Abstract
Background/Objectives: Cytomegalovirus (CMV) infection is a frequent complication after lung transplantation, especially in high-risk donor-positive/recipient-negative (D+/R−) patients. CMV-specific hyperimmunoglobulin (CMV-HIG), administered either with antivirals or as monotherapy, may be beneficial for preventing or treating CMV infection in selected clinical scenarios. This study evaluated CMV-HIG indications and their impact on clinical outcomes in our lung transplant unit. Methods: We retrospectively analyzed adult lung transplant recipients (2010–2023) who received ≥2 doses of CMV-HIG for universal prophylaxis, monotherapy prophylaxis, preemptive therapy, or treatment of invasive disease. Results: CMV-HIG was administered to 204 out of 336 recipients (61%). CMV-HIG was well tolerated, with no treatment-related adverse events. Indications were preemptive therapy (63%), universal prophylaxis (24%), monotherapy prophylaxis (7%), and treatment of invasive disease (6%). CMV-HIG was well tolerated, with no treatment-related adverse events. No patients developed invasive disease during combination prophylaxis or preemptive treatment. The combination treatment of patients with invasive disease was also effective, and no cases of VGC resistance were detected. CMV-HIG monoprophylaxis has allowed us to delay or prevent viral replication in recipients who developed VGC side effects. Rates of acute rejection, Chronic Lung Allograft Dysfunction (CLAD), and overall survival were similar across CMV risk groups. Conclusions: Our results showed that the combined use of CMV-HIG and antiviral agents is effective in preventing CMV infection and disease in high-risk lung transplant recipients. This combination is also useful in treating invasive disease and preventing VGC resistance. Additionally, CMV-HIG monoprohylaxis can delay or prevent viral replication in recipients experiencing VGC-related side effects. These findings support the use of CMV-HIG in selected clinical settings, although prospective studies are needed to define its potential benefits within the current therapeutic armamentarium.
1. Introduction
Cytomegalovirus (CMV) infection is one of the most frequent and clinically significant complications following lung transplantation. Its incidence and severity are primarily determined by the serological status of the donor (D) and recipient (R), with seronegative recipients of seropositive donors (D+/R−) at greatest risk [1,2,3]. CMV infection ranges from asymptomatic viral replication to severe invasive disease, most commonly affecting the lungs and gastrointestinal tract [4,5,6]. Beyond invasive disease, CMV exerts additional clinically relevant effects through interactions with the immune system, including acute rejection, chronic lung allograft dysfunction (CLAD), and other opportunistic infections [7,8,9,10,11]. Registry data indicate that high-risk recipients (D+/R−) develop CLAD more frequently and experience poorer long-term survival [12].
Therapeutic options for CMV prevention and treatment include antivirals such as ganciclovir (GCV), valganciclovir (VGC), foscarnet (FOS), cidofovir (CDV), letermovir (LTV), maribavir (MVR), and CMV-specific hyperimmunoglobulin (CMV-HIG) [13,14,15,16]. International guidelines recommend the use of antiviral prophylaxis with VGC during the first post-transplant year in patients identified as being at increased risk of CMV infection [16]. Letermovir has emerged as a potential alternative to valganciclovir for CMV prophylaxis in lung transplant recipients, particularly in patients with contraindications to VGC or those who develop VGC-related adverse effects. The favorable safety profile of letermovir, characterized by a substantially lower incidence of hematological toxicity, particularly myelosuppression, makes it an attractive option for recipients who are highly susceptible to treatment-related adverse events [17,18]. Nevertheless, the clinical evidence supporting the use of newer antiviral drugs in the specific setting of lung transplantation remains limited, as large-scale randomized trials are still lacking. For this reason, GCV/VGC are still the first-line choices [16].
The recommended duration of antiviral prophylaxis ranges between 6 and 12 months, depending on the individual risk profile of the recipient, including donor–recipient CMV serostatus and the intensity of immunosuppressive therapy [16,19]. However, a major limitation in real-world practice is that a considerable proportion of patients are unable to complete the full prophylactic course. It has been reported that almost half of lung transplant recipients experience premature discontinuation of therapy, mainly due to the occurrence of hematological adverse effects, with myelotoxicity representing the most frequent and clinically significant complication. Interruptions in VGCV prophylaxis have been estimated to affect up to 50% of recipients [20,21,22]. In this regard, data from a previous study conducted at our center revealed that 51.8% of lung transplant recipients developed at least one episode of neutropenia during prophylaxis. The severity of neutropenia was classified as mild in 50.57% of cases, moderate in 36.88%, and severe in 12.54% of patients [20].
CMV-specific hyperimmunoglobulin (CMV-HIG) is derived from donors exhibiting high titers of neutralizing antibodies against cytomegalovirus. It was originally developed in the 1970 s for both the prevention and treatment of CMV infection in immunocompromised patients [23,24,25]. The availability of highly effective antiviral agents has significantly limited the use of CMV-HIG in routine clinical practice [13,16]. However, several retrospective studies and meta-analyses suggests that the combination of CMV-HIG with antiviral therapy may further reduce the risk of CMV disease in solid organ transplant recipients [26,27,28]. The precise therapeutic role of CMV-HIG in the era of new and highly effective antivirals, such as letermovir and maribavir, remains to be fully defined. The latest international guidelines indicate that some clinicians continue to administer CMV-HIG in combination with antiviral therapy for high-risk recipients [16,17,18]. The latest international guidelines describe that some physicians use CMV-HIG in combination with antivirals in high-risk recipients [16].
Apart from prophylaxis, CMV-HIG could also be beneficial in combined treatment with antivirals for patients with invasive CMV disease, as an alternative prophylaxis for patients with VGC-related side effects, and for patients with hypogammaglobulinemia [24,28,29,30]. Other potential benefits of CMV-HIG are related to the immunomodulatory properties of gammaglobulins [24,31,32,33,34], which could help mitigate the increased post-transplant morbidity and mortality observed in high-risk recipients [12].
The optimal use of CMV-HIG for the prevention and treatment of CMV infection remains controversial [35]. Furthermore, there is limited real-world evidence regarding its application and the potential impact on long-term patient outcomes [29,36]. Over the past decade, our center has accumulated extensive experience using CMV-HIG for various indications in a large cohort of lung transplant recipients. The objective of this study was to describe the utilization of CMV-HIG in our Lung Transplant Unit and to report patient outcomes, including CMV infection, incidence of acute rejection, development of chronic lung allograft dysfunction (CLAD), and overall survival.
2. Materials and Methods
2.1. Study Design
We conducted an observational, retrospective, analytical study including all adult patients (≥18 years) who underwent lung transplantation at the Puerta de Hierro Majadahonda University Hospital (Madrid, Spain) between 1 January 2010, and 31 December 2023 with regular follow-up in our unit. The study protocol was approved by the Ethics Committee of the Puerta de Hierro Majadahonda University Hospital, on 20 November 2023 (No. 196/23).
Our standard approach to immunosuppression includes basiliximab and a life-time triple immunosuppression regimen with tacrolimus (goal levels 5–15 depending on time post-transplant), mycophenolate mofetil (500–1000 mg twice a day depending of myelotoxicity), and prednisone (doses adjusted to time post-transplant).
Antibacterial prophylaxis is based on the results of the isolates obtained from both the donor and the recipient at the time of surgery. Additional prophylaxis includes the administration of sulfamethoxazole/trimethoprim three times per week and nebulized liposomal amphotericin B (6 mL) every 48 h until hospital discharge, followed by weekly administration thereafter.
Universal CMV prophylaxis consists of intravenous GCV followed by oral VGC, with doses adjusted for renal function, for 6–12 months depending on recipient risk.
The CMV-HIG indications in our unit are as follows:
- Universal prophylaxis in high-risk recipients (D+/R−): Administered in combination with GCV/VGC, 100 mL per dose every 48 h during the first week post-transplant, weekl during the first month, then monthly for the first year.
- Alternative to GCV/VGC due to adverse effects: 100 mL per dose administered every 48 h or weekly, depending on patient-specific factors and clinical judgment.
- Preemptive therapy in combination with VGC for persistent and/or high-level CMV DNAemia: 100 mL per dose administered weekly until resolution.
- Treatment of invasive CMV disease in combination with GCV/VGC: 100 mL per dose every 48 h during the first week, then weekly until clinical and virological resolution.
CMV viral load was assessed, es previously described by our group [21] at the end of prophylaxis period and monitored weekly or every 2–4 weeks according to patient risk and physician discretion. From January 2009 to April 2017, plasma CMV DNA was quantified in copies/mL using the COBAS AmpliPrep/COBAS TaqMan CMV Test (Roche Diagnostics®, Basel, Switzerland), and thereafter in IU/mL with the COBAS 6800 platform, following the WHO International Standard (NIBSC 09/162; conversion factor: 1.1 copies/mL or 0.91 IU per copy).
Electronic medical records were reviewed to collect data on patient demographics, underlying disease, type of transplant, donor and recipient CMV serostatus, prophylaxis start and end dates, date of first viral load determination, presence of invasive disease, grade of acute rejection, CLAD status, and date of death or last follow-up.
2.2. Inclusion Criteeria
Patients who received at least two consecutive doses of CMV-HIG during the post-transplant period were included and classified into one of the following groups according to the initial indication:
- Universal prophylaxis in high-risk recipients (D+/R−).
- Monotherapy prophylaxis in patients with intolerance or adverse effects to GCV/VGC.
- Preemptive therapy in patients with persistent and/or high-level CMV DNAemia.
- Treatment of invasive CMV disease.
2.3. Exclusion Criteria
Patients who received fewer than two doses of CMV-HIG and those who were not regularly followed up in our transplant unit were excluded from the study.
2.4. Definitions
The definitions applied in this study have also been described previously by our group [21]. Briefly, high-level DNAemia was defined as ≥1000 IU/mL requiring antiviral treatment [37], low-level DNAemia as 35–999 IU/mL, and persistent DNAemia as ≥2 consecutive CMV DNA determinations above the cutoff. Recurrent DNAemia referred to new CMV detection in a previously infected patient after at least 4 weeks without detectable virus during active surveillance [38]. Invasive disease was defined as histologically proven or probable based on clinical criteria [39].
Acute and chronic lung allograft rejection were defined according to the International Society for Heart and Lung Transplantation (ISHLT) criteria. Acute rejection was classified and graded based on biopsy findings [40], while chronic lung allograft dysfunction (CLAD) was defined as a persistent decline of ≥20% in FEV1 from the reference baseline values [41].
2.5. Statistical Analysis
Descriptive analysis was performed on all variables recorded. For quantitative variables, both Student’s t-test and non-parametric tests (MannWhitney U-test or Kruskal–Wallis) were used if the distributions of the variables did not meet the assumptions of normality. The normality assumption was tested through the Shapiro–Wilk test. The Cumulative Incidence Function (CIF) was used to estimate time to CLAD detection in the presence of competing risks. Differences between risk groups were assessed using the Fine and Gray subdistribution hazard model, and results were reported as subdistribution hazard ratios (SHR) with corresponding p-values. Post-transplant survival of different patient groups was analyzed with Kaplan–Meier tests and compared using log-rank tests. The significant level was defined as a p value < 0.05. All statistical analyses were performed using R (version 4.3.3) and Stata (version 18.0; StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX, USA: StataCorp LLC).
3. Results
During the study period, 204 out of the 336 lung transplant recipients (61%) in our unit received CMV-HIG. Demographic characteristics of treated and untreated patients are summarized in Table 1, and the distribution of recipients according to CMV infection risk is presented in Table 2.

Table 1.
Demographic characteristics of treated and untreated patients with CMV-HIG.

Table 2.
Demographic characteristics by CMV infection risk groups.
3.1. Indications of CMV-HIG
The initial indication for CMV-HIG was universal prophylaxis in 48 high-risk recipients (24%), monotherapy prophylaxis in 15 recipients (7%) due to VGC adverse effects, preemptive therapy in 129 recipients (63%) with persistent or recurrent CMV DNAemia, and combined treatment in 12 patients (6%) with invasive CMV disease. Fifty-four patients (26%) presented more than one indication, either simultaneously or at different time points during the study period and the more frequent combination was preemptive treatment in patients with neutropenia. No adverse events related to CMV-HIG infusion were observed.
3.1.1. Universal Prophylaxis in High-Risk Recipients
Four out the 48 high-risk recipients developed DNAemia during the prophylactic period. Two patients presented a very low-level DNAemia (<35 UI/mL) while receiving combined prophylaxis with CMV-HIG and VGC; both cases resolved after increasing the VGC dose. The other two recipients were on CMV-HIG monotherapy prophylaxis due to VGC adverse effects. DNAemia was detected on days 7 and 12 post-CMV-HIG administration, with viral loads of 583 and 924 UI/mL, respectively. In both cases, viral load resolved following reintroduction of GCV along with concomitant administration of granulocyte colony-stimulating factor (G-CSF).
3.1.2. Monotherapy Prophylaxis Due to VGC Adverse Effects
Fifteen recipients received CMV-HIG monotherapy prophylaxis due to VGC-related adverse effects, mainly neutropenia. Ten patients (67%) experienced DNAemia-free intervals longer than 15 days following CMV-HIG administration. The remaining five patients (33%) had shorter DNAemia-free periods, with two cases lasting less than one week and three cases under two weeks. All detected DNAemia levels were very low (<35 UI/mL) and resolved after reintroduction of VGC along with concomitant administration of granulocyte colony-stimulating factor (G-CSF).
3.1.3. Preemptive Therapy
The most frequent indication for CMV-HIG in our Lung Transplant Unit was preemptive therapy administered in combination with VGC to recipients with persistent or recurrent DNAemia. All 129 recipients treated under this indication were asymptomatic at the time of viral detection, and none progressed to invasive CMV disease.
3.1.4. Treatment of Invasive CMV Disease
At the end of prophylaxis period, 12 recipients (3.5% of the total cohort) developed CMV disease, with six cases confirmed and six suspected. The most common site of infection was the gastrointestinal tract (9 cases), followed by the lungs (2 cases) and CMV syndrome (1 case). All recipients were successfully treated with a combination of GCV/VGC, CMV-HIG and a reduction in immunosuppression until symptoms resolution and viral clearance. No cases of VGC resistance were observed during the study period.
3.2. Patients and Allograft Evolution
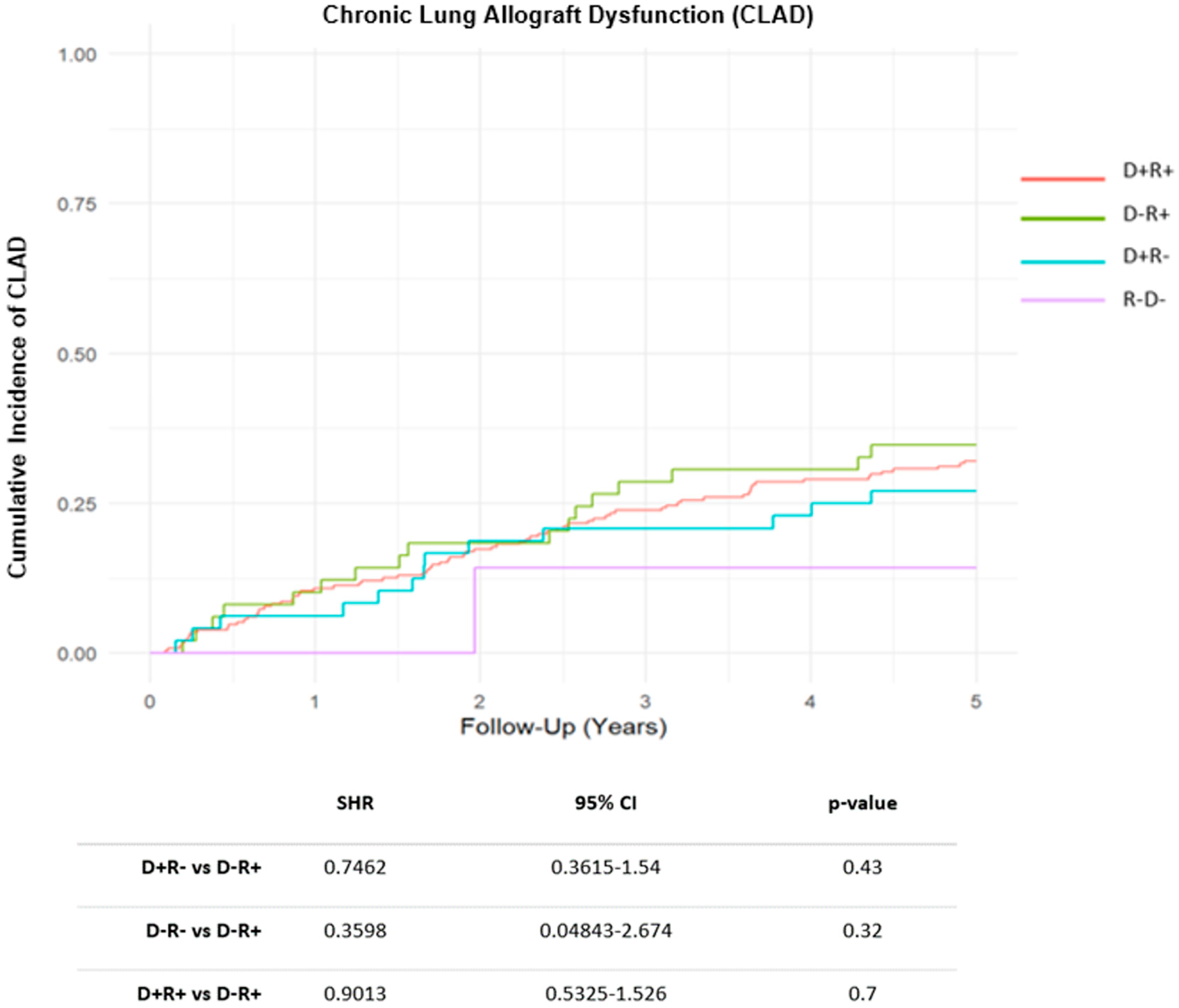
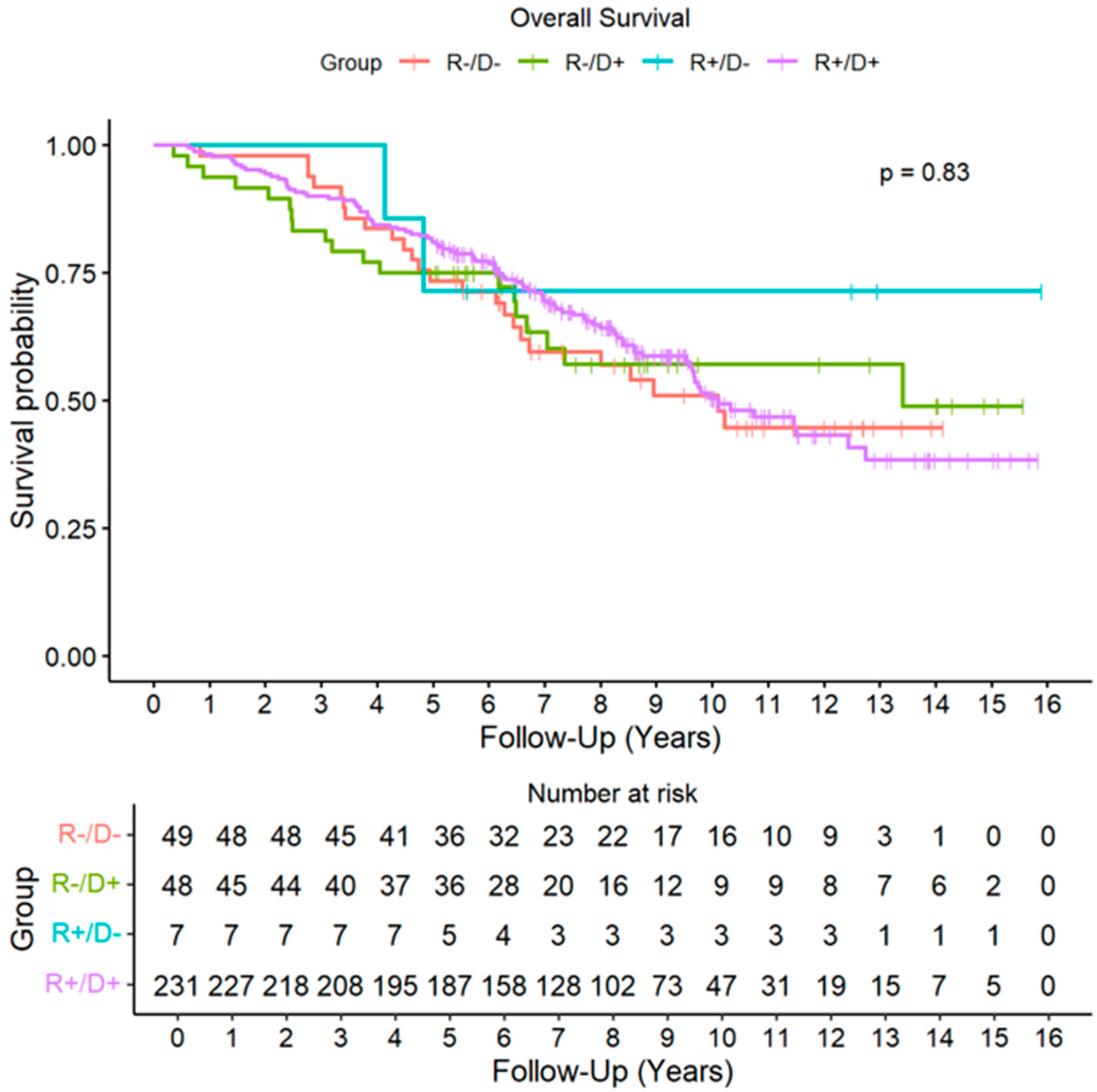
During the follow-up period, acute rejection of grade ≥ A2 was detected in 139 recipients (41%), with no significant differences across CMV risk groups. A total of 115 patients (34%) developed some degree of CLAD during the follow-up. The cumulative incidence (CIF) of CLAD was similar across CMV risk groups (Figure 1). By the end of follow-up period, 143 recipients (43%) had died, with CLAD progression being the leading cause of death. No significant differences in overall survival were found among the different CMV risk categories (HR D+R+: 0.90, CI 95%: 0.58–1.41, p = 0.650; HR D+R−: 0.92, CI 95%: 0.50–1.67, p = 0.775; HR D−R−: 0.53, CI 95% 0.12–2.24, p = 0.386) (Figure 2).
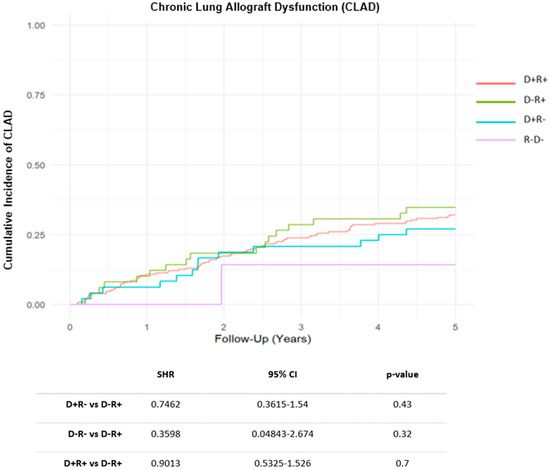
Figure 1.
Cumulative Incidence Function (CIF) of Chronic Lung Allograft Dysfunction (CLAD) by Cytomegalovirus risk groups. Abbreviations: SHR (subhazard ratio); CI (confidence interval); D (donor); R (recipient).
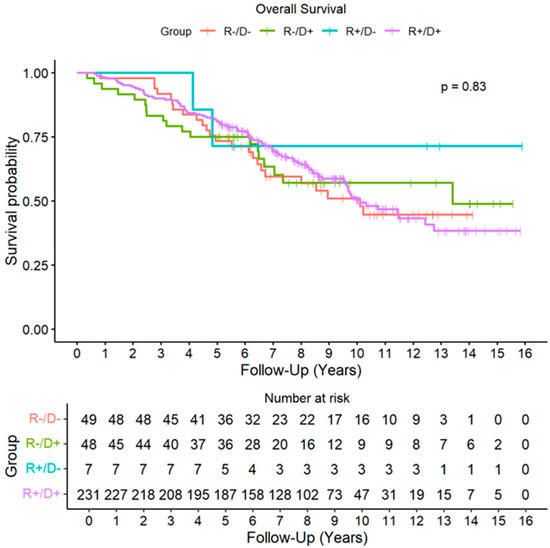
Figure 2.
Overall Survival by Cytomegalovirus risk groups. Abbreviations: D (donor); R (recipient).
4. Discussion
CMV-HIG has been extensively employed in our Lung Transplant Unit for both the prevention and treatment of CMV infection, reflecting its broad applicability in clinical practice. Beyond standard prophylaxis, alternative indications have included preemptive therapy for recipients with persistent or recurrent DNAemia, as well as monotherapy prophylaxis in patients unable to receive valganciclovir (VGC) due to toxicity or intolerance. This therapeutic strategy, integrated within our specific CMV prevention protocol, may have contributed to improved post-transplant outcomes in high-risk recipients, delayed the onset of DNAemia in patients unable to receive standard antiviral therapy, and potentially helped prevent the emergence of antiviral resistance, which was not observed in our cohort.
The most frequent use of CMV-HIG in solid organ transplantation has been for the prevention of CMV infection [26,27]. The potential benefit of combining CMV-HIG with GCV/VGC was first described by Valantine et al. in 2002 [25], who reported fewer CMV infections and improved post-transplant outcomes in lung transplant recipients receiving combined prophylaxis. However, subsequent studies have reported conflicting results, and the additional protection conferred by CMV-HIG in patients already receiving effective antiviral therapy remains a subject of debate [23,24,42]. The latest international guidelines reflect this uncertainty, noting that “some experts add CMV-HIG to prophylaxis” in high-risk recipients [16]. In our cohort, the favorable outcomes observed in D+/R− recipients suggest that combined prophylaxis with CMV-HIG and antivirals may be particularly beneficial in this subgroup. Nevertheless, the absence of a control group limits the ability to draw definitive conclusions regarding the additive effect of CMV-HIG in this context.
CMV-HIG may be used as an alternative prophylaxis in patients who develop adverse effects to VGC [15,28,29,30]. In such cases, CMV-HIG could provide a temporary protective window, allowing spontaneous hematological recovery or adjustment of antiviral therapy. In our cohort, more than half of intermediate-risk recipients receiving CMV-HIG monotherapy due to VGC side-effects remained free of viral replication during the two weeks following infusion. Conversely, two high-risk recipients on CMV-HIG monotherapy prophylaxis developed DNAemia on days 7 and 14 post-infusion, respectively. These observations suggest that the duration of viral suppression achieved with CMV-HIG alone may be shorter in high-risk patients, emphasizing the need for close monitoring and individualized treatment strategies.
The viral replication-free period observed in our patients aligns with previously reported CMV-HIG pharmacokinetic data. Ganzinger et al. analyzed blood levels of CMV-HIG in six volunteers (three seropositive and three seronegative). Following infusion of 7 g of CMV-HIG, anti-CMV antibody levels in the seropositive volunteers increased by 1.3 to 2.3 times, with a mean AUC of 3950 per day. Levels slowly increased until day 9, and remained unchanged thereafter until day 15. Notably, this increase was not accompanied by a corresponding rise in neutralizing activity. In contrast, seronegative volunteers achieved significantly lower anti-CMV antibody levels, with a mean AUC of 647 per day; levels decreased slightly until day 8, and remained relatively stable until day 15. Unlike the seropositive volunteers, the neutralizing capacity in seronegative individuals increased up to sixfold post-infusion and declined gradually during the following two weeks [43]. Metzelaar et al. describe similar kinetics in seronegative heart transplant recipients receiving CMV-HIG at multiple time points post-transplant (days 2, 7, 14, 35, 56 and 77. They observed that after four doses of CMV-HIG, blood antibody levels tended to stabilize. Based on these findings, the authors suggested that following an initial loading phase, CMV-HIG administration could potentially be spaced to a bi-weekly schedule [44]. These pharmacokinetic insights support the use of CMV-HIG as short-term prophylaxis in carefully selected patients.
In our Lung Transplant Unit, combined preemptive therapy for persistent or recurrent DNAemia was the most frequent CMV-HIG indication. Although there are currently no formal guidelines endorsing the combined use of VGC and CMV-HIG in this setting, none of our patients progressed to invasive disease or developed antiviral resistance. These findings suggest that such a strategy may offer potential benefits in preventing disease progression and preserving antiviral efficacy.
All patients in our cohort who developed invasive CMV disease (3.5%) responded favorably to combined treatment and adjustment of immunosuppression. Notably, the frequency of invasive CMV disease observed in our series is lower than previously reported in the literature [1,3,4]. These outcomes may be partially attributed to the extended prophylaxis period and the proactive early intervention with combined therapy in recipients with recurrent or persistent DNAemia. However, further studies are needed to confirm these findings.
Antiviral resistance to GCV/VGC in lung transplant recipients has been reported in 5–12% of cases and is associated with increased morbidity and mortality [13,29,45]. In our cohort, no CMV resistance was observed during either prophylaxis or treatment of invasive disease. These findings suggest that the development of resistance can be minimized through combined prophylaxis in at-risk recipients, appropriate management of invasive disease with combination therapy, and close clinical monitoring.
CMV infection has been associated with poorer post-transplant outcomes, particularly in high-risk recipients [8,9,10,12]. The release of pro-inflammatory cytokines during infection may contribute to both acute and chronic allograft rejection. In our cohort, post-transplant outcomes of high-risk recipients were comparable to those of other groups, with no significant differences in the incidence of acute rejection, chronic lung allograft dysfunction (CLAD), or overall survival. These favorable results likely reflect the combination of our prophylaxis protocol, early detection of DNAemia, and proactive management strategies, highlighting the potential benefits of integrating CMV-HIG into clinical practice for selected patients.
The main limitations of this study are its retrospective, single-center design. The observational nature of our cohort limits the ability to establish causal relationships between CMV-HIG use and clinical outcomes. Variability in the timing, dosing, and indications for CMV-HIG administration may also affect the generalizability of our findings. Other important limitations include the lack of a control group that did not receive CMV-HIG prophylaxis or treatment, and the small number of patients included in some subgroups, such as monotherapy prophylaxis and CMV disease treatment. In patients who received preemptive therapy, viral load progression could not be analyzed statistically due to the inhomogeneity of follow-up time and the presence of confounding factors such as risk mismatch and comorbidity. Future prospective studies and randomized trials are warranted to delineate the optimal use of CMV-HIG in combination with both traditional and novel antiviral therapies, to better define its role in contemporary lung transplant practice, and to evaluate its impact on long-term graft function, infection rates, and survival.
5. Conclusions
In summary, our results showed that the combined use of CMV-HIG and antiviral agents is an effective strategy for preventing CMV infection and disease in high-risk lung transplant recipients. This therapeutic combination has also been useful in treating patients with invasive disease and in preventing resistance to VGC. Additionally, CMV-HIG monoprohylaxis has been useful for delaying or preventing viral replication in selected clinical settings when no other therapeutic alternatives were available. While definitive conclusions are limited by the observational nature of the study and the absence of a control group, these findings provide real-world evidence supporting the judicious use of CMV-HIG in contemporary lung transplant practice. Further prospective studies and randomized trials are warranted to establish optimal dosing schedules, patient selection criteria, and long-term efficacy in the current therapeutic armamentarium.
Author Contributions
Conceptualization, M.P.U.G.; methodology, M.P.U.G. and S.G.-M.F.; software, E.R.R.; validation, S.G.-M.F., E.R.R., A.S.G. and C.A.S.; formal analysis, R.S.F.d.S., S.G.-M.F., E.R.R. and M.P.U.G.; investigation, R.S.F.d.S., R.L.H., M.A.P., C.G.F., M.T.L.C.d.l.F., A.S.G., C.A.S. and M.P.U.G.; resources, M.P.U.G.; data curation, S.G.-M.F.; writing—original draft preparation, R.S.F.d.S. and M.P.U.G.; writing—review and editing, R.S.F.d.S., S.G.-M.F., R.L.H., M.A.P., C.G.F., M.T.L.C.d.l.F., E.R.R., A.S.G., M.P.U.G. and C.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Puerta de Hierro Majadahonda University Hospital. (protocol code 196/23) on 20 November 2023.
Informed Consent Statement
Patient consent was waived because the data are anonymized and the study is observational and retrospective.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hammond, S.P.; Martin, S.T.; Roberts, K.; Gabardi, S.; Fuhlbrigge, A.L.; Camp, P.C.; Goldberg, H.; Marty, F.; Baden, L. Cytomegalovirus disease in lung transplantation: Impact of recipient seropositivity and duration of antiviral prophylaxis. Transpl. Infect. Dis. 2012, 15, 163–170. [Google Scholar] [CrossRef]
- Chang, A.; Musk, M.; Lavender, M.; Wrobel, J.; Yaw, M.; Lawrence, S.; Chirayath, S.; Boan, P. Cytomegalovirus viremia in lung transplantation during and after prophylaxis. Transpl. Infect. Dis. 2019, 21, e13069. [Google Scholar] [CrossRef] [PubMed]
- Mitsani, D.; Nguyen, M.H.; Kwak, E.J.; Silveira, F.P.; Vadnerkar, A.; Pilewski, J.; Crespo, M.; Toyoda, Y.; Bermudez, C.; Clancy, C.J. Cytomegalovirus disease among donor-positive/recipient-negative lung transplant recipients in the era of valganciclovir prophylaxis. J. Heart Lung Transplant. 2010, 29, 1014–1020. [Google Scholar] [CrossRef]
- Avery, R.K.; Silveira, F.P.; Benedict, K.; Cleveland, A.A.; Kauffman, C.A.; Schuster, M.G.; Dubberke, E.R.; Husain, S.; Paterson, D.L.; Chiller, T.; et al. Cytomegalovirus infections in lung and hematopoietic cell transplant recipients in the Organ Transplant Infection Prevention and Detection Study: A multi-year, multicenter prospective cohort study. Transpl. Infect. Dis. 2018, 20, e12877. [Google Scholar] [CrossRef] [PubMed]
- Lopez Garcia-Gallo, C.; García Fadul, C.; Laporta, R.; Portero, F.; Millan, I.; Ussetti, P. Cytomegalovirus Immunoglobulin for Prophylaxis and Treatment of Cytomegalovirus Infection in the (Val)Ganciclovir Era: A Single-Center Experience. Ann. Transplant. 2015, 20, 661–666. [Google Scholar] [CrossRef] [PubMed]
- García-Gallo, C.L.; Gil, P.U.; Laporta, R.; Carreño, M.C.; De Pablo, A.; Ferreiro, M.J. Is Gammaglobulin Anti-CMV Warranted in Lung Transplantation? Transplant. Proc. 2005, 37, 4043–4045. [Google Scholar] [CrossRef]
- Kawashima, M.; Ma, J.; Huszti, E.; Levy, L.; Berra, G.; Renaud-Picard, B.; Takahagi, A.; Ghany, R.; Sato, M.; Keshavjee, S.; et al. Association between cytomegalovirus viremia and long-term outcomes in lung transplant recipients. Am. J. Transplant. 2024, 24, 1057–1069. [Google Scholar] [CrossRef]
- Bennett, D.; Bergantini, L.; Ferrara, P.; Cusi, M.G.; Scolletta, S.; Montagnani, F.; Paladini, P.; Sestini, P.; Refini, R.M.; Luzzi, L.; et al. Cytomegalovirus Infection Is Associated with Development of Chronic Lung Allograft Dysfunction. Lung 2022, 200, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Beam, E.; Lesnick, T.; Kremers, W.; Kennedy, C.C.; Razonable, R.R. Cytomegalovirus disease is associated with higher all-cause mortality after lung transplantation despite extended antiviral prophylaxis. Clin. Transplant. 2016, 30, 270–278. [Google Scholar] [CrossRef]
- Stern, M.; Hirsch, H.; Cusini, A.; van Delden, C.; Manuel, O.; Meylan, P.; Boggian, K.; Mueller, N.J.; Dickenmann, M. Cytomegalovirus Serology and Replication Remain Associated with Solid Organ Graft Rejection and Graft Loss in the Era of Prophylactic Treatment. Transplantation 2014, 98, 1013–1018. [Google Scholar] [CrossRef]
- Solak, Y.; Biyik, Z.; Cizmecioglu, A.; Genc, N.; Ozbek, O.; Gaipov, A.; Yeksan, M. Cytomegalovirus and Aspergillus spp. coinfection in organ transplantation: A case report and review of the literature. CEN Case. Rep. 2013, 2, 59–67. [Google Scholar] [CrossRef]
- Chambers, D.C.; Perch, M.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report—2021; Focus on recipient characteristics. J. Heart Lung Transplant. 2021, 40, 1060–1072. [Google Scholar] [CrossRef]
- Stewart, A.G.; Kotton, C.N. What’s New: Updates on Cytomegalovirus in Solid Organ Transplantation. Transplantation 2024, 108, 884–897. [Google Scholar] [CrossRef]
- Bottino, P.; Pastrone, L.; Curtoni, A.; Bondi, A.; Sidoti, F.; Zanotto, E.; Cavallo, R.; Solidoro, P.; Costa, C. Antiviral Approach to Cytomegalovirus Infection: An Overview of Conventional and Novel Strategies. Microorganisms 2023, 11, 2372. [Google Scholar] [CrossRef]
- Grossi, P.A.; Peghin, M. Recent advances in cytomegalovirus infection management in solid organ transplant recipients. Curr. Opin. Organ. Transplant. 2024, 29, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kumar, D.; Manuel, O.; Chou, S.; Hayden, R.T.; Danziger-Isakov, L.; Asberg, A.; Tedesco-Silva, H.; Humar, A. The Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. Transplantation 2025, 109, 1066–1110. [Google Scholar] [CrossRef]
- Martinez, S.; Sindu, D.; Nailos, M.D.; Cherrier, L.; Tokman, S.; Waila, R.; Goodlet, K. Evaluating the efficacy and safety of letermovir compared to valganciclovir for the prevention of human cytomegalovirs disease in adult lung tansplanbt recipients. Transplant. Inffec. Dis. 2024, 26, e14279. [Google Scholar] [CrossRef]
- Mezochow, A.; Clausen, E.; Whitaker, K.; Claridge, T.; Blumberg, E.; Coutrwright, T. Letermovir should be first-line cytomegalovirus prophylaxis in lung transplant recipients. Am. J. Tranplat. 2025, 25, 908–915. [Google Scholar] [CrossRef]
- Monforte, V.; Sintes, H.; López-Gallo, C.; Delgado, M.; Santos, F.; Zurbano, F.; Solé, A.; Gavaldá, J.; Borro, J.M.; Redel-Montero, J.; et al. Risk factors, survival, and impact of prophylaxis length in cytomegalovirus-seropositive lung transplant recipients: A prospective, observational, multicenter study. Transpl. Infect. Dis. 2017, 19, e12694. [Google Scholar] [CrossRef] [PubMed]
- Sanabrias Fernández de Sevilla, R.; Sánchez Cerviño, A.C.; Laporta Hernández, R.; Aguilar Pérez, M.; García Fadul, C.; García-Masedo Fernández, S.; Sánchez Guerrero, A.; Ussetti Gil, M.P. Impact of Neutropenia on Clinical Outcomes after Lung Transplantation. Med. Sci. 2024, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- García-Masedo Fernández, S.; Laporta, R.; García Fadul, C.; Aguilar Pérez, M.; Anel Pedroche, J.; Sanabrias Fernández de Sevilla, R.; Royuela, A.; Sánchez Romero, I.; Ussetti Gil, M.P. CMV Infection Risk Factors and Viral Dynamics After Valganciclovir Prophylaxis: 10 Years of Experience in Lung Transplant Recipients. Microorganisms 2024, 12, 2360. [Google Scholar] [CrossRef]
- Wiita, A.P.; Roubinian, N.; Khan, Y.; Chin-Hong, P.V.; Singer, J.P.; Golden, J.A.; Miller, S. Cytomegalovirus disease and infection in lung transplant recipients in the setting of planned indefinite valganciclovir prophylaxis. Transpl. Infect. Dis. 2012, 14, 248–258. [Google Scholar] [CrossRef]
- Rea, F.; Potena, L.; Yonan, N.; Wagner, F.; Calabrese, F. Cytomegalovirus Hyper Immunoglobulin for CMV Prophylaxis in Thoracic Transplantation. Transplantation 2016, 100 (Suppl. S3), S19–S26. [Google Scholar] [CrossRef]
- Grossi, P.; Mohacsi, P.; Szabolcs, Z.; Potena, L. Cytomegalovirus Immunoglobulin After Thoracic Transplantation: An Overview. Transplantation 2016, 100 (Suppl. 3S), S1–S4. [Google Scholar] [CrossRef]
- Valantine, H.A.; Luikart, H.; Doyle, R.; Theodore, J.; Hunt, S.; Oyer, P.; Robbins, R.; Berry, G.; Reitz, B. Impact of Cytomegalovirus Hyperimmune Globulin on Outcome After Cardiothoracic Transplantation: A Comparative Study of Combined Prophylaxis with CMV Hyperimmune Globulin Plus Ganciclovir Versus Ganciclovir Alone. Transplantation 2001, 72, 1647–1652. [Google Scholar] [CrossRef]
- Barten, M.J.; Baldanti, F.; Staus, A.; Hüber, C.M.; Glynou, K.; Zuckermann, A. Effectiveness of Prophylactic Human Cytomegalovirus Hyperimmunoglobulin in Preventing Cytomegalovirus Infection following Transplantation: A Systematic Review and Meta-Analysis. Life 2022, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Bonaros, N.; Mayer, B.; Schachner, T.; Laufer, G.; Kocher, A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: A meta-analysis. Clin. Transplant. 2008, 22, 89–97. [Google Scholar] [CrossRef]
- Schulz, U.; Solidoro, P.; Müller, V.; Szabo, A.; Gottlieb, J.; Wilkens, H.; Enseleit, F. CMV Immunoglobulins for the Treatment of CMV Infections in Thoracic Transplant Recipients. Transplantation 2016, 100 (Suppl. S3), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Santhanakrishnan, K.; Yonan, N.; Callan, P.; Karimi, E.; Al-Aloul, M.; Venkateswaran, R. The use of CMVIg rescue therapy in cardiothoracic transplantation: A single-center experience over 6 years (2011–2017). Clin. Transplant. 2019, 33, e13655. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Parquin, F.; Messika, J.; Véronique, B.; Brugière, O.; Degot, T.; Feuillet, S.; Lepavec, J.; Tissot, A.; Dromer, C.; et al. Use of anti-CMV immunoglobulins in lung transplant recipients: The French experience. Transpl. Infect. Dis. 2021, 23, e13754. [Google Scholar] [CrossRef]
- Carbone, J. The Immunology of Posttransplant CMV Infection: Potential Effect of CMV Immunoglobulins on Distinct Components of the Immune Response to CMV. Transplantation 2016, 100 (Suppl. S3), S11–S18. [Google Scholar] [CrossRef]
- Van Gent, R.; Metselaar, H.J.; Kwekkeboom, J. Immunomodulation by hyperimmunoglobulins after solid organ transplantation: Beyond prevention of viral infection. Transplant. Rev. 2017, 31, 78–86. [Google Scholar] [CrossRef]
- Desai, R.; Collett, D.; Watson, C.J.E.; Johnson, P.J.; Moss, P.; Neuberger, J. Impact of Cytomegalovirus on Long-term Mortality and Cancer Risk After Organ Transplantation. Transplantation 2015, 99, 1989–1994. [Google Scholar] [CrossRef]
- Solidoro, P.; Libertucci, D.; Delsedime, L.; Ruffini, E.; Bosco, M.; Costa, C.; Rinaldi, M.; Baldi, S. Combined Cytomegalovirus Prophylaxis in Lung Transplantation: Effects on Acute Rejection, Lymphocytic Bronchitis/Bronchiolitis, and Herpesvirus Infections. Transplant. Proc. 2008, 40, 2013–2014. [Google Scholar] [CrossRef]
- Mora, V.M.; Ussetti, P.; de Pablo, A.; Iturbe, D.; Laporta, R.; Alonso, R.; Aguilar, M.; Quezada, C.A.; Cifrián, J.M. Evaluation of Two Different CMV-Immunoglobulin Regimens for Combined CMV Prophylaxis in High-Risk Patients following Lung Transplant. Microorganisms 2022, 11, 32. [Google Scholar] [CrossRef]
- Banga, N.; Kanade, R.; Kappalayil, A.; Timofte, I.; Lawrence, A.; Bollineni, S.; Kaza, V.; Torres, F. Long-Term Outcomes Among Lung Transplant Recipients with High-Risk Cytomegalovirus Mismatch Managed with a Multimodality Regimen. Clin. Transplant. 2025, 39, e70219. [Google Scholar] [CrossRef]
- Navarro, D.; San-Juan, R.; Manuel, O.; Giménez, E.; Fernández-Ruiz, M.; Hirsch, H.H.; Grossi, P.A.; Aguado, J.M.; ESGICH CMV Survey Study Group, on behalf of the European Study Group of Infections in Compromised Hosts (ESGICH) from the Society of Clinical Microbiology and Infectious Diseases (ESCMID). Cytomegalovirus infection management in solid organ transplant recipients across European centers in the time of molecular diagnostics: An ESGICH survey. Transpl. Infect. Dis. 2017, 19, e12773. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Griffiths, P.; Paya, C. Definitions of Cytomegalovirus Infection and Disease in Transplant Recipients. Clin. Infect. Dis. 2002, 34, 1094–1097. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Verleden, G.M.; Hendriks, J.M.H.; Verleden, S.E. The diagnosis and management of chronic lung allograft dysfunction. Curr. Opin. Pulm. Med. 2024, 30, 377–381. [Google Scholar] [CrossRef]
- Hodson, E.M.; Jones, C.A.; Strippoli, G.F.; Webster, A.C.; Craig, J.C. Immunoglobulins, vaccines or interferon for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Kidney and Transplant Group, editor. Cochrane Database Syst. Rev. 2007, 2, CD005129. [Google Scholar]
- Ganzinger, U.; Martindale, J.J.; Gaudera, E.; Millendorfer, A.; Scriba, M.; Bachmayer, H. Pharmacokinetics of an Anti-Cytomegalovirus Hyperimmunoglobulin after Single Intravenous Administration to Healthy Volunteers. Vox Sang. 1991, 60, 203–206. [Google Scholar] [CrossRef]
- Metselaar, H.J.; Velzing, J.; Rothbarth, P.H.; Simoons, M.L.; Bos, E.; Weimar, W. A pharmacokinetic study of anti-cytomegalovirus hyperimmunoglobulins in cytomegalovirus seronegative cardiac transplant recipients. Transplant. Proc. 1987, 19, 4063–4065. [Google Scholar]
- Majeed, A.; Latif, A.; Kapoor, V.; Sohail, A.; Florita, C.; Georgescu, A.; Zangeneh, T. Resistant Cytomegalovirus Infection in Solid-organ Transplantation: Single-center Experience, Literature Review of Risk Factors, and Proposed Preventive Strategies. Transplant. Proc. 2018, 50, 3756–3762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).