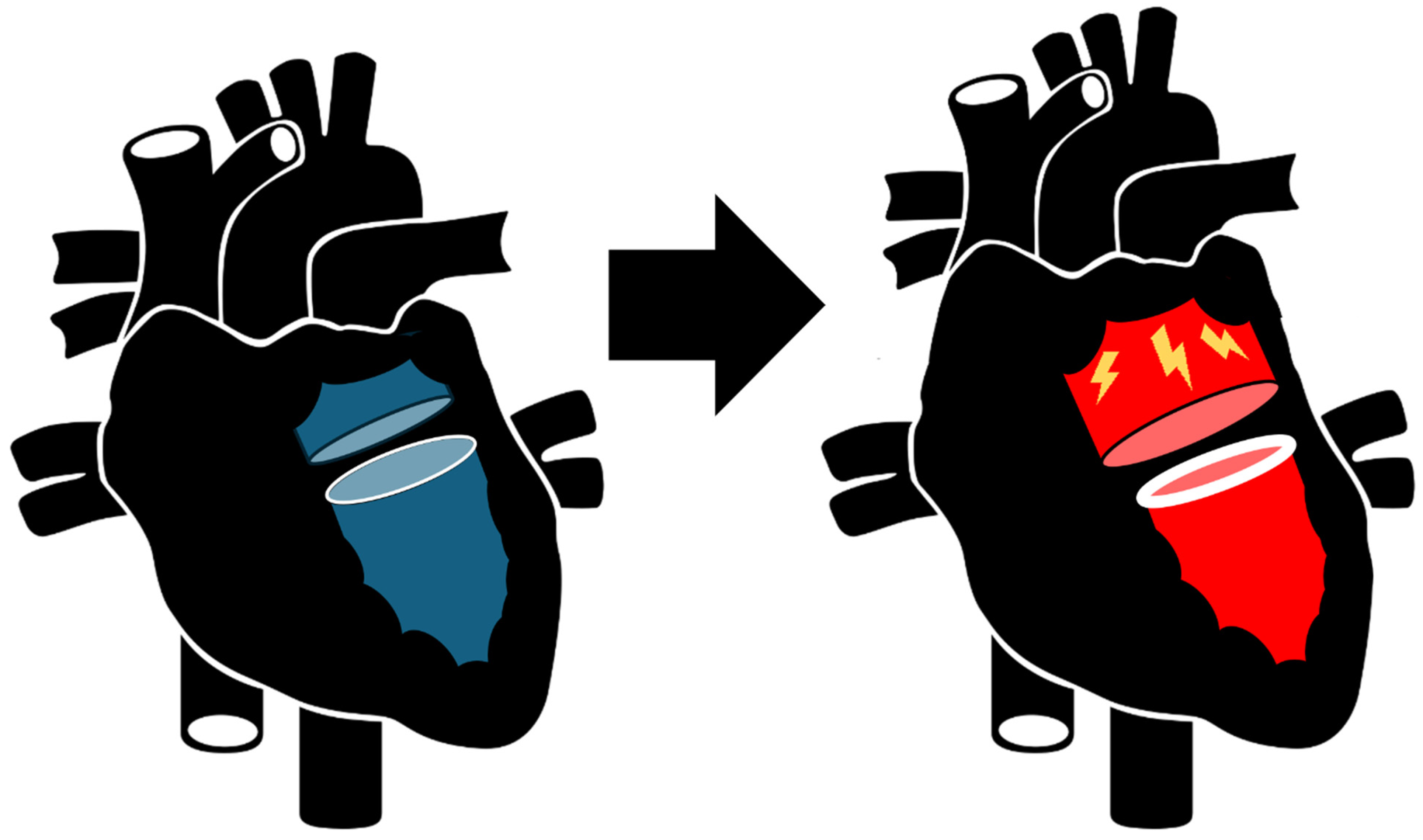
Management of Left Atrial Tachyrhythms in the Setting of HFpEF with Pulsed-Field Ablation: Treating Fire with Water?
Abstract
1. Introduction
1.1. Heart Failure
1.2. Atrial Fibrillation
1.3. Ablation Techniques for Atrial Fibrillation
1.4. Correlation between Heart Failure and Atrial Fibrillation
2. The Fire of HFpEF and AF
2.1. Heart Failure with Preserved Ejection Fraction
2.2. Atrial Fibrillation in the Setting of HFpEF
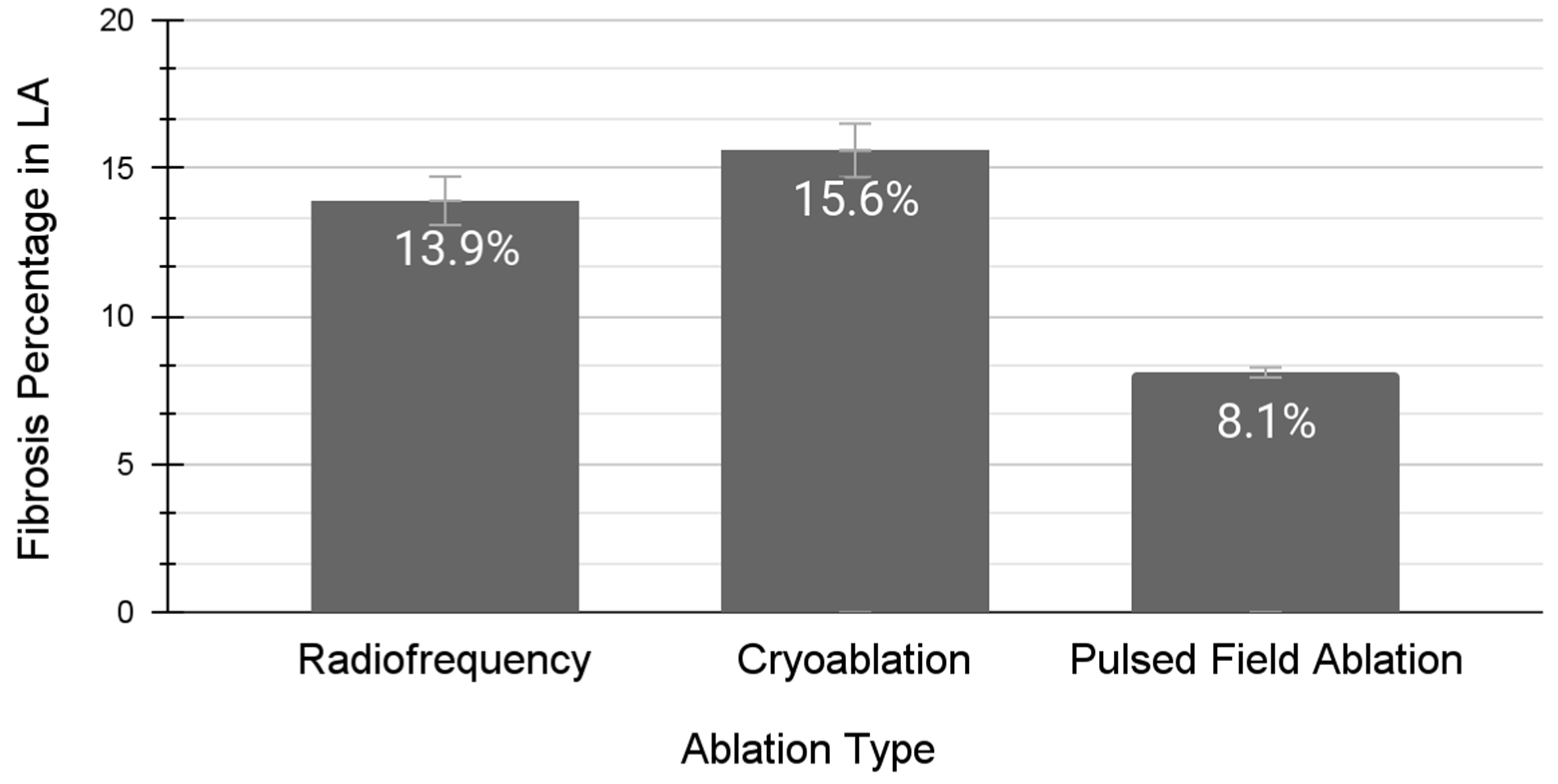
2.3. Pulsed-Field Ablation
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, M.S.; Shahid, I.; Bennis, A.; Rakisheva, A.; Metra, M.; Butler, J. Global epidemiology of heart failure. Nat. Rev. Cardiol. 2024, 21, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunlay, S.M.; Roger, V.L. Understanding the epidemic of heart failure: Past, present, and future. Curr. Heart Fail. Rep. 2014, 11, 404–415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Global Cardiovascular Risk Consortium; Magnussen, C.; Ojeda, F.M.; Leong, D.P.; Alegre-Diaz, J.; Amouyel, P.; Aviles-Santa, L.; De Bacquer, D.; Ballantyne, C.M.; Bernabé-Ortiz, A.; et al. Global Effect of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Chinyere, I.R.; Balakrishnan, M.; Hutchinson, M.D. The emerging role of cardiac contractility modulation in heart failure treatment. Curr. Opin. Cardiol. 2022, 37, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Colilla, S.; Crow, A.; Petkun, W.; Singer, D.E.; Simon, T.; Liu, X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 2013, 112, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J.-Qual. Care Clin. Outcomes 2021, 7, 574–582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanaka, Y.; Shah, N.S.; Passman, R.; Greenland, P.; Lloyd-Jones, D.M.; Khan, S.S. Trends in Cardiovascular Mortality Related to Atrial Fibrillation in the United States, 2011 to 2018. J. Am. Heart Assoc. 2021, 10, e020163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Epicoco, G.; Sorgente, A. Predictors of Atrial Fibrillation Recurrence after Catheter Ablation. J. Atr. Fibrillation 2014, 6, 1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, J.L.; Jaquiss, R.D.; Schuessler, R.B.; Boineau, J.P. Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J. Thorac. Cardiovasc. Surg. 1995, 110, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kearney, K.; Stephenson, R.; Phan, K.; Chan, W.Y.; Huang, M.Y.; Yan, T.D. A systematic review of surgical ablation versus catheter ablation for atrial fibrillation. Ann. Cardiothorac. Surg. 2014, 3, 15–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandey, A.; Kim, S.; Moore, C.; Thomas, L.; Gersh, B.; Allen, L.A.; Kowey, P.R.; Mahaffey, K.W.; Hylek, E.; Peterson, E.; et al. Predictors and Prognostic Implications of Incident Heart Failure in Patients With Prevalent Atrial Fibrillation. JACC Heart Fail. 2017, 5, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Martinez, F.; Calderon, J.M.; Fernandez, A.; Sauri, I.; Uso, R.; Trillo, J.L.; Redon, J.; Forner, M.J. Incidence and impact of atrial fibrillation in heart failure patients: Real-world data in a large community. ESC Heart Fail. 2022, 9, 4230–4239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, A.; Brito, D.; Vaqar, S.; Chhabra, L. Congestive Heart Failure. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Watson, R.D.; Gibbs, C.R.; Lip, G.Y. ABC of heart failure. Clinical features and complications. BMJ 2000, 320, 236–239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chinyere, I.R.; Moukabary, T.; Goldman, S.; Juneman, E. Electrical and mechanical alternans during ventricular tachycardia with moderate chronic heart failure. J. Electrocardiol. 2018, 51, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.; Methawasin, M.; Strom, J.; Nair, P.; Hutchinson, K.; Granzier, H. Alternative Splicing of Titin Restores Diastolic Function in an HFpEF-Like Genetic Murine Model (TtnΔIAjxn). Circ. Res. 2016, 119, 764–772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henning, R.J. Diagnosis and treatment of heart failure with preserved left ventricular ejection fraction. World J. Cardiol. 2020, 12, 7–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Loon, T.; Knackstedt, C.; Cornelussen, R.; Reesink, K.D.; Brunner La Rocca, H.P.; Delhaas, T.; van Empel, V.; Lumens, J. Increased myocardial stiffness more than impaired relaxation function limits cardiac performance during exercise in heart failure with preserved ejection fraction: A virtual patient study. Eur. Heart J. Digit. Health 2020, 1, 40–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Chen, Q.; Ma, S. Left atrial fibrosis in atrial fibrillation: Mechanisms, clinical evaluation and management. J. Cell. Mol. Med. 2021, 25, 2764–2775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veenhuyzen, G.D.; Simpson, C.S.; Abdollah, H. Atrial fibrillation. Can. Med Assoc. J. CMAJ 2004, 171, 755–760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nesheiwat, Z.; Goyal, A.; Jagtap, M. Atrial Fibrillation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Pellman, J.; Sheikh, F. Atrial fibrillation: Mechanisms, therapeutics, and future directions. Compr. Physiol. 2015, 5, 649–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ludhwani, D.; Wieters, J.S. Paroxysmal Atrial Fibrillation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Han, S.; Jia, R.; Cen, Z.; Guo, R.; Zhao, S.; Bai, Y.; Xie, M.; Cui, K. Early rhythm control vs. rate control in atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 978637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alobaida, M.; Alrumayh, A. Rate control strategies for atrial fibrillation. Ann. Med. 2021, 53, 682–692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parameswaran, R.; Al-Kaisey, A.M.; Kalman, J.M. Catheter ablation for atrial fibrillation: Current indications and evolving technologies. Nat. Rev. Cardiol. 2021, 18, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Rottner, L.; Metzner, A. Langzeiterfolg nach Vorhofflimmerablation [Long-term success after catheter ablation of atrial fibrillation]. Herzschrittmacherther Elektrophysiol. 2023, 34, 286–290. (In German) [Google Scholar] [CrossRef] [PubMed]
- Wiggins, B.S.; ACC Solution Set Oversight Committee; Cibotti-Sun, M.; Moore, M.M. 2023 Atrial Fibrillation Guideline-at-a-Glance. J. Am. Coll. Cardiol. 2024, 83, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156, Erratum in Circulation 2024, 149, e167. Erratum in Circulation 2024, 149, e936. Erratum in Circulation 2024, 149, e1413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Monaco, A.; Vitulano, N.; Troisi, F.; Quadrini, F.; Romanazzi, I.; Calvi, V.; Grimaldi, M. Pulsed Field Ablation to Treat Atrial Fibrillation: A Review of the Literature. J. Cardiovasc. Dev. Dis. 2022, 9, 94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Repp, M.L.; Chinyere, I.R. Opportunities and Challenges in Catheter-Based Irreversible Electroporation for Ventricular Tachycardia. Pathophysiology 2024, 31, 32–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iyengar, S.K.; Iyengar, S.; Srivathsan, K. The promise of pulsed field ablation and the challenges ahead. Front. Cardiovasc. Med. 2023, 10, 1235317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, X.; Liu, S.; Yin, H.; He, Q.; Xue, Z.; Lu, C.; Su, S. Study on Optimal Parameter and Target for Pulsed-Field Ablation of Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 690092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chinyere, I.R.; Mori, S.; Hutchinson, M.D. Cardiac Blood Vessels and Irreversible Electroporation: Findings from Pulsed Field Ablation. Vessel. Plus 2024, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Hartl, S.; Reinsch, N.; Füting, A.; Neven, K. Pearls and Pitfalls of Pulsed Field Ablation. Korean Circ. J. 2023, 53, 273–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ha, F.J.; Han, H.C.; Sanders, P.; Teh, A.W.; O’Donnell, D.; Farouque, O.; Lim, H.S. Prevalence and prevention of oesophageal injury during atrial fibrillation ablation: A systematic review and meta-analysis. Europace 2019, 21, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Arbogast, A.; Chinyere, I.R. Pulsed Field Ablation and Neurocardiology: Inert to Efferents or Delayed Destruction? Rev. Cardiovasc. Med. 2024, 25, 106. [Google Scholar] [CrossRef]
- McBride, S.; Avazzadeh, S.; Wheatley, A.M.; O’Brien, B.; Coffey, K.; Elahi, A.; O’Halloran, M.; Quinlan, L.R. Ablation Modalities for Therapeutic Intervention in Arrhythmia-Related Cardiovascular Disease: Focus on Electroporation. J. Clin. Med. 2021, 10, 2657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suárez, A.G.; Hornero, F.; Berjano, E.J. Mathematical modeling of epicardial RF ablation of atrial tissue with overlying epicardial fat. Open Biomed. Eng. J. 2010, 4, 47–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakatani, Y.; Sridi-Cheniti, S.; Cheniti, G.; Ramirez, F.D.; Goujeau, C.; André, C.; Nakashima, T.; Eggert, C.; Schneider, C.; Viswanathan, R.; et al. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace 2021, 23, 1767–1776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- King, J.B.; Azadani, P.N.; Suksaranjit, P.; Bress, A.P.; Witt, D.M.; Han, F.T.; Chelu, M.G.; Silver, M.A.; Biskupiak, J.; Wilson, B.D.; et al. Left Atrial Fibrosis and Risk of Cerebrovascular and Cardiovascular Events in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Badger, T.J.; Daccarett, M.; Akoum, N.W.; Adjei-Poku, Y.A.; Burgon, N.S.; Haslam, T.S.; Kalvaitis, S.; Kuppahally, S.; Vergara, G.; McMullen, L.; et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: Lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ. Arrhythm. Electrophysiol. 2010, 3, 249–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyle, P.M.; Sarairah, S.; Kwan, K.T.; Scott, G.D.; Mohamedali, F.; Anderson, C.A.; Bifulco, S.F.; Ordovas, K.G.; Prutkin, J.; Robinson, M.; et al. Elevated fibrosis burden as assessed by MRI predicts cryoballoon ablation failure. J. Cardiovasc. Electrophysiol. 2023, 34, 302–312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sohns, C.; Fink, T.; Braun, M.; Sciacca, V.; Piran, M.; Khalaph, M.; Hamriti, M.E.; Guckel, D.; Imnadze, G.; Sommer, P. Lesion formation following pulsed field ablation for pulmonary vein and posterior wall isolation. Pacing Clin. Electrophysiol. 2023, 46, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Torlapati, P.G.; Casella, M.; Della Rocca, D.G.; Schiavone, M.; Doty, B.; La Fazia, V.M.; Pahi, S.; Pierucci, N.; Valeri, Y.; et al. Redefining the blanking period following pulsed-field ablation in patients with atrial fibrillation. Heart Rhythm. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Thermal Ablation | Pulsed-Field Ablation | |
|---|---|---|
| Mechanism of Action | Necrosis of all local cells along with destruction of extracellular matrix and acellular connective tissue | Irreversible electroporation of membrane-bound cells including cardiomyocytes without disruption of extracellular matrix and acellular connective tissue |
| Specific Contraindications | Intracardiac locations at a high risk for fistula formation | Higher than average propensity to vasospasm coronary arteries |
| Blanking Period | 3 months | Estimated to be 1 month presently |
| Long-term Durability | High, potentially curative after serial ablations | Unknown presently, potentially high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinyere, T.C.; Chinyere, I.R. Management of Left Atrial Tachyrhythms in the Setting of HFpEF with Pulsed-Field Ablation: Treating Fire with Water? Therapeutics 2024, 1, 42-51. https://doi.org/10.3390/therapeutics1010006
Chinyere TC, Chinyere IR. Management of Left Atrial Tachyrhythms in the Setting of HFpEF with Pulsed-Field Ablation: Treating Fire with Water? Therapeutics. 2024; 1(1):42-51. https://doi.org/10.3390/therapeutics1010006
Chicago/Turabian StyleChinyere, Tyler Chinedu, and Ikeotunye Royal Chinyere. 2024. "Management of Left Atrial Tachyrhythms in the Setting of HFpEF with Pulsed-Field Ablation: Treating Fire with Water?" Therapeutics 1, no. 1: 42-51. https://doi.org/10.3390/therapeutics1010006
APA StyleChinyere, T. C., & Chinyere, I. R. (2024). Management of Left Atrial Tachyrhythms in the Setting of HFpEF with Pulsed-Field Ablation: Treating Fire with Water? Therapeutics, 1(1), 42-51. https://doi.org/10.3390/therapeutics1010006






