals CD47 and SIRPα have not been identified in zebrafish, indicating that other primitive signals may influence macrophage behavior [21,22,23]. This quality assurance mechanism may also operate in adulthood, responding to environmental stress, marrow transplantation, or clonal stem cell disorders such as myelodysplasia and leukemia. Macrophages may selectively expand or remove clones of tissue-specific stem cells in other systems, similar to the findings in HSPCs. This mechanism is crucial for adequate tissue regeneration, and manipulating it could have significant therapeutic implications for stem cell disorders and tissue regeneration [20].
Recent advancements include creating zebrafish with green fluorescent stem cells using an enhancer from the Runx1 gene, allowing visualization of stem cell origins in the aorta [1,24]. Upon landing in the niche, the stem cell undergoes transmigration outside the blood vessels, with blood vessel cells wrapping around the stem cell in a process called endothelial cuddling. This process is critical for stem cell division, with one stem cell remaining in the niche while the other enters circulation. Electron microscopy revealed that the circulating stem cell attaches to a stromal cell, with its divisions perpendicular to the stromal cell plane [1]. The niche comprises five blood vessel cells, forming a specialized environment. RNA tomography [Tomoseq] has provided insights into the genes adjacent to stem cells within the niche. Endothelial-specific genes, identified through this technique, were predominantly found near the stem cells in the tail region. These endothelial cells form specialized veins known as venous sinusoids. By using promoters of specific genes, such as E-selectin and the mannose receptor, researchers drove green fluorescent protein expression in transgenic fish with red blood vessels, isolating the venous sinusoids. ATAC-seq, a technique to analyze open chromatin regions, revealed open chromatin specific to venous sinusoids. This allowed researchers to isolate small DNA segments, such as a 125-base pair region, responsible for the tissue-specific expression. These findings underscore the importance of the vascular niche in HSC maintenance and function, contributing to a deeper understanding of stem cell biology and therapeutic potential [1]. Another study [25] investigates the transcription factor [TF] code that specifies the vascular endothelial cell [EC] fate within the hematopoietic stem and progenitor cell [HSPC] niche in the bone marrow, a key area for supporting blood formation. By comparing ECs from zebrafish kidney marrow [a primary hematopoietic site] and liver [a non-niche site], the researchers identified TFs uniquely upregulated in marrow ECs, including the mafbb, foxp4, irf8, and hoxb8a. Overexpression of tfec (Transcription Factor EC) and mafbb (MAF bZIP transcription factor B-b, zebrafish paralog of human MAFB) in zebrafish liver sinusoidal ECs resulted in significant upregulation of HSPC niche-supportive genes, such as mrc1a, lyve1b, and dab2. These findings were validated in human iPSC-derived ECs, where overexpression of TFEC (Transcription Factor EC) and MAFB (MAF bZIP transcription factor B) led to the upregulation of similar niche-supportive genes, including CXCL12 (C-X-C motif chemokine ligand 12), PTN (pleiotrophin), and JAG1 (Jagged canonical Notch ligand 1). Functional assays demonstrated enhanced HSPC colony-forming ability when co-cultured with TF-overexpressing ECs. This highlights that a specific TF code that distinguishes marrow ECs from non-niche ECs can offer potential therapeutic targets to enhance HSPC (Hematopoietic Stem and Progenitor Cells) support in clinical settings [25].
According to a study [26], during development, a specific number of hematopoietic stem cell [HSC] clones form and expand to create the adult stem cell pool, balancing self-renewal and differentiation to sustain lifelong hematopoiesis. The transcription factor RUNX1, crucial for definitive hematopoiesis, relies on the cofactor CBFβ for DNA binding stability. Disruptions in RUNX1 or CBFβ reduce clonal diversity. Chemical screening in zebrafish identified Ro5-3335 as a RUNX1 inhibitor that increases HSC divisions and enhances chimerism upon cell transplantation. In human CD34+ cells, Ro5-3335 remodels the RUNX1 complex by binding to ELF1 independently of CBFβ, promoting self-renewal and preventing differentiation by activating specific cell cycles and hematopoietic genes. This study is the first to demonstrate that pharmacological intervention can increase stem cell clone numbers in vivo, revealing new potential treatments to enhance clonal diversity in blood diseases [26].
Drug discovery is costly and laborious, with significant gaps between laboratory and clinical settings hindering development [27]. Zebrafish, sharing drug responses with humans, serve as a powerful preclinical model comparable to mammalian models like mice. This shared pharmacology positions zebrafish as valuable in drug repurposing and discovering new chemical entities [NCEs], potentially leading to new drug classes. Zebrafish can be used to explore drug exposure–response relationships, on-target and adverse effects, and internal exposure, supporting their scalability to other vertebrates. Developing zebrafish pharmacology will improve drug screening protocols, provide insights into drug metabolism, and identify adverse events, enhancing their role in the pharmaceutical industry [27].
The Role of MicroRNAs in Hematopoietic Stem Cell Regulation and Therapy
MicroRNAs (miRNAs) are essential post-transcriptional regulators that profoundly influence hematopoietic stem cell (HSC) biology. Several miRNAs are selectively enriched in long-term HSCs and modulate self-renewal, quiescence, and lineage commitment. For example, the miR-125 family (miR-125a/b) enhances HSC repopulation and confers resistance to apoptosis, although overexpression can predispose to leukemogenesis [28]. miR-126, highly expressed in primitive HSCs, maintains quiescence by dampening PI3K/AKT signaling, ensuring long-term engraftment [29]. In contrast, miR-29a promotes myeloid differentiation and is elevated during HSC aging [30]. Zebrafish models have further illuminated the role of miRNAs during embryonic HSC emergence: miR-142-3p regulates definitive HSC formation by targeting Irf7, while miR-223 limits the overexpansion of hemogenic endothelium, preserving proper niche size and HSPC emergence [31,32]. These findings underscore the conserved role of miRNAs in endothelial-to-hematopoietic transition and niche maintenance.
Beyond development, miRNAs like miR-146a maintain HSC integrity by buffering inflammatory responses; its loss leads to bone marrow failure and increased inflammatory cytokines [33]. Intriguingly, many miRNAs have therapeutic potential. miR-34a, for instance, supports DNA damage response and HSC survival following irradiation [34]. Targeting or mimicking such miRNAs offers a potential strategy for modulating HSC behavior in transplantation, mitigating clonal expansion of mutated HSCs in CHIP, or enhancing hematopoietic recovery. Incorporating miRNA modulation into engineered HSCs or extracellular vesicle strategies could improve the precision of regenerative therapies. Collectively, these findings support miRNAs as both biomarkers and therapeutic tools in HSC-based interventions.
3. Advancements in Understanding Clonality and Hematopoietic Stem Cell Therapies
Clonality is an important factor to consider when working with stem cells, which describes how a population of cells is derived from a single progenitor. In the context of hematopoietic stem cells (HSCs), clonality refers to the expansion of specific HSC clones, which can influence the effectiveness of stem cell therapies. HSC transplants are commonly used to treat various blood disorders; however, they come with significant challenges. One of the primary obstacles is the inflammation of the host niche, which can cause stress to the transplanted cells, leading to limited engraftment capacity and transplant rejection [1,35]. Additionally, only some of the clone cells adapt and contribute to the host following the transplant [1]. Other complications include Graft vs. Host Disease (GvHD), which can occur when the transplanted cells identify the host’s cells as foreign and mount an immune response against them [36]; transplant rejection, which occurs when the host immune system body rejects the transplanted cells and attacks them [37]; and limited engraftment capacity, meaning that the transplanted stem cells are only able to regenerate a limited amount of clones [38]. However, in vivo, cellular barcoding is a newly found research method which could lead to future therapies being investigated. In vivo cellular barcoding comes with significant advantages, such as the lack of need for a transplant, the lack of stress on cells, and the stem cells being in their native niche, and it provides for an accurate assessment of clonality [1]. In vivo, cellular barcoding works by tagging individual cells through unique DNA or mRNA sequences to effectively track them over time and allows for the unique DNA or mRNA sequences to be reproduced throughout the system [39]. To look at and assess these clones, it is required to bleed the specimen or patient monthly and conduct a marrow analysis for an accurate assessment of clonality [1].
Hematopoietic stem cells and progenitor cells (HSPCs) are polyclonal, and many humans, as they age, end up with mutations in epigenetic regulators such as DNMT3A, TET2, and ASXL1. This condition, known as Clonal Hematopoiesis of Indeterminate Potential (CHIP), refers to the presence of these mutations, which do not always lead to immediate health issues but have the potential to progress into more serious blood disorders [1]. CHIP mutations increase the clonal expansion of mutated cells, which, over time, may accumulate additional genetic changes that lead to myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) [1]. This progression is particularly important for clinicians to monitor, as identifying patients with CHIP may allow for early intervention before the onset of these more severe conditions. MDS is a type of blood cancer where the bone marrow fails to produce healthy, mature blood cells, resulting in a low number of functional blood cells [40]. AML is another form of blood cancer where the bone marrow produces abnormal white blood cells, known as myeloblasts, that do not mature, which reduces space for healthy blood cell development [40,41]. Both of these conditions can develop due to the CHIP-associated mutations driving the unchecked replication of defective cells, which outcompete normal blood cells. It was found that approximately 13% of 70-year-olds have mutated stem cells, a figure that increases with age [1]. Since CHIP is associated with inflammation, individuals with this condition are at a higher risk of developing blood clots, heart attacks, or strokes due to the abnormal blood cell shapes impairing normal circulation [1,40]. Given these risks, studying alternative treatment methods to manage CHIP and prevent the progression to MDS, AML, and related cardiovascular issues is crucial for improving patient outcomes.
While zebrafish models provide invaluable insights into HSC quality, understanding clonal hematopoiesis is vital for assessing the long-term behavior of these cells. The study of clonal hematopoiesis offers a lens into stem cell regulation, disease progression, and therapeutic potential.
The study of clonal hematopoiesis highlights the risk of certain genetic mutations driving blood disorders. By understanding the mechanisms underlying clonal diversity and mutation-driven cell expansion, we can inform strategies to mitigate these risks in clinical settings. Clonal hematopoiesis serves as a key aspect of HSC regulation. Understanding the balance between self-renewal and differentiation is crucial for developing targeted therapies, as these mechanisms impact HSC quality and long-term health outcomes. Integrating research on clonal hematopoiesis with insights gained from zebrafish models could help identify therapeutic targets for improved HSC function.
Several systems allow researchers to properly study stem cells and clones [1,42]. Looking specifically at the Zebrabow system, different stem cells can be marked with different colors to appropriately track the clones [1,42]. This is achieved through the utilization of the fluorescent proteins RFP, CFP, and YFP, which are then used to label the stem cells and see how they combine and multiply throughout the system [42]. As cells multiply and combine, the different colors are pronounced and reflect how the recombination and multiplication that has occurred can be viewed using microscopy [42]. The colored stem cells can be tracked later in the peripheral blood as the cells clone and mutate throughout the body to many different tissues and organs [1,42]. It was found that just 24 h following the stem cell barcoding, it was predicted that there would be 21 clones [1]. This system in particular can be used to study clonal hematopoiesis.
Several studies have found that mutant stem cells create mutant macrophages and neutrophils. These mutant cells then produce inflammatory cytokines (IL-1, TNFα), which in turn inflame the bone marrow and ultimately result in preleukemia cells [1,43,44]. One study in particular found that due to the presence of CHIP and these mutated cells producing the inflammatory cytokines, there was nearly double the risk of coronary heart disease in humans [43]. This further emphasized the need for a solution to prevent the further cloning of these CHIP cells [1].
The presence of CHIP and inflammatory cytokines from the mutated cells resulted in nearly double the risk of coronary heart disease [43]. This further corroborates the need for a treatment avenue that prevents further cloning of these CHIP cells [1]. One solution is Tissue Editing With Inducible Stem Cell Tagging Via Recombination (TWISTR). TWISTR is a method that injects guide RNAs via Crisper Cas9 to prevent clonal hematopoiesis by knocking down the clonal hematopoiesis-causing genes. When the cells reach adulthood, the peripheral blood and marrow can be collected and sequenced—which allows for the dominant clone to be sorted out [1,45]. This study showed that in cases of mutation, 60% of the blood can be monoclonal-dominated (with asx11 being the most dominant cluster type) [1,45]. This study was able to confirm that mutant cells were producing inflammatory cytokines. Moreover, it was able to identify that stem cells were producing anti-inflammatory modulators (soc3sa, nr4a1, atf3); these modulators have been shown to confer a survival advantage for the cell—explaining why CHIP primarily impacts older individuals [1,45].
Looking at a specific method, Tissue Editing With Inducible Stem Cell Tagging Via Recombination (TWISTR), guide RNAs are injected with Crisper-Cas9 to initiate a gene knockout of the genes that cause clonal hematopoiesis in humans [1,45]. Once the cells reach adulthood, the peripheral blood and the marrow are collected and sequenced, which allows for the dominant clone to be sorted out [1,45]. It was found in this process that in cases of mutation, 60% of the blood was dominated by one stem cell only, which shows clonal dominance [1,45]. In particular, it was found that typically asxl1 was the dominant stem cell cluster in these mutant cells [1,45]. This study was able to confirm that the mutant cells were producing inflammatory cytokines, but it was also able to identify that stem cells in return were producing anti-inflammatory modulators (socs3a, nr4a1, atf3), which was found to be a survival advantage for the cell, which explains why CHIP tends to impact primarily older individuals [1,45]. It was further found in this study that by removing nr4a1 through knockout, no effect was found on normal stem cells but the size of the clones was significantly reduced. There is more research to be done, but the current research suggests that nr4a1 could be a therapeutic [1,45].
Despite the promise of CRISPR and barcoding techniques, they come with several limitations and risks that must be carefully considered. CRISPR, while powerful, can lead to off-target effects, where the Cas9 protein cuts DNA at unintended locations, potentially causing harmful mutations. Another challenge is the delivery of CRISPR components (Cas9 protein and guide RNA) into target cells, with methods like viral vectors introducing additional risks [46,47]. Furthermore, CRISPR raises ethical concerns, particularly regarding germline editing, which could have long-term, heritable impacts. The immune response to the Cas9 protein is another risk, as the body might recognize it as foreign, reducing treatment efficacy and potentially causing side effects.
While these advancements hold great promise, both CRISPR and barcoding techniques come with limitations. CRISPR, for instance, carries risks such as off-target effects and immune responses to the Cas9 protein [46,47]. Additionally, barcoding techniques can be complex and expensive, limiting their scalability [48]. Nonetheless, continued research in these areas is likely to yield new therapeutic options for managing clonal hematopoiesis and improving HSC transplant outcomes.
4. Macrophages: Essential Regulators of Tissue Repair, Stem Cell Clonality, and Hematopoietic Homeostasis
Macrophages are highly versatile immune cells that play a critical role in defending the body against pathogens and clearing cellular debris. Traditionally recognized for their capacity to phagocytize harmful particles, recent research has significantly broadened our understanding of their diverse functions, including maintaining tissue homeostasis, facilitating tissue repair, and regulating stem cell clonality [49]. These cells exhibit remarkable plasticity, adapting their behavior according to the specific needs of the microenvironment during the tissue repair process. Macrophages progress through distinct phases—initiation, maintenance, and resolution—during this process [50,51]. For instance, following the subsidence of inflammation, macrophages actively contribute to the resolution of inflammation by secreting anti-inflammatory cytokines, such as IL-10 and TGF-β, which promote tissue healing [52]. Moreover, they release various growth factors that stimulate the proliferation, differentiation, and activation of epithelial, endothelial, and stem cells, thereby supporting overall tissue regeneration [50]. A critical aspect of macrophage function is their ability to adopt different phenotypes, commonly described as M1 and M2 polarization [51]. M1 macrophages, or “classically activated” macrophages, are induced by pro-inflammatory signals such as IFN-γ and lipopolysaccharides (LPS). These cells produce inflammatory cytokines and reactive oxygen species that aid in combating infections and initiating tissue repair [53,54]. In contrast, M2 macrophages, or “alternatively activated” macrophages, are stimulated by anti-inflammatory signals, including IL-4 and IL-13. M2 macrophages play an essential role in resolving inflammation and promoting wound healing by secreting anti-inflammatory cytokines and metabolizing arginine, leading to the production of ornithine and polyamines, which further encourage cell growth and tissue regeneration [55,56]. Understanding the balance between M1 and M2 macrophages is critical for developing targeted therapies aimed at modulating immune responses in various diseases. By focusing on macrophage polarization, researchers aim to devise innovative treatments for conditions such as chronic inflammation, tissue injury, and cancer.
Clonality is the preservation of a stem cell population that can self-renew and differentiate into progeny. Clonal hematopoiesis (CH) is defined by HSCs and differentiated progeny spreading into the blood cell population [57]. HSCs and hematopoietic stem and progenitor cells (HSPCs) are the parent cells that are found in the bone marrow. HSC and HSPC differentiation is controlled by growth factors and cytokines released by cells in the bone marrow niche.
HSPCs are cells within the bone marrow that differentiate into various types of blood cells [58,59]. Macrophages remove material from HSPCs, causing HSPCs to die or divide. Macrophages phagocytose HSCs that are undergoing apoptosis or have high levels of ROS. High levels of ROS potentially indicate cellular damage; macrophages remove them to ensure that only healthy HSCs will differentiate into blood cells [60]. Dysfunctional HSCs can lead to hematological disorders (e.g., leukemia); macrophages prevent it [61]. On the other hand, macrophages induce HSC differentiation by remodeling the niche. A stem cell niche is a specialized microenvironment in which stem cells receive stimuli that determine their fate: engulfment, differentiation, etc. [62]. Macrophages exist within various bone marrow niches. CXCL12 transcription is increased when macrophages are present. CXCL12 is crucial for HSC retention in the niche; therefore, if macrophages are not present, then HSCs will withdraw from their respective niche [63].
One method employed by researchers to study macrophage–HSC interactions is irf8 knockdown. Irf8 (Interferon Regulatory Factor 8) is a transcription factor that plays a vital role in the development and function of macrophages and dendritic cells [64]. The irf8 protein has a weak DNA-binding affinity and requires a DNA-binding domain with an IRF association domain (IAD). Without the IAD, irf8 will be unable to form a transcription complex. Irf8 is crucial for HSC differentiation of various myeloid cells: macrophages and dendritic cells. Irf8 function is largely controlled by the expression of interacting protein counterparts (PU.1). HSC differentiation occurs because irf8 contributes to dendritic cell or lymphoid differentiation via the creation of distinct heterodimers [65]. The heterodimerization between irf8 and its transcription factors (e.g., PU.1) results in the upregulation of genes involved in monocyte differentiation [66]. Shia et al. successfully knocked down irf8 by adding a morpholino (morpholino oligonucleotide that prevents mRNA translation) into zebrafish [67]. Zebrafish with knocked-down IRF8 did not have an embryonic macrophage population. Zebrafish are often chosen as a medium of study for experiments involving gene knockdown, as Zebrafish DNA can be altered easily via gene knockdown with morpholinos; they also contain transparent embryos, providing live imaging of cellular imaging and interactions [68]. When macrophages are removed via irf8 knockdown, the number of HSCs clones is less than its sibling-control group [69]. The reduction in HSC clones compared to the control group highlights the importance macrophages play in maintaining stem cell clonality. Also, the reduction reiterates that macrophages help sustain HSC populations.
Macrophages affect stem cells during mammalian embryogenesis by regulating HSC and HSPC homing. Vascular cell adhesion molecule-1 (VCAM-1) macrophages are necessary for the HSPC homing to its respective vascular niche [63]. HSPCs that enter from the intersegmental vessel or caudal venous plexus always flow into caudal hematopoietic tissue while passing the capillary confluence point, leading to frequent interactions between VCAM-1 macrophages and HSPCs. Li et al. showed that 40% of HSPCs would remain in the caudal hematopoietic tissue because of VCAM-1 macrophage assistance; in other words, macrophages play a vital role in HSPC retention [63]. VCAM-1 assists with homing by guiding lodged HSPCs from the dorsal venous capillaries to the venous capillaries. Once HSPCs get to their respective niche, interactions between VCAM-1 and B1 integrin subunits anchor HSPCs to endothelial cells [70]. Potocnick et al. showed that in cell culture with reduced B1 integrin subunits, fewer HSPCs remained anchored to endothelial cells [71]. Proper stem cell homing is vital for effective hematopoiesis and immune system development; if HSPCs cannot get to their specific niche, they cannot receive signals for maintenance, self-renewal, and differentiation.
Chemokine signaling of CXCL12/CXCR4 exemplifies the importance of HSPC-macrophage interactions in niche localization. CXCL12 is synthesized in the bone marrow and creates a gradient that guides HSPCs to their respective niches via the CXCR4 receptor. CXCR4 activation leads to the recruitment of intracellular G-proteins, initiating several signaling cascades, including the Akt (also known as Protein Kinase B, PKB) and MAPK (mitogen-activated protein kinase) pathways [72,73]. These pathways regulate HSPC survival and polarity. Priming factors (i.e., cationic antimicrobial peptides (CAMPs) such as C3a, cathelicidin (LL-37), and β2-defensin) activate complement cascades that sensitize HSPCs to the CXCL12 gradient [59]. HSPCs migrate away from the area of high concentrations (stromal cells in the bone marrow niche) via the extracellular matrix and interstitial fluid. HSPCs sense the gradient through their CXCR4 receptors; receptor activation is higher on the side of the cell with a higher CXCL12 concentration [74]. The differential receptor activation results in HSPC polarization and niche localization. The Golgi apparatus plays a vital role in cell polarity as it regulates the centrosome and leading edge of a migrating HSPC [75]. When CXCL12 binds to CXCR4, Rac1 promotes polymerization of actin filaments near the cell membrane [76]. The leading edge (lamellipodia and filopodia) protrudes toward the direction with higher chemokine concentration—concerning the CXCL12 gradient—and pushes the cell in its respective direction [77]. On the opposite side of the cell, RhoA forms actin-myosin contractile fibers, which retract the trailing end as the HSPC moves forward. Deletion of the CXCR4 gene in the bone marrow niche resulted in loss of HSPC retention, quiescence, and repopulation [78]. Furthermore, Sugiyama et al. showed that induced deletion of CXCR4 resulted in a drastic reduction in HSC numbers in the bone marrow niche [79]. If CXCR4 is not present, then the CXCL12 gradient will have limited effect; without the gradient’s influence, migrating HSPCs will lack a direction to orient their leading edges and will be unable to localize to their respective niche. Macrophages release HMGB1, a versatile protein that functions both as a nuclear protein and an extracellular signaling molecule [80]. HMGB1 forms a heterocomplex with CXCL12, enhancing CXCL12’s presentation to CXCR4 receptors and enhancing the signaling efficiency of CXCL12 [59]. Increased CXCL12 signaling efficiency would likely lead to increased HSPC niche localization efficiency [79] (Figure 2).
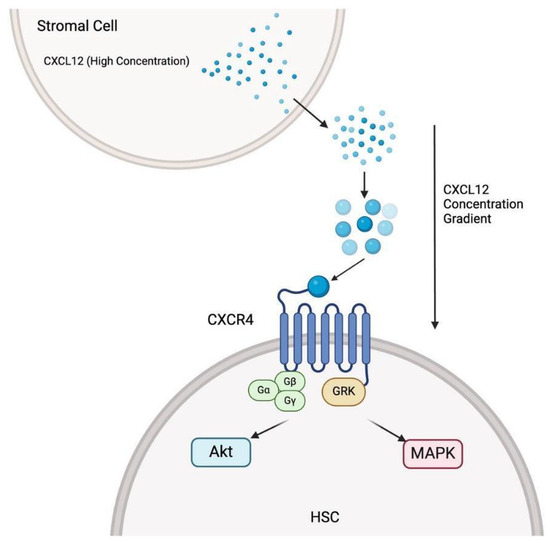
Figure 2.
The stromal cells in the bone marrow secrete high concentrations of CXCL12, establishing a concentration gradient that attracts HSCs via CXCL12/CXCR4 binding. The binding triggers downstream signaling pathways (i.e., Akt and MAPK). The signaling pathways are vital for HSC (hematopoietic stem cell) localization, survival, and migration.
HMOX1 (heme oxygenase-1) has been clinically proven to be an effective marker for myeloid cells, especially when they are under oxidative stress [81]. Alaluf et al. showed the genes that were upregulated in cells that were expressing HMOX1 were APOEI, FTH, or FTLI. The listed genes are commonly expressed in TAMs (tumor-associated macrophages). By evaluating the HMOX1 expression, researchers determined that HMOX1 is a crucial feature of macrophages in the tumor microenvironment [82]. As a cellular adaptive response, HMOX1 is upregulated in the presence of ROS compounds. Thus, HMOX1 as a marker holds more promise for macrophage populations under oxidative stress: classically activated macrophages, TAMs, inflammatory macrophages, and monocyte-derived macrophages [83,84,85,86].
Calreticulin (CALR) is an endoplasmic reticulum (ER) protein that can migrate to the cell membrane of stem cells and become an apoptotic cell clearance signal [1,87]. The calreticulin interacts with LRP1 receptors on macrophages. The CALR KDEL sequence serves as a retention signal for the ER [88]. Wattrus et al. knocked down calr3a and cal3b and substantially reduced the amount of HSPC-macrophage interactions. The study showed that HSPCs with overexpressed CALR had more macrophage interaction than the control group [58]. Calreticulin, therefore, is an important mediator of the interactions between HSPC and macrophages.
Reactive oxygen species (ROS) levels can affect HSC niche localization. ROS results in increased calreticulin levels on the cell membrane of stem cells. When CALR reacts with LRP1 receptors, it causes macrophages to phagocytose the stem cell. High levels of ROS lead to a state of “doom” in HSPCs: cytotoxic ROS levels are detrimental to stem cell populations; thus, the increased CALR marks the ROS-rich cell for phagocytosis. Macrophages produce and secrete IL-1 when medium ROS levels are detected in stem cells. Interleukin-1 (IL-1) binds to the IL-1r receptor on HSCs, activating the MAPK pathway [89]. In the context of quality assurance, MAPK leads to cell proliferation and differentiation [1,90].
With the help of macrophages, quiescent HSCs remain dormant in hypoxic regions of the bone marrow. Under high-stress conditions within the bone marrow, macrophages exhibit elevated levels of cyclooxygenase-2 (COX-2), a key regulator of HSC quiescence [88]. COX-2 catalyzes the production of prostaglandin E2 (PGE2), which plays a significant role in the maintenance of quiescent HSC by inhibiting the Akt pathway and preventing intracellular ROS production in HSC [60]. Juntilla et al. showed that Akt1- and Akt2-deficient HSCs had decreased ROS levels [91]. This reduction in ROS impairs the HSC’s ability to differentiate and maintain HSC quiescence. Increased ROS levels, on the other hand, lead to activation of the MAPK pathway and increased sensitivity to the previously mentioned CXCL12 gradient [92]. Higher levels of ROS lead to increased CXCL12 levels, which in turn enhances the gradient.
Macrophages are indispensable as they maintain HSC function and homeostasis. Macrophages modulate inflammation and facilitate tissue repair. Healthy and successful hematopoiesis cannot be performed without macrophages, as they model niches, secrete cytokines, and interact with HSCs. Targeting macrophage-HSC/HSPC interaction is a promising avenue of study that can improve patient outcomes by enhancing cell function and treating diseases like leukemia.
5. Conclusions and Future Perspectives
HSCs are pivotal for blood formation and offer immense therapeutic potential. This review has explored the intricate regulatory mechanisms of HSCs, emphasizing their interactions within the bone marrow niche, and the implications of clonal hematopoiesis on disease progression. Advances in zebrafish models have deepened our understanding of stem cell quality control while emerging tools like CRISPR and cellular barcoding pave the way for enhancing clonal diversity and improving stem cell transplant outcomes.
Despite these advancements, challenges remain. Future research should focus on elucidating the molecular crosstalk between HSCs and their niche, particularly in the context of inflammation and therapeutic interventions. Additionally, developing pharmacological strategies to mitigate the expansion of mutant clones, especially those associated with clonal hematopoiesis, will be key. By addressing these areas, we can advance stem cell biology, optimizing the therapeutic potential of HSCs for hematologic diseases.
HSCs are fundamental for blood formation and hold immense therapeutic promise. This review has explored the complex regulatory mechanisms that govern HSC function, including transcription factors, microRNAs, and epigenetic modifications. The bone marrow niche, with its intricate cellular interactions, emerges as a critical environment for maintaining HSC function, emphasizing the need to decode these dynamics for future therapeutic applications. Recent technological advancements, such as single-cell RNA sequencing and multiphoton intravital microscopy, have greatly enhanced our understanding of hematopoiesis, offering new possibilities for targeted therapeutic interventions and heralding a new era in stem cell research [93,94].
Additionally, the review has underscored the pivotal roles of endothelial and stromal cells in HSC regulation and the impact of extrinsic factors like ionization and obesity on HSC function. Zebrafish models have further expanded our insights into blood stem cell development and macrophage interactions, presenting promising therapeutic avenues for blood disorders. Emerging findings on clonal hematopoiesis, especially its relationship with aging and disease progression, provide new opportunities for the development of therapies aimed at targeting clonal hematopoiesis. Advanced techniques like in vivo cellular barcoding and CRISPR-mediated gene editing hold vast potential for treating conditions such as clonal hematopoiesis of indeterminate potential (CHIP), myelodysplastic syndromes, and leukemia [95,96].
Despite these advancements, significant challenges persist. Our understanding of the exact roles of transcription factors and microRNAs in HSC regulation—especially in therapeutic contexts—is still incomplete [97]. Furthermore, the influence of factors such as inflammation and extracellular vesicle trafficking on HSC behavior and stem cell therapy outcomes demands deeper investigation. Future research should focus on deciphering the molecular crosstalk between HSCs and their niche, which will be critical in optimizing stem cell therapies [98]. Additionally, developing more specific markers for identifying functional HSCs will enable more accurate targeting and monitoring in both research and clinical applications.
Looking ahead, pharmacokinetic models in zebrafish and other systems will be crucial in addressing the gap between preclinical findings and clinical applications, ensuring that therapies are both safe and effective. Identifying novel pharmacological targets to mitigate the expansion of mutant stem cell clones, particularly those associated with inflammatory conditions like CHIP—which elevate the risk of cardiovascular disease and leukemia—will also be a key area of future research [99].
By addressing these areas, we can advance the field of stem cell biology and exploit HSCs’ full therapeutic potential. Continued research, together with innovative technologies, will lead to groundbreaking treatments, revolutionizing hematology and regenerative medicine.
Author Contributions
Conceptualization: V.C., R.H.A. and H.C. Methodology: V.C., R.H.A. and H.C. Formal Analysis: V.C., R.H.A., H.C., J.G., A.T., C.H.P. and Y.L. Investigation: V.C., R.H.A., H.C., J.G., A.T., C.H.P., and Y.L. Resources: V.C., R.H.A., H.C., J.G., A.T., C.H.P. and Y.L. Data Curation: V.C., R.H.A., H.C., A.T., C.H.P. and Y.L. Writing—Original Draft Preparation: V.C., R.H.A., H.C., J.G., A.T., C.H.P. and Y.L. Writing—Review and Editing: V.C., R.H.A. Visualization: V.C., R.H.A., H.C., J.G., A.T., C.H.P. and Y.L. Supervision: V.C., C.H.P. and Y.L. Project Administration: V.C., C.H.P. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No patient data was directly utilized in this study.
Acknowledgments
We thank Leonard Zon for the opportunity to learn from a global leader in Medicine. We are grateful to be part of MedNews Week. We would like to express our sincere gratitude to Jill Gregory for her assistance in significantly improving the figures of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zon, L. Stem Cell Clonality and the Niche. In Proceedings of the MedNews Week Keynote Conference, Online, 17 November 2021; Available online: https://www.youtube.com/watch?v=GjbpBZRL2rA&t=262s (accessed on 1 August 2024).
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), S131–193. [Google Scholar] [CrossRef]
- Pereira, A.L.; Galli, S.; Nombela-Arrieta, C. Bone marrow niches for hematopoietic stem cells. HemaSphere 2024, 8, e133. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulou, T.; Scadden, D.T. Stem-cell ecology and stem cells in motion. Blood 2008, 111, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R. The Relationship Between the Spleen Colony-Forming Cell and the Hemopoietic Stem Cell. Blood Cells 1978, 4, 7–25. Available online: https://pubmed.ncbi.nlm.nih.gov/747780/ (accessed on 1 August 2024). [PubMed]
- Sánchez-Lanzas, R.; Kalampalika, F.; Ganuza, M. Diversity in the bone marrow niche: Classic and novel strategies to uncover niche composition. Br. J. Haematol. 2022, 199, 647–664. [Google Scholar] [CrossRef]
- Gomariz, A.; Isringhausen, S.; Helbling, P.M.; Nombela-Arrieta, C. Imaging and spatial analysis of hematopoietic stem cell niches. Ann. New York Acad. Sci. 2019, 1466, 5–16. [Google Scholar] [CrossRef]
- Mann, Z.; Sengar, M.; Verma, Y.K.; Rajalingam, R.; Raghav, P.K. Hematopoietic stem cell factors: Their functional role in self-renewal and clinical aspects. Front. Cell Dev. Biol. 2022, 10, 664261. [Google Scholar] [CrossRef]
- Papa, L.; Djedaini, M.; Hoffman, R. Ex vivo HSC expansion challenges the paradigm of unidirectional human hematopoiesis. Ann. New York Acad. Sci. 2020, 1466, 39–50. [Google Scholar] [CrossRef]
- Araki, D.; Fu, J.F.; Huntsman, H.; Cordes, S.; Seifuddin, F.; Alvarado, L.J.; Cheruku, P.S.; Cash, A.; Traba, J.; Li, Y.; et al. NOTCH-mediated ex vivo expansion of human hematopoietic stem and progenitor cells by culture under hypoxia. Stem Cell Rep. 2021, 16, 2336–2350. [Google Scholar] [CrossRef]
- Batsali, A.K.; Georgopoulou, A.; Mavroudi, I.; Matheakakis, A.; Pontikoglou, C.G.; Papadaki, H.A. The role of bone marrow mesenchymal stem cell derived extracellular vesicles (MSC-EVs) in normal and abnormal hematopoiesis and their therapeutic potential. J. Clin. Med. 2020, 9, 856. [Google Scholar] [CrossRef]
- Sarvar, D.P.; Effatpanah, H.; Akbarzadehlaleh, P.; Shamsasenjan, K. Mesenchymal stromal cell-derived extracellular vesicles: Novel approach in hematopoietic stem cell transplantation. Stem Cell Res. Ther. 2022, 13, 202. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Pereira, M.; Del Tatto, M.; Quesenberry, P. Mesenchymal stem cell-derived extracellular vesicles improve survival and enhance hematopoietic recovery in mice exposed to high-dose irradiation. Stem Cells Dev. 2025, 34, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Tamplin, O.J.; Durand, E.M.; Carr, L.A.; Childs, S.J.; Hagedorn, E.J.; Li, P.; Yzaguirre, A.D.; Speck, N.A.; Zon, L.I. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 2015, 160, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Daley, G.Q. Stem cells in the treatment of disease. New Engl. J. Med. 2019, 380, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fraticelli, A.E.; Weinreb, C.; Wang, S.-W.; Migueles, R.P.; Jankovic, M.; Usart, M.; Klein, A.M.; Lowell, S.; Camargo, F.D. Single-cell lineage tracing unveils a role for TCF15 in haematopoiesis. Nature 2020, 583, 585–589. [Google Scholar] [CrossRef]
- Fast, E.M.; Sporrij, A.; Manning, M.; Rocha, E.L.; Yang, S.; Zhou, Y.; Guo, J.; Baryawno, N.; Barkas, N.; Scadden, D.; et al. External signals regulate continuous transcriptional states in hematopoietic stem cells. Elife 2021, 10, e66512. [Google Scholar] [CrossRef]
- Chen, A.T.; Zon, L.I. Zebrafish blood stem cells. J. Cell. Biochem. 2009, 108, 35–42. [Google Scholar] [CrossRef]
- Chow, A.; Lucas, D.; Hidalgo, A.; Mendez-Ferrer, S.; Hashimoto, D.; Scheiermann, C.; Battista, M.; Leboeuf, M.; Prophere, C.; Rooijen, N.V.; et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011, 208, 261–271. [Google Scholar] [CrossRef]
- Travnickova, J.; Chau, V.T.; Julien, E.; Mateos-Langerak, J.; Gonzalez, C.; Lelievre, E.; Lutfalla, G.; Tavian, M.; Kissa, K. Primitive macrophages control HSPC mobilization and definitive hematopoiesis. Nat. Commun. 2015, 6, 6227. [Google Scholar] [CrossRef]
- Feng, M.; Chen, J.Y.; Weissman-Tsukamoto, R.; Volkmer, J.-P.; Ho, P.Y.; McKenna, K.M.; Cheshire, S.; Zhang, M.; Guo, N.; Gip, P.; et al. Macrophages eat cancer cells using their own calreticulin as a guide: Roles of TLR and Btk. Proc. Natl. Acad. Sci. USA 2015, 112, 2145–2150. [Google Scholar] [CrossRef]
- Feng, M.; Marjon, K.D.; Zhu, F.; Weissman-Tsukamoto, R.; Levett, A.; Sullivan, K.; Kao, K.S.; Markovic, M.; Bump, P.A.; Jackson, H.M.; et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat. Commun. 2018, 9, 3194. [Google Scholar] [CrossRef]
- Chao, M.P.; Jaiswal, S.; Weissman-Tsukamoto, R.; Alizadeh, A.A.; Gentles, A.J.; Volkmer, J.; Weiskopf, K.; Willingham, S.B.; Raveh, T.; Park, C.Y.; et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2010, 2, 63ra94. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.E.; Galloway, J.L.; Smith, A.C.H.; Keefe, M.D.; Cashman, T.J.; Paik, E.J.; Mayhall, E.A.; Amsterdam, A.H.; Zon, L.I. A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood 2009, 113, 5776–5782. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Gunage, R.D.; Jing, R.; Corbin, A.F.; Chen, K.Z.M.; Tang, Y.; Stockard, O.; Yang, S.; Zhou, Y.; Daley, G.Q.; et al. In Vivo Reprogramming of Adult Liver Sinusoidal Vascular Endothelial Cells into a Hematopoietic Stem and Progenitor Cell Niche. Blood 2023, 142 (Suppl. 1), 4071. [Google Scholar] [CrossRef]
- Robertson, A.L.; Yue, L.; Choudhuri, A.; Kubaczka, C.; Wattrus, S.J.; Mandelbaum, J.; Avagyan, S.; Yang, S.; Freeman, R.J.; Chan, V.; et al. Hematopoietic stem cell division is governed by distinct RUNX1 binding partners. bioRxiv 2024. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Ooi, A.G.L.; Sahoo, D.; Adorno, M.; Wang, Y.; Weissman, I.L.; Park, C.Y. MicroRNA-125b expands hematopoietic stem cells and enriches for the lymphoid-balanced and lymphoid-biased subsets. Proc. Natl. Acad. Sci. USA 2010, 107, 21505–21510. Available online: http://www.jstor.org/stable/25756914 (accessed on 1 August 2024). [CrossRef]
- Guo, S.; Lu, J.; Schlanger, R.; Zhang, H.; Wang, J.Y.; Fox, M.C.; Purton, L.E.; Fleming, H.H.; Cobb, B.; Merkenschlager, M.; et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc. Natl. Acad. Sci. USA 2010, 107, 14229–14234. [Google Scholar] [CrossRef]
- Han, Y.; Park, C.Y.; Bhagat, G.; Zhang, J.; Wang, Y.; Fan, J.; Liu, M.; Zou, Y.; Weissman, I.L.; Gu, H. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J. Exp. Med. 2010, 207, 475–489. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, L.; Lozano-Gil, J.M.; Lachaud, C.; Mesa-Del-Castillo, P.; Cayuela, M.L.; García-Moreno, D.; Pérez-Oliva, A.B.; Mulero, V. Zebrafish Models to Study Inflammasome-Mediated Regulation of Hematopoiesis. Trends Immunol. 2020, 41, 1116–1127. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; He, Q.; Gao, J.; Gao, Y.; Liu, B.; Liu, F. miR-142-3p regulates the formation and differentiation of hematopoietic stem cells in vertebrates. Cell Res. 2013, 23, 1356–1368. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.L.; Rao, D.S.; Boldin, M.P.; Taganov, K.D.; O’Connell, R.M.; Baltimore, D. NF-κB dysregulation in microRNA-146a–deficient mice drives the development of myeloid malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 9184–9189. [Google Scholar] [CrossRef]
- Zeng, H.; Hu, M.; Lu, Y.; Zhang, Z.; Xu, Y.; Wang, S.; Chen, M.; Shen, M.; Wang, C.; Chen, F.; et al. MicroRNA 34a promotes ionizing radiation–induced DNA damage repair in murine hematopoietic stem cells. FASEB J. 2019, 33, 8138–8147. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M. Inflammation: A key regulator of hematopoietic stem cell fate in health and disease. Blood 2017, 130, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Graft, vs. Host Disease. Available online: https://my.clevelandclinic.org/health/diseases/10255-graft-vs-host-disease-an-overview-in-bone-marrow-transplant (accessed on 1 August 2024).
- Transplant Rejection. Available online: https://uvahealth.com/services/transplant/transplant-rejection (accessed on 1 August 2024).
- Cilloni, D.; Carlo-Stella, C.; Falzetti, F.; Sammarelli, G.; Regazzi, E.; Colla, S.; Rizzoli, V.; Aversa, F.; Martelli, M.F.; Tabilio, A. Limited engraftment capacity of bone marrow-derived mesenchymal cells following T-cell-depleted hematopoietic stem cell transplantation. Blood 2000, 96, 3637–3643. [Google Scholar] [CrossRef] [PubMed]
- Kebschull, J.M.; Zador, A.M. Cellular barcoding: Lineage tracing, screening and beyond. Nat. Methods 2018, 15, 871–879. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Acute Myeloid Leukemia Treatment. Available online: https://www.cancer.gov/types/leukemia/patient/adult-aml-treatment-pdq (accessed on 1 August 2024).
- Pan, Y.A.; Freundlich, T.; Weissman, T.A.; Schoppik, D.; Wang, X.C.; Zimmerman, S.; Ciruna, B.; Sanes, J.R.; Lichtman, J.W.; Schier, A.F. Zebrabow: Multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development 2013, 140, 2835–2846. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shavartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Fuster, J.J.; Maclauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.-L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef]
- Avagyan, S.; Henninger, J.E.; Mannherz, W.P.; Mistry, M.; Yoon, J.; Yang, S.; Weber, M.C.; Moore, J.L.; Zon, L.I. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 2021, 374, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C. CRISPR-Cas9: What Are the Pros and Cons? Available online: https://www.idtdna.com/pages/education/decoded/article/crispr-cas9-what-are-the-pros-and-cons (accessed on 1 August 2024).
- Rasul, M.F.; Hussen, B.M.; Salihi, A.; Ismael, B.S.; Jalal, P.J.; Zanichelli, A.; Jamali, E.; Baniahmad, A.; Ghafouri-Fard, S.; Basiri, A.; et al. Strategies to Overcome the Main Challenges of the Use of CRISPR/Cas9 as a Replacement for Cancer Therapy. Mol. Cancer 2022, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, J.; Ge, S.; Lai, L. CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research. Front. Med. 2021, 8, 649896. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef]
- Yu, Y.; Yue, Z.; Xu, M.; Zhang, M.; Shen, X.; Ma, Z.; Li, J.; Xie, X. Macrophages play a key role in tissue repair and regeneration. PeerJ 2022, 10, e14053. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 Macrophages Derived from THP-1 Cells Differentially Modulate the Response of Cancer Cells to Etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Schaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophase Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage Polarisation: An Immunohistochemical Approach for Identifying M1 and M2 Macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef]
- Goldman, E.A.; Spellman, P.T.; Agarwal, A. Defining clonal hematopoiesis of indeterminate potential: Evolutionary dynamics and detection under aging and inflammation. Cold Spring Harb. Mol. Case Stud. 2023, 9, a006251. [Google Scholar] [CrossRef] [PubMed]
- Wattrus, S.J.; Smith, M.L.; Rodrigues, C.P.; Hagedorn, E.J.; Kim, J.W.; Budnik, B.; Zon, L.I. Quality assurance of hematopoietic stem cells by macrophages determines stem cell clonality. Science 2022, 377, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Serwin, K.; Schneider, G. Innate immunity derived factors as external modulators of the CXCL12-CXCR4 axli, Library is and their role in stem cell homing and mobilization. Theranostics 2013, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef]
- Colom Díaz, P.A.; Mistry, J.J.; Trowbridge, J.J. Hematopoietic stem cell aging and leukemia transformation. Blood 2023, 142, 533–542. [Google Scholar] [CrossRef]
- Ferraro, F.; Celso, C.L.; Scadden, D. Adult stem cels and their niches. Adv. Exp. Med. Biol. 2010, 695, 155–168. [Google Scholar] [CrossRef]
- Li, D.; Xue, W.; Li, M.; Dong, M.; Wang, J.; Wang, X.; Li, X.; Chen, K.; Zhang, W.; Wu, S.; et al. VCAM-1+ macrophages guide the homing of HSPCs to a vascular niche. Nature 2018, 564, 119–124. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. (n.d.). IRF8 Interferon Regulatory Factor 8 [Homo Sapiens (Human)]—Gene—NCBI. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/3394 (accessed on 1 August 2024).
- Moorman, H.R.; Reategui, Y.; Poschel, D.B.; Liu, K. IRF8: Mechanism of Action and Health Implications. Cells 2022, 11, 2630. [Google Scholar] [CrossRef]
- Ahamed, F.; Eppler, N.; Jones, E.; He, L.; Zhang, Y. Small Heterodimer Partner Modulates Macrophage Differentiation during Innate Immune Response through the Regulation of Peroxisome Proliferator Activated Receptor Gamma, Mitogen-Activated Protein Kinase, and Nuclear Factor Kappa B Pathways. Biomedicines 2023, 11, 2403. [Google Scholar] [CrossRef]
- Shiau, C.E.; Kaufman, Z.; Meireles, A.M.; Talbot, W.S. Differential requirement for irf8 information of embryonic and adult macrophages in zebrafish. PLoS ONE 2015, 10, e0117513. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Karchner, S.I.; Hahn, M.E. Gene Knockdown by Morpholino-Modified Oligonucleotides in the Zebrafish (Danio rerio) Model: Applications for Developmental Toxicology; Methods in Molecular Biology; Clifton, N.J., Ed.; Humana: Totowa, NJ, USA, 2012; Volume 889, pp. 51–71. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Moore, J.B., IV; Uchida, S. A novel macrophage subtype directs hematopoietic stem cell homing and retention. Ann. Transl. Med. 2019, 7 (Suppl. 3), S79. [Google Scholar] [CrossRef]
- Chiu, Y.G.; Aljitawi, O.S. VCAM-1+ macrophages usher hematopoietic stem and progenitor cell to vascular niche “hotspots”. Ann. Transl. Med. 2019, 7 (Suppl. 3), S116. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, A.J.; Brakebusch, C.; Fässler, R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 2000, 12, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Mestas, J.; Gharaee-Kermani, M.; Burdick, M.D.; Sica, A.; Belperio, J.A.; Keane, M.P.; Strieter, R.M. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J. Biol. Chem. 2005, 280, 22473–22481. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, Z.; Ma, L.; Pei, G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J. Biol. Chem. 2002, 277, 49212–49219. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. (n.d.). CXCR4 C-X-C Motif Chemokine Receptor 4 [Mus Musculus (House Mouse)]—Gene—NCBI. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/12767 (accessed on 1 August 2024).
- Florian, M.C.; Geiger, H. Concise review: Polarity in stem cells, disease, and aging. Stem Cells 2010, 28, 1623–1629. [Google Scholar] [CrossRef]
- García-Cuesta, E.M.; Rodríguez-Frade, J.M.; Gardeta, S.R.; D’Agostino, G.; Martínez, P.; Soler Palacios, B.; Cascio, G.; Wolf, T.; Mateos, N.; Ayala-Bueno, R.; et al. Altered CXCR4 dynamics at the cell membrane impairs directed cell migration in WHIM syndrome patients. Proc. Natl. Acad. Sci. USA 2022, 119, e2119483119. [Google Scholar] [CrossRef]
- Garner, R.M.; Theriot, J.A. Leading edge maintenance in migrating cells is an emergent property of branched actin network growth. eLife 2022, 11, e74389. [Google Scholar] [CrossRef]
- Singh, P.; Mohammad, K.S.; Pelus, L.M. CXCR4 expression in the bone marrow microenvironment is required for hematopoietic stem and progenitor cell maintenance and early hematopoietic regeneration after myeloablation. Stem Cells 2020, 38, 849–859. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Yang, H.; Antoine, D.J.; Andersson, U.; Tracey, K.J. The many faces of HMGB1: Molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 2013, 93, 865–873. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. (n.d.-a). HMOX1 Heme Oxygenase 1 [Homo Sapiens (Human)]—Gene—NCBI. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/3162 (accessed on 1 August 2024).
- Alaluf, E.; Vokaer, B.; Detavernier, A.; Azouz, A.; Splittgerber, M.; Carrette, A.; Boon, L.; Libert, F.; Soares, M.; Le Moine, A.; et al. Heme oxygenase-1 orchestrates the immunosuppressive program of tumor-associated macrophages. JCI Insight 2020, 5, e133929. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.B.; Greten, F.R. Immune cell—Produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef] [PubMed]
- De Groot, L.E.S.; Van Der Veen, A.; Martinez, F.O.; Hamann, J.; Lutter, R.; Melgert, B.N. Oxidative stress and macrophages: Driving forces behind exacerbations of asthma and chronic obstructive pulmonary disease? Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L369–L384. [Google Scholar] [CrossRef]
- Huang, X.; He, C.; Hua, X.; Kan, A.; Mao, Y.; Sun, S.; Duan, F.; Wang, J.; Huang, P.; Li, S. Oxidative stress induces monocyte-to-myofibroblast transdifferentiation through p38 in pancreatic ductal adenocarcinoma. Clin. Transl. Med. 2020, 10, e41. [Google Scholar] [CrossRef]
- Goicoechea, S.M.; Murphy-Ullrich, J.E. Cell surface calreticulin: Role in signaling thrombospondin anti-adhesive activity. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6333/ (accessed on 1 August 2024).
- Seyfried, A.N.; Maloney, J.M.; MacNamara, K.C. Macrophages Orchestrate Hematopoietic Programs and Regulate HSC Function During Inflammatory Stress. Front. Immunol. 2020, 11, 1499. [Google Scholar] [CrossRef]
- Srinivasan, D.; Yen, J.H.; Joseph, D.J.; Friedman, W. Cell type-specific interleukin-1beta signaling in the CNS. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6482–6488. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Juntilla, M.M.; Patil, V.D.; Calamito, M.; Joshi, R.P.; Birnbaum, M.J.; Koretzky, G.A. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 2010, 115, 4030–4038. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, S.-T.; Zhang, X.-Y.; Ding, H.-R.; Yuan, Y.; He, J.-J.; Wang, M.-S.; Yang, B.; Li, Y.-B. The Evolution of Single-Cell RNA Sequencing Technology and Application: Progress and Perspectives. Int. J. Mol. Sci. 2023, 24, 2943. [Google Scholar] [CrossRef] [PubMed]
- Hor, J.L.; Germain, R.N. Intravital and High-Content Multiplex Imaging of the Immune System. Trends Cell Biol. 2022, 32, 406–420. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, S.; Schreuder, J.; Tran, J.; Metcalf, D.; Sargeant, T.; Ng, A.P.; Weber, T.S.; Naik, S. A Multi-Track Landscape of Haematopoiesis Informed by Cellular Barcoding and Agent-Based Modelling. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Khorramian-Ghahfarokhi, M.; Shafieizadeh, M.; Mahmoudi, E.; Eskandari, F.; Rashidi, M.; Arshi, A.; Mokhtari-Farsani, A. Comprehensive Review of CRISPR-Based Gene Editing: Mechanisms, Challenges, and Applications in Cancer Therapy. Mol. Cancer 2024, 23, 9. [Google Scholar] [CrossRef]
- Ortiz, G.G.R.; Mohammadi, Y.; Nazari, A.; Ataeinaeini, M.; Kazemi, P.; Yasamineh, S.; Al-Naqeeb, B.Z.T.; Zaidan, H.K.; Gholizadeh, O. A State-of-the-Art Review on the MicroRNAs Roles in Hematopoietic Stem Cell Aging and Longevity. Cell Commun. Signal. 2023, 21, 85. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S. Molecular Interactome between HSCs and Their Niches. Blood 2019, 134, 1197–1198. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Bick, A.G. Clonal Hematopoiesis of Indeterminate Potential: An Expanding Genetic Cause of Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).