Abstract
Background: Endoscopic retrograde cholangiopancreatography (ERCP) is preferred for biliary drainage in malignant distal biliary obstruction (MDBO). Endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) is considered a rescue therapy for failed ERCP. This study aims to evaluate the safety and efficacy of this technique as the primary modality for MDBO biliary drainage. Methods: An electronic database search was conducted following PRISMA guidelines to identify studies on EUS-CDS for primary biliary drainage in MDBO. A meta-analysis was performed using random and fixed effects models. Results: We extracted data from 10 eligible studies comprising 519 patients. The mean age for the study was 70 years ± SD 2.66. The pooled technical success rate was 92.36% (95% CI = 88.39–95.56), and the clinical success rate was 88.91% (95% CI = 85.22–92.13). The pooled stent dysfunction rate was 13.66% (95% CI = 7.47–21.35), and the reintervention rate was 15.91% (95% CI = 11.00–21.54) of patients. The mean stent patency duration was 229.20 days ± SD 113.9. The total pooled adverse events rate was 17.50% (95% CI = 12.90–22.64), and 9.03% (95% CI = 4.43–15.05) was considered moderate to severe. Procedure-related pancreatitis had a pooled rate of 0%. The pooled adverse event rate of acute cholangitis was 6.84% (95% CI = 3.69–10.88), and for acute cholecystitis it was 2.61% (95% CI = 1.06–4.83). Conclusions: EUS-CDS demonstrates favorable outcomes when used as a primary approach in MDBO. With a long stent patency duration and no procedure-related acute pancreatitis, it may be considered the primary technique when expertise is available.
1. Introduction
Malignant distal biliary obstruction (MDBO) often develops with tumors arising from the distal common bile duct, ampulla, duodenum, or pancreas. Many patients present with symptomatic biliary obstruction at diagnosis, which manifests as painless jaundice. Endoscopic transpapillary stenting for preoperative biliary drainage is recommended when MDBO is complicated by cholangitis, severe symptomatic jaundice (>15 mg/dL), and expected delay in surgery, and in jaundiced patients awaiting neoadjuvant chemotherapy [1]. Endoscopic therapy for palliative biliary drainage is also recommended in unresectable cases of MDBO [1].
Endoscopic retrograde cholangiopancreatography (ERCP) followed by transpapillary stent placement remains the primary treatment modality for achieving biliary drainage in MDBO [1,2,3,4]. In the United States, approximately 500,000 ERCPs are completed yearly, with a high success rate ranging from 82% to 98% [2,3,4]. However, ERCP may be unsuccessful in subjects with ampullary lesions, periampullary diverticulum, gastroduodenal obstruction, or anatomic variations [5,6]. Additionally, pancreatitis is a significant adverse event of ERCP. Previous studies have reported that post-ERCP pancreatitis occurs in about 9.7% of all patients and up to 14% of high-risk patients [7]. Percutaneous biliary drainage (PBD) is the standard alternative to a failed or complicated ERCP. However, the risks of the long-standing external biliary drain, long recovery times, and patient discomfort have been associated with this procedure [8,9].
Since the early 2000s, following Giovanni’s report, endoscopic ultrasound-guided biliary drainage (EUS-BD) has gained popularity and is now extensively used as an alternative to PBD for biliary drainage when ERCP fails [10]. In a meta-analysis by Khan et al., the reported technical and clinical success rates of EUS-BD were 90% and 94%, respectively [11]. EUS-BD techniques include EUS-guided hepaticogastrostomy, choledochoduodenostomy, and gallbladder drainage [12]. Hepaticogastrostomy involves a transmural fistula created between the gastric lumen and the left hepatic duct, while in EUS-CDS, a fistula is created between the duodenal lumen and the dilated common bile duct. These procedures are established by creating a biliodigestive fistula and deploying a self-expanding metallic stent under EUS guidance [12]. In addition to EUS-guided transmural approaches, an EUS-assisted ERCP technique can also be employed in failed ampullary canulation in MDBO [13]. This EUS-rendezvous procedure involves the introduction of a guidewire into the biliary tract under EUS guidance, which is followed by advancement into the duodenum across the ampulla. An ERCP can then be completed over this guidewire [13,14].
The EUS-CDS approach is preferred for patients with mid- or distal biliary obstruction or those with insufficient intrahepatic bile duct dilation [15]. When coupled with an electrocautery-enhanced lumen-apposing metal stent (EC-LAMS), this technique achieves higher success rates and minimal adverse events [16,17]. Due to its relative ease, shorter procedure times, and favorable outcome data, more endoscopists prefer this technique (EUS-CDS with EC-LAMS) to other EUS-BD techniques [18]. By avoiding the ampulla, the risk of procedure-related pancreatitis is almost non-existent with EUS-CDS compared to ERCP [19]. EUS-CDS also has a reduced risk of stent dysfunction or obstruction, which may result from tumor ingrowth or overgrowth, as the region of malignancy is typically not involved [19]. EUS-BD has also been shown to have fewer adverse events compared to PBD [20]. Numerous studies have evaluated the safety and efficacy of EUS-CDS as a salvage technique after a failed ERCP [12,21,22,23,24]. However, at this time, there are limited data on using EUS-CDS as the primary technique for biliary drainage in malignant distal biliary obstruction. This meta-analysis aims to assess the safety and efficacy of primary EUS-CDS for MDBO.
2. Methods
2.1. Search Strategy
This meta-analysis adhered to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. However, the review was not registered. A literature search was conducted using electronic database engines such as PubMed, EMBASE, Cochrane Library, and Ovid from January 2005 through December 2023 to identify studies that evaluated EUS-CDS as the primary intervention for treating biliary obstruction due to MDBO. The keywords used were “endoscopic ultrasound-guided choledochoduodenostomy”, “malignant distal biliary drainage”, “MDBO”, “endoscopic ultrasound-guided biliary drainage”, “EUS-BD”, and “EUS-CDS”. References of reviewed articles were then scanned for similar studies. Included searches were required to report technical and clinical success rates, reintervention rates, and adverse events. The selected studies were assessed to exclude potential duplicates.
2.2. Inclusion and Exclusion Criteria
Prospective and retrospective studies that evaluated endoscopic EUS-CDS as the primary modality for biliary drainage in MDBO were included in the meta-analysis. Alternate methods of EUS-BD, such as hepaticogastrostomy and EUS-guided gall bladder drainage, were excluded. Previous studies have evaluated the utility of EUS-guided biliary drainage as a rescue intervention after failed ERCP. The primary aim of our study was to evaluate the safety and efficacy of EUS-CDS as the primary approach for biliary intervention. Hence, we excluded studies that looked at EUS-CDS in failed ERCP because they were likely to confound the results, as outcomes of EUS-CDS may be influenced by prior ERCP attempts. Abstracts with incomplete data, animal-based studies, and comments were also excluded. The articles were reviewed independently by two authors (E.A., H.G.). A third author was invited to review when a mutual agreement was not reached (S.P.).
2.3. Data Extraction
The following data were extracted from the selected studies for this meta-analysis: (1) study characteristics (primary author, period of study, study design, year of publication, and country of the population studied), (2) patient characteristics (number of patients enrolled, participant demographics), (3) intervention details (indications, type of stent used, stent diameter), (4) outcomes (technical success, clinical success rates, procedure duration, stent dysfunction and patency, adverse events, re-intervention rate).
2.4. Quality Assessment
The included studies were assessed using a modified Newcastle–Ottawa scale. The studies with a score of 5 or more out of eight items in selection, comparability, and outcome were selected for the meta-analysis. (Table 1). Five non-randomized studies were included in this study, and each had a score of at least 5. The randomized controlled trials were also assessed for quality using the Jadad score scale. This is a seven-item scale based on randomization, allocation concealment, blinding, dropouts, and withdrawals. A study that met a score of 3 or more criteria was considered a good-quality randomized controlled trial (Table 2). Five randomized trials were included in the study, and all had Jadad scores of 3 or above.

Table 1.
Newcastle–Ottawa quality assessment.

Table 2.
Jadad scale for randomized controlled trials.
2.5. Outcomes of Study
This study aimed to assess the safety and efficacy of choledochoduodenostomy when used as the primary approach for malignant distal biliary obstruction. The outcomes of interest were technical and clinical success rates, reintervention rate, stent patency, and adverse events. Procedural time was recorded as the time from scope insertion to scope removal at the end of the procedure. The stent patency was the mean time interval from stent insertion to stent dysfunction. The adverse events, when reported, were graded on a severity scale based on the American Society of Gastrointestinal Endoscopy.
2.6. Statistical Analysis
This meta-analysis was conducted by calculating pooled estimates. With the Freeman–Turkey double arcsine transformation, individual estimates were transformed into one. The pooled proportion was then calculated using the inverse arcsine variance weights and DerSimonian–Laird weights for the fixed and random effects models, respectively. For this study, findings were reported based on the random effects model. Forest plots showed individual estimates in each study and the pooled estimate. The widths of the point estimates represented the weights of the studies. Cochran’s Q test and the I2 statistic were employed to assess the heterogeneity of the studies. I2 values of 0–39% had non-significant heterogeneity, values of 40–75% were moderate, and 76–100% were termed considerable heterogeneity. The null hypothesis assumes that there is heterogeneity, and a p-value > 0.10 indicates that there is no statistical evidence of heterogeneity. The effects of publication and selection bias were tested using the Egger bias and the Begg–Mazumdar bias indicators. Potential publication bias was also assessed using funnel plots. Cohen’s κ was utilized to assess interobserver variability. The statistical analysis was done using Microsoft Excel 2019.
3. Results
The initial search strategy identified 3893 publications, of which 841 relevant articles were reviewed. Subsequently, data were extracted from 10 studies comprising 519 patients who had met the inclusion criteria to be included in the final analysis. The PRISMA flow diagram in Figure 1 describes the details of the review process.
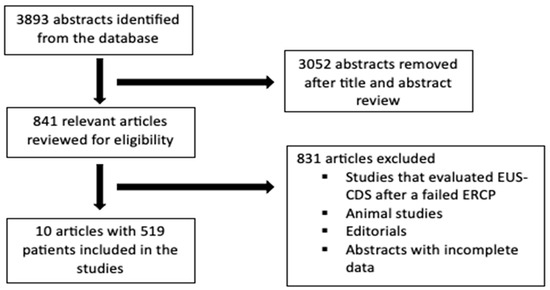
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart describing study selection process.
Among the 10 selected studies, 8 were prospective and 2 were retrospective. A total of 519 patients were included in the meta-analysis, and the study characteristics are listed in Table 3. Indications for EUS-CDS in these patients were pancreatic cancer (87%), cholangiocarcinoma (4%), gallbladder cancer (1%), ampullary cancer (2%), and other gastrointestinal cancers (6%). The mean patient age was 70 years ± SD 2.66, with females comprising 47.09%. The average common bile duct diameter was 14.8 mm ± SD 1.47. The mean procedure time was 16.9 min ± SD 5.80. Average stent patency was 229.20 days ± SD 113.91, and there was a stent dysfunction rate of 13.66% (95% CI = 7.47–21.35).

Table 3.
Demographics and indications for the included studies.
The pooled technical success rate was 92.36% (95% CI = 88.39–95.56). A forest plot representing the individual and pooled estimates is shown in Figure 2. The pooled clinical success rate was 88.91% (95% CI = 85.22–92.13). There was no evidence of significant heterogeneity, with an I2 value of 20.80% (95% CI = 0–62). A forest plots showing the individual and pooled estimates for clinical success can be seen in Figure 3. The Begg–Mazumdar bias indicator yielded a Kendall’s tau b value of −0.11 (p = 0.60), suggesting no publication bias. There was also no evidence of publication bias when calculated using the Egger bias indicator, which yielded a value of −0.72 (95% CI = −0.37–4.00, p = 0.592).

Figure 2.
Forest plot showing the technical success rate for EUS-CDS [19,24,25,26,27,28,29,30,31,32].
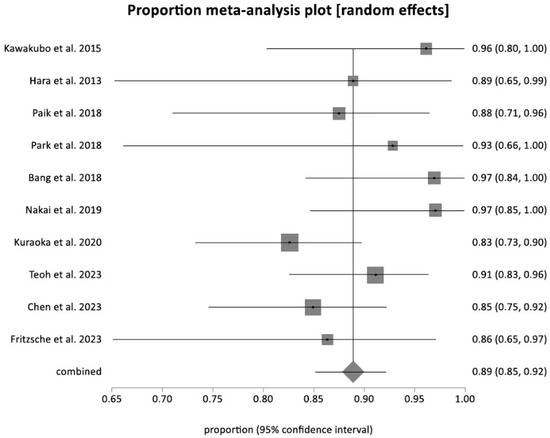
Figure 3.
Forest plot showing the clinical success rate for EUS-CDS [19,24,25,26,27,28,29,30,31,32].
The pooled reintervention rate was 15.91% (95% CI = 11.00–21.54). Figure 4 shows a forest plot demonstrating individual and pooled estimates for the reintervention rate. Reasons for reintervention included acute cholangitis, cholecystitis, stent clogging and migration, and tumor overgrowth. The pooled rate of all the adverse events was 17.50% (95% CI = 12.90–22.64) (Figure 5). Figure 6 shows a funnel plot evaluating the publication bias for overall adverse events.

Figure 4.
Forest plot showing the reintervention rate for EUS-CDS [19,24,25,26,27,28,29,30,31,32].
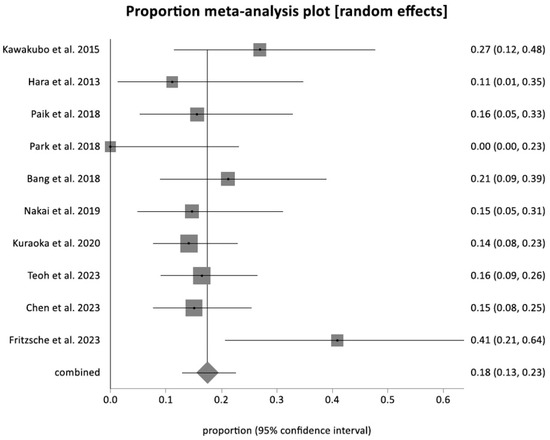
Figure 5.
Forest plot showing the adverse event rate for EUS-CDS [19,24,25,26,27,28,29,30,31,32].
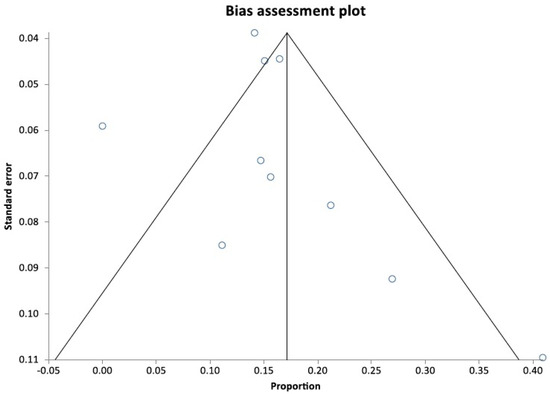
Figure 6.
Funnel plot evaluating publication bias [19,24,25,26,27,28,29,30,31,32].
There were no reports of pancreatitis associated with EUS-CDS, yielding a pooled rate of 0%. Adverse events were classified based on severity, with 9.03% (95% CI = 4.43–15.05) being moderate to severe. The pooled adverse event rate for acute cholangitis was 6.84% (95% CI = 3.69–10.88), and for acute cholecystitis it was 2.61% (95% CI = 1.06–4.83).
4. Discussion
ERCP-guided biliary drainage is the current standard of care for managing malignant distal biliary obstruction due to its high success rate and limited negative outcomes [4]. However, many patients with MDBO present with duodenal invasion or alteration of the biliary tract anatomy, which may hinder the success of this procedure [5,6]. Endoscopic ultrasound-guided biliary drainage has been established as a safe and successful strategy for managing MDBO in cases of failed ERCP [5,33]. Endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) is a commonly used EUS-BD technique that has similar success and adverse event rates to ERCP without the risk of pancreatitis [7,19,34]. It also has a shorter intervention time than ERCP and PBD [20]. In addition, EUS-CDS using electrocautery-enhanced lumen-apposing metal stents is evidently faster and safer than other EUS-BD methods [18,34,35,36,37]. However, EUS-CDS is currently considered to be primarily a salvage therapy after a failed ERCP. This meta-analysis evaluated the efficacy and safety of EUS-CDS when performed as the primary intervention for MDBO.
This report included 10 studies with patients who underwent primary EUS-CDS for biliary drainage. Feasibility and efficacy were assessed using technical success, clinical success, and reintervention rates. Technical success was defined uniformly by the included studies as the successful placement of a transmural stent across the duodenum and bile flow through the stent. The clinical success definitions varied slightly across the included studies. These definitions were all within what is considered standard based on current literature. We adopted the outcome data of clinical success as defined by the included studies and merged them into one definition as follows: Clinical success in this study was defined as a reduction in serum bilirubin by 50% or more from its pre-treatment level within the first month. The adverse event rate was used to evaluate the procedure’s safety, and a sub-analysis was done to assess individual complications.
In our study, the pooled technical success rate for EUS-CDS in patients with MDBO was 92%. The clinical success rate was 89%, indicating that most participants had significantly reduced serum bilirubin levels after the study. These values are comparable with the technical and clinical success rates with ERCP in patients with MDBO [19,24,31]. Additionally, ERCP has an overall technical success rate ranging from 85% to 95% and a clinical success rate as high as 96% [38,39]. However, studies have shown that ERCP frequently fails in patients with duodenal involvement or periampullary invasion due to difficulty in cannulating the papilla [5,6,30]. The EUS-CDS technique offers an advantage over ERCP for biliary drainage in this patient group because it does not rely on access to the papilla [12]. Thus, this technique is a preferred option in patients with MDBO and duodenal invasion. Additionally, EUS-CDS has significantly higher clinical success rates than PBD, a standard alternative after failed ERCP, although the technical success rates are comparable with both techniques [20].
The pooled adverse event rate in our meta-analysis was around 17%. On sub-group analysis, the reported rates for acute cholangitis and acute cholecystitis are about 7% and 9%, respectively. Notably, the rate of acute pancreatitis was 0% in our study. Overall, the rate of adverse events for ERCP is similar to that of EUS-CDS, with a range of 7% to 12% [4]. However, acute pancreatitis is the most common adverse event in patients who undergo ERCP [4]. The incidence of post-ERCP pancreatitis ranges from 2% to 15%, with up to 5% of the cases classified as severe [4,40,41]. Post-ERCP pancreatitis can result in significant complications, prolonged hospital stays, and death in severe cases [42]. From our meta-analysis findings, no case of pancreatitis was reported in any of the studies, as the major papilla was not cannulated in this procedure. This highlights the significant role EUS-CDS plays in averting post-procedural pancreatitis in patients with MDBO, hence lowering their risks. EUS-CDS also has lower adverse event and reintervention rates than PBD.
This study has some notable strengths. To our knowledge, this is the first meta-analysis evaluating EUS-CDS alone as the primary approach in managing MDBO. While previous meta-analyses included studies where EUS-CDS was carried out after failed ERCPs, this meta-analysis only included studies that assessed EUS-CDS as a primary treatment method for MDBO, thus eliminating the possible confounding effect of prior ERCP attempts. Based on the above findings, it is recommended that EUS-CDS be the primary intervention, particularly in high-risk patients, as it has comparable clinical outcomes with ERCP while completely eliminating the risk of post-procedural pancreatitis, which can be fatal in this patient group. Additionally, many patients with MDBO would also have a duodenal invasion or altered biliary tract anatomy, and it would be futile to attempt an ERCP in such patients when EUS-CDS, which completely bypasses the papilla, exists.
This study has some limitations, such as variability in different aspects of the included studies, particularly study designs, stent types, and clinical settings, which may have introduced heterogeneity, measured as the I2 value. The studies applied in this meta-analysis had both prospective and retrospective designs. There was also variability in the type of stents used. Earlier studies reported self-expandable metallic stents deployed in a multistep fashion, while later studies used biliary EC-LAMS with a single-step approach. [19,29,32]. Different stent types yield varying clinical outcomes, and this could affect the generalizability of the study in a larger setting where multiple other stent types are used. Furthermore, the interventions were performed almost exclusively by experts in high-volume centers. This can result in selection bias, as the outcomes from these expert centers might be challenging to replicate in a different setting. Additionally, EUS-CDS is a highly advanced endoscopic procedure with a steep learning curve and the potential to cause severe adverse events. Hence, use of this technique as a primary approach in MDBO, although feasible with potential advantages over ERCP in centers with expertise, will remain largely unavailable in many community centers. This also limits the generalizability of our findings. Future studies with larger sample sizes, standardized clinical outcome definitions, and randomly assigned stent types should be considered, as these will improve the reliability and generalizability of the findings across all clinical settings.
5. Conclusions
EUS-CDS demonstrates favorable outcomes when used as a primary approach in MDBO. With a long stent patency duration and no procedure-related acute pancreatitis, it may be considered the primary technique when expertise is available.
Author Contributions
E.A., study concept and design, data acquisition, analysis and interpretation of data, drafting of the manuscript. H.G., study concept and design, data acquisition, analysis and interpretation of data, drafting of the manuscript. I.V. data acquisition, drafting of the manuscript. S.R.P. statistical analysis, critical revision of the manuscript. Relevant guidelines/checklists—PRISMA guidelines. The manuscript has been read and approved by all the authors, and the requirements for authorship have been met. Each author believes that the manuscript represents honest work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its subsequent amendments.
Informed Consent Statement
This study does not contain identifying information about the patients.
Data Availability Statement
This study is exempt from the need for institutional review board approval, as it is a systematic review and meta-analysis of previously published data.
Conflicts of Interest
The authors of this article do not have any conflict of interest with the publication of this manuscript or any institution or product mentioned in this manuscript, nor is it essential to the outcome of the study presented. The other authors also do not have any conflict of interest with products that compete with those mentioned in this manuscript. None of the other authors had any financial relationship within the last three years with a biotechnology manufacturer, a pharmaceutical company, or other commercial entity that has any interest in the subject matter, materials, or process(es) discussed in the manuscript.
References
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; van Hooft, J.E. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline–Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar]
- Silviera, M.L.; Seamon, M.J.; Porshinsky, B.; Prosciak, M.P.; Doraiswamy, V.A.; Wang, C.F.; Lorenzo, M.; Truitt, M.; Biboa, J.; Jarvis, A.M.; et al. Complications related to endoscopic retrograde cholangiopancreatography: A comprehensive clinical review. J. Gastrointestin. Liver. Dis. 2009, 18, 73–82. [Google Scholar]
- Cappell, M.S.; Friedel, D.M. Stricter national standards are required for credentialing of endoscopic-retrograde-cholangiopancreatography in the United States. World J. Gastroenterol. 2019, 25, 3468–3483. [Google Scholar] [CrossRef]
- Demir, T.; Ustaoglu, M. Evaluation of the success and complication rates of endoscopic retrograde cholangiography according to the difficulty of the procedure. Precis. Med. Sci. 2023, 12, 4–9. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914. [Google Scholar] [CrossRef]
- Perdue, D.G.; Freeman, M.L.; ERCOST Study Group. Failed biliary ERCP: A prospective multicenter study of risk factors, complications, and resource utilization. Gastrointest. Endosc. 2004, 59, P192. [Google Scholar] [CrossRef]
- Kochar, B.; Akshintala, V.S.; Afghani, E.; Elmunzer, B.J.; Kim, K.J.; Lennon, A.M.; Khashab, M.A.; Kalloo, A.N.; Singh, V.K. Incidence, severity, and mortality of post-ERCP pancreatitis: A systematic review by using randomized, controlled trials. Gastrointest. Endosc. 2015, 81, 143–149.e9. [Google Scholar] [CrossRef]
- Kedia, P.; Gaidhane, M.; Kahaleh, M. Endoscopic guided biliary drainage: How can we achieve efficient biliary drainage? Clin. Endosc. 2013, 46, 543–551. [Google Scholar] [CrossRef]
- Hathorn, K.E.; Bazarbashi, A.N.; Sack, J.S.; McCarty, T.R.; Wang, T.J.; Chan, W.W.; Thompson, C.C.; Ryou, M. EUS-guided biliary drainage is equivalent to ERCP for primary treatment of malignant distal biliary obstruction: A systematic review and meta-analysis. Endosc. Int. Open 2019, 7, E1432–E1441. [Google Scholar] [CrossRef]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef]
- Khan, M.A.; Akbar, A.; Baron, T.H.; Khan, S.; Kocak, M.; Alastal, Y.; Hammad, T.; Lee, W.M.; Sofi, A.; Artifon, E.L.A.; et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. Dig. Dis. Sci. 2016, 61, 684–703. [Google Scholar] [CrossRef]
- Uemura, R.S.; Khan, M.A.; Otoch, J.P.; Kahaleh, M.; Montero, E.F.; Artifon, E.L. EUS-guided choledochoduodenostomy versus hepaticogastrostomy: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2018, 52, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Itoi, T.; Sofuni, A.; Tonozuka, R.; Mukai, S. Endoscopic ultrasonography-guided rendezvous technique. Dig. Endosc. 2016, 28, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sonthalia, N.; Rodge, G.A.; Shah, B.B.; Patil, V.; Goenka, M.K. Endoscopic ultrasonography-guided rendezvous technique for removal of a long biliary ascariasis: A challenging case. VideoGIE 2021, 6, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, S.; Liu, X.; Wang, S.; Ge, N.; Wang, G. Endoscopic ultrasound-guided biliary drainage using a fully covered metallic stent after failed endoscopic retrograde cholangiopancreatography. Gastroenterol. Res. Pr. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Dasari, C.S.; Chandrasekar, V.T.; Priyan, H.; Jayaraj, M.; Law, J.; Larsen, M.; Kozarek, R.; Ross, A.; Irani, S. Effectiveness and safety of EUS-guided choledochoduodenostomy using lumen-apposing metal stents (LAMS): A systematic review and meta-analysis. Surg. Endosc. 2020, 34, 2866–2877. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.W.; Jo, S.J.; Moon, J.H.; Lee, Y.N.; Park, J.K.; Lee, T.H.; Cha, S.-W.; Cho, Y.D.; Park, S.-H.; Park, S.I.; et al. A novel electrocautery-enhanced delivery system for one-step endoscopic ultrasound-guided drainage of the gallbladder and bile duct using a lumen-apposing metal stent: A feasibility study. Endoscopy 2021, 53, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Privat, J.; Pinard, F.; Fumex, F.; Chaput, U.; Valats, J.-C.; Cholet, F.; Jezequel, J.; Grandval, P.; Legros, R.; et al. EUS-guided choledochoduodenostomy by use of electrocautery-enhanced lumen-apposing metal stents: A French multicenter study after implementation of the technique (with video). Gastrointest. Endosc. 2020, 92, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-I.; Sahai, A.; Donatelli, G.; Lam, E.; Forbes, N.; Mosko, J.; Paquin, S.C.; Donnellan, F.; Chatterjee, A.; Telford, J.; et al. Endoscopic ultrasound-guided biliary drainage of first intent with a lumen-apposing metal stent vs endoscopic retrograde cholangiopancreatography in malignant distal biliary obstruction: A multicenter randomized controlled study (ELEMENT Trial). Gastroenterology 2023, 165, 1249–1261.e5. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhara, V.; Sawas, T.; Bailey, N.; Yeung, K.K.A.; James, T.; Reddy, S.; Fleming, C.; Marya, N.; Storm, A.; Abu Dayyeh, B.; et al. Comparison of EUS-guided choledochoduodenostomy and percutaneous drainage for distal biliary obstruction: A multicenter cohort study. Endosc. Ultrasound 2022, 11, 223–230. [Google Scholar] [CrossRef]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef]
- Hara, K.; Yamao, K.; Mizuno, N.; Hijioka, S.; Imaoka, H.; Tajika, M.; Tanaka, T.; Ishihara, M.; Okuno, N.; Hieda, N.; et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J. Gastroenterol. 2016, 22, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.A.; Messallam, A.A.; Penas, I.; Nakai, Y.; Modayil, R.J.; De la Serna, C.; Hara, K.; El Zein, M.; Stavropoulos, S.N.; Perez-Miranda, M.; et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open 2016, 4, E175–E181. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.-H.; Kim, S.-O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: A multicenter randomized clinical trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Yamao, K.; Hijioka, S.; Mizuno, N.; Imaoka, H.; Tajika, M.; Kondo, S.; Tanaka, T.; Haba, S.; Takeshi, O.; et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy 2013, 45, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, K.; Kawakami, H.; Kuwatani, M.; Kubota, Y.; Kawahata, S.; Kubo, K.; Sakamoto, N. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy 2015, 48, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Nakai, Y.; Kawakami, H.; Ishiwatari, H.; Kitano, M.; Ito, Y.; Yasuda, I.; Kato, H.; Matsubara, S.; Irisawa, A.; et al. Prospective multicenter study of primary EUS-guided choledochoduodenostomy using a covered metal stent. Endosc. Ultrasound 2019, 8, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, N.; Hara, K.; Okuno, N.; Kuwahara, T.; Mizuno, N.; Shimizu, Y.; Niwa, Y.; Terai, S. Outcomes of EUS-guided choledochoduodenostomy as primary drainage for distal biliary obstruction with covered self-expandable metallic stents. Endosc. Int. Open 2020, 8, E861–E868. [Google Scholar] [CrossRef] [PubMed]
- Voermans, R.P.; Van Wanrooij, R.L.J.; Fritzsche, J.A.; Fockens, P.; Besselink, M.G.; Busch, O.R.; Daams, F.; Montazeri, N.S.M.; Wilmink, J.W. Endoscopic ultrasound-guided choledochoduodenostomy using single-step lumen-apposing metal stents for primary drainage of malignant distal biliary obstruction (SCORPION-p): A prospective pilot study. Endoscopy 2023, 56, 47–52. [Google Scholar] [CrossRef]
- Park, J.K.; Woo, Y.S.; Noh, D.H.; Yang, J.-I.; Bae, S.Y.; Yun, H.S.; Lee, J.K.; Lee, K.T.; Lee, K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018, 88, 277–282. [Google Scholar] [CrossRef]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest. Endosc. 2018, 88, 9–17. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Napoleon, B.; Kunda, R.; Arcidiacono, P.G.; Kongkam, P.; Larghi, A.; Van der Merwe, S.; Jacques, J.; Legros, R.; Thawee, R.-E.; et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology 2023, 165, 473–482.e2. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kitano, M.; Omoto, S.; Kadosaka, K.; Kamata, K.; Miyata, T.; Yamao, K.; Sakamoto, H.; Harwani, Y.; Kudo, M. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest. Endosc. 2016, 84, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Anderloni, A.; Fugazza, A.; Pellegatta, G.; Troncone, E.; Attardo, S.; Cappello, A.; Repici, A. Endoscopic choledochoduodenostomy by lumen-apposing metal stent in jaundice recurrence after transpapillary metal stent placement. Endoscopy 2019, 51, E239–E240. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Privat, J.; Pinard, F.; Fumex, F.; Valats, J.-C.; Chaoui, A.; Cholet, F.; Godard, B.; Grandval, P.; Legros, R.; et al. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents: A retrospective analysis. Endoscopy 2019, 51, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Anderloni, A.; Fugazza, A.; Troncone, E.; Auriemma, F.; Carrara, S.; Semeraro, R.; Maselli, R.; Di Leo, M.; D’amico, F.; Sethi, A.; et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest. Endosc. 2019, 89, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kunda, R.; Pérez-Miranda, M.; Will, U.; Ullrich, S.; Brenke, D.; Dollhopf, M.; Meier, M.; Larghi, A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg. Endosc. 2016, 30, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-K.; Pan, K.-T.; Lee, M.H.; Lin, C.-H. Endoscopic salvage therapy after failed biliary cannulation using advanced techniques: A concise review. World J. Gastroenterol. 2022, 28, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.J.; Arrubla, A.; Jusué, V.; Estremera, F.; González de la Higuerra, B.; Carrascosa, J.; Ibáñez Beroiz, B.; Rodríguez Mendiluce, I.; Hervás, N.; Prieto, C.; et al. The volume of ERCP per endoscopist is associated with a higher technical success and a lower post-ERCP pancreatitis rate. A prospective analysis. Rev. Española De Enfermedades Dig. 2022, 115, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, S.-O.; So, H.; Shin, E.; Kim, D.U.; Park, D.H. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16551. [Google Scholar] [CrossRef]
- Cahyadi, O.; Tehami, N.; De-Madaria, E.; Siau, K. Post-ERCP Pancreatitis: Prevention, Diagnosis and Management. Medicina 2022, 58, 1261. [Google Scholar] [CrossRef]
- Trap, R.; Adamsen, S.; Hart-Hansen, O.; Henriksen, M. Severe and Fatal Complications After Diagnostic and Therapeutic ERCP: A Prospective Series of Claims to Insurance Covering Public Hospitals. Endoscopy 1999, 31, 125–130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).