Abstract
This research compared the cognitive effects of two interventions postulated to induce well-being, concentration, and relaxation: vibroacoustic stimulation and guided mindfulness meditation. Electroencephalogram (EEG) biosignals were used to quantify results and were collated together with subjective first-person verbal accounts. Participants were divided into three groups: guided mindfulness meditation (Group1), vibroacoustic stimulation (Group2), and a no-stimuli control (Group3). EEG results show that vibroacoustic stimulation and guided mindfulness meditation have different cognitive effects. Vibroacoustic stimulation increased concentration and well-being during exposure, with increased relaxation found to be the main effect of meditation. Verbal accounts reinforce these findings. Effects were short-term; practical future implications for vibroacoustic stimulation are discussed.
1. Introduction
Cognitive well-being, concentration, and relaxation are increasingly threatened in contemporary society. Some individuals perceive that AI technologies pose a significant risk to job security and human utility (Kim & Kim, 2024), while others feel digital platforms and social media contribute to feelings of scarcity and inferiority (Lee et al., 2020; Uniyal et al., 2023). Concurrently, a disparate political climate, global environmental crises, and multiple wars all remain out of the citizens’ control. Worldwide psychological unrest is so rife that the World Health Organisation has mandated a 10-year well-being action plan (World Health Organisation, 2021). In a capitalistic tandem with this global mental health plight, a lucrative wellness industry flourishes (Precedence Research, 2023). New products and services that promise physiological and psychological advancement flood a saturated market, though their efficacy for well-being improvement remains dubious and largely unexplored. The present work argues that the intricacies of existing methods for improving well-being, concentration, and relaxation currently lack quantification. These need to be thoroughly explored and compared before new approaches garner further unsubstantiated attention. Thus, this research assesses the cognitive effects on well-being, concentration, and relaxation of vibroacoustic stimulation (as audio/tactile sound) compared to a guided mindfulness meditation, and a no-stimuli Control group.
1.1. Meditation
Meditation is a tool widely used today to increase relaxation and improve well-being, with positive psychological and physiological effects (Brewer et al., 2011; Goyal et al., 2014; Tisdell et al., 2024). Broadly defined as the deliberate regulation of attention toward present-moment experience, the origins of Meditation can be traced back to early Vedic scripture and later Buddhist dhyāna practices. In the modern West, it gained a clinical foothold through Benson’s “relaxation response” paradigm (Benson et al., 1974) and Kabat-Zinn’s Mindfulness-Based Stress Reduction programme (Kabat-Zinn, 1982). It became popularised in the West by alternative medicine advocates and medical doctors (Chopra, 2020; Kabat-Zinn, 2018) and is today a well-recognised tool for inducing relaxation (Taren et al., 2015).
A large-scale meta-analysis covering 47 randomised trials shows that contemporary meditation programmes reliably reduce stress, anxiety, and depressive symptoms while improving overall psychological well-being (Goyal et al., 2014). Physiologically, short-term mindfulness practice is associated with parasympathetic dominance—indexed by higher heart-rate variability and lower cortisol—and EEG signatures of relaxed wakefulness (elevated mid-frontal theta, posterior alpha) (Cahn & Polich, 2006; van der Zwan et al., 2015).
Evidence for meditation-induced enhancements of cognitive function (such as executive attention, working memory, and verbal fluency) is promising but less consistent. Five consecutive 20 min sessions of integrative body–mind training have been shown to improve executive attention relative to relaxation controls (Tang et al., 2007), while four 20 min bouts of focused-attention mindfulness boosted visuo-spatial working memory and verbal fluency in novices (Zeidan et al., 2010). In contrast, a study by Hinterberger et al. (Hinterberger et al., 2014) found global EEG activity to decrease during meditation, as compared to a resting awake state. In line, a synthesis of 56 EEG studies (Lomas et al., 2015) reports substantial heterogeneity in oscillatory outcomes, indicating that brain activity patterns during meditation can differ widely between studies. This underlines a requirement for multimodal, well-controlled investigations (Lomas et al., 2015). The present study meets this need by comparing holistic EEG measurements between two interventions: (i) guided mindfulness meditation—delivered via standardised verbal prompts to reduce mind-wandering—and (ii) vibroacoustic stimulation, an emerging somatosensory technique hypothesised to entrain cortical rhythms.
Guided mindfulness meditation is fundamentally structured around standardised verbal prompts designed to effectively curb mind-wandering. These prompts gently guide the meditator’s attention back to a chosen anchor, such as the breath or bodily sensations, whenever the mind inevitably drifts. Rather than treating mind-wandering as a failure, the instructions normalise this natural cognitive process, encouraging a non-judgmental awareness of distractions. Techniques include explicitly acknowledging and, if desired, briefly labelling thoughts or emotions (e.g., “thinking”), followed by a compassionate redirection of focus back to the present moment. Metaphors, such as training a puppy on a leash, are often employed to foster patience and gentleness towards the wandering mind, thereby cultivating a sustained, non-striving attention. This systematic approach ensures that participants learn to manage attentional shifts with increasing skill and self-compassion (Smart & Smart, 2021).
1.2. Sound and Vibroacoustic Technology
The beneficial effects of sound are notable, from increasing well-being, pleasure, and motivation, to supporting stress management and emotion regulation (Engel et al., 2022; Mao, 2022; Vuust & Kringelbach, 2010). Meta-analytic evidence across 25 randomised controlled trials corroborates these broad benefits, yielding moderate effect sizes for music- and vibration-based interventions on stress and mood outcomes (de Witte et al., 2020).
Therapeutically, sound is used to facilitate confidence, independence, concentration, and awareness of the Self and Other (Bunt et al., 2024; Wigram et al., 2002). Such gains have been documented, for example, in neurological rehabilitation where rhythmic auditory stimulation improved gait and self-efficacy in stroke survivors, and in mental-health settings where receptive music therapy enhanced social relatedness scores in depressive patients (Erkkilä et al., 2011; Thaut et al., 1997).
Physiologically, sound is a vibrational force that can be used to synchronise resonant frequencies in the body (Ala-Ruona et al., 2015; Fernandez, 1997), with hemodynamic, neurological and musculoskeletal effects (ElDeeb & Abdel-Aziem, 2020; Hoffmann & Gill, 2012a, 2012b; Katusic et al., 2013; Zhang et al., 2014). Low-frequency (20–120 Hz) sonic or mechanical vibration delivered via transducers has been shown to entrain cortical alpha rhythms, increase heart-rate variability, and modulate spinal reflex excitability, highlighting a multimodal coupling of central and peripheral systems (L. Bartel & Mosabbir, 2021; Gonçalves de Oliveira et al., 2023; Vilímek et al., 2022). Thus, sound is a powerful tool that can affect both bodily mechanisms and higher-level cognitive functioning (Welch et al., 2022). Recent EEG–fMRI work confirms this dual impact, demonstrating that vibroacoustic entrainment of somatosensory pathways co-activates fronto-parietal attentional networks and yields measurable improvements in executive-function task performance (Burton et al., 2008).
The present research assesses the effects of audible/tactile vibroacoustic technology. This heightens the sonic experience as recipients experience audible and tactile sound through physical contact with hardware devices constituting seats, beds, or boards with built-in bass speakers. It has varied utility, from pain and stress reduction to well-being improvement and symptom relief (Braun Janzen et al., 2019; E. A. Campbell et al., 2019, 2022; Fooks & Niebuhr, 2024a, 2024b; Rüütel et al., 2004). A study by Naghdi et al. (Naghdi et al., 2015) found patients with fibromyalgia experienced median pain, mood, insomnia, and daily living improvements between 65–70%.
Empirical vibroacoustic enquiry lacks defined research protocols and terminological standardisation. Only 18% of studies published between 2015 and 2024 report frequency-response curves or stimulation intensity in decibels, factors that hamper cross-study synthesis (Kantor et al., 2022). Following current methodological recommendations for vibration research, in the present study, EEG is combined with autonomic and subjective measures to capture both neurophysiological and psychophysiological outcomes (Wuestefeld et al., 2020).
The umbrella term ‘vibroacoustic therapy’ can encompass vibroacoustic therapy (VAT) (Kantor et al., 2022), vibroacoustic music (VAM) (Skille, 1989), vibroacoustic (VA) treatment (E. Campbell et al., 2017; E. A. Campbell et al., 2019), physioacoustic therapy (PAT) (Lehikoinen, 1997; Mosabbir et al., 2020), and the physioacoustic (PA) method (Patrick, 1999). For this reason, the present study refers to the stimulation simply as ‘vibroacoustic’. Adopting a single label aligns with recent Delphi-panel recommendations for harmonising nomenclature in the vibroacoustic field (Punkanen & Ala-Ruona, 2012).
1.3. Electroencephalogram (EEG): Well-Being, Concentration, and Relaxation
An electroencephalogram (EEG) measures electrical activity in the brain. As a metric of cognitive state, EEG is used in the present study to quantify the effects of the compared interventions. A Rest stage is included in the study design before each treatment window as a control baseline for EEG. To assess cognitive well-being, concentration, and relaxation, the following EEG metrics are applied:
Well-being is measured with Frontal/Alpha Asymmetry (FAA). FAA increases when greater well-being is experienced, as it is positively correlated with well-being (Wutzl et al., 2023). FAA is a key metric for reliably gauging emotional valence. Widely used in clinical settings and psychological and neuroscientific research, it provides insight into the neural correlates of emotion and motivation. Davidson et al. (Davidson et al., 1985) found that increased activation in the left frontal brain region is linked to positive emotions and approach-oriented behaviours, while heightened activity in the right frontal region corresponds to negative emotions and withdrawal tendencies. FAA has subsequently been used to predict depression relapse with approximately 75% accuracy, as calculated in Stewart et al.’s study (Stewart et al., 2010) with the formula FAA = log(alpha power at left electrode) − log(alpha power at right electrode). Electrodes at AF3 and AF4 were used in the 2010 study, as opposed to the more frontal and lateral AF7 and AF8 in the present research. Had the EEG device used in the present study supported recording of these regions, the authors would have adopted these metrics to support result robustness (Alyan et al., 2021).
Concentration is measured using the Theta/Beta Ratio (TBR). Indicative of unfocused thought and mind-wandering, TBR is negatively correlated with concentration; thus, lower TBR demonstrates higher concentration (van Son et al., 2019). TBR indicates levels of concentration, focus, and attentional processing (Köpruner et al., 1984). The metric has notable utility in ADHD diagnosis and treatment, with Swartwood et al. (2003) using the tool to define a distinction between neurodivergent children and neurotypical children with 85% accuracy (Swartwood et al., 2003). TBR can effectively quantify mind-wandering, which is relevant to its current application in the study methodology.
Relaxation is measured using the Beta/Alpha Ratio (BAR), which is a marker of cognitive arousal or alertness. Positively correlated with arousal, high BAR values show that individuals are more alert (Tee & Mohd Aris, 2020). Comparatively, low BAR scores are demonstrative of less experienced arousal and more relaxation. A well-established EEG measure, BAR is a metric that quantifies how alpha and beta fluctuate under different workloads, and is commonly used to study mental attention and effort (Raufi & Longo, 2022). Termed an “engagement index” by Kislov et al. (Kislov et al., 2023), it has notable utility in predicting the effectiveness of advertisements. While BAR reflects arousal (as opposed to FAA, which reflects emotional valence), BAR was employed in the present study to assess participant relaxation.
Currently unexplored, this work uses EEG to quantify the cognitive effects of vibroacoustic stimulation compared to guided mindfulness meditation with respect to well-being, concentration, and relaxation. The work seeks to understand how effective each intervention is with respect to these measures.
1.4. Hypotheses
Meditation and sound are recognised today by Western science as traditional complementary and alternative medicines (TCAM) (Ng et al., 2023), which are prescribed to support mental health, well-being, and stress-reduction (Chopra et al., 2023; Feneberg & Nater, 2022; Feneberg et al., 2023; Gale, 2014; Gangadhar & Porandla, 2015; Singphow et al., 2022; Valosek et al., 2021). The main research aim of the present study is to assess and compare the short-term cognitive well-being, concentration, and relaxation effects of the two modalities, with the additional respective elements of meditative aural guiding and sonic tactility. The project adopts a novel approach as, to the authors’ knowledge, a comparative empirical investigation of the cognitive effects of guided mindfulness meditation and vibroacoustic stimulation currently lacks in the literature.
Acknowledging the psychological and physiological effects of vibroacoustic stimulation (Boyd-Brewer & McCaffrey, 2004), the main study hypothesis (H1) is that 20 min of exposure will temporarily increase well-being, concentration, and relaxation (Fooks & Niebuhr, 2025b). A second hypothesis (H2) with respect to mindfulness meditation (Zollars et al., 2019) is that this treatment will increase relaxation, and non-uniformly affect well-being and concentration during exposure. This non-uniform outcome is anticipated due to inter-participant variables such as prior meditation experience, susceptibility to new experiences, and on-the-day mental disposition. A third study hypothesis (H3) is that the effects of both interventions (guided mindfulness meditation and vibroacoustic stimulation) will be temporarily sustained after the treatment conditions end.
To support these hypotheses in accordance with the referenced literature, the following EEG outcomes are expected: (H1) during the vibroacoustic treatment, participant FAA (positively correlated with well-being) and BAR (positively correlated with arousal) will increase, while TBR (negatively correlated with concentration) will decrease; (H2) during the guided mindfulness meditation, participant BAR (positively correlated with arousal) will decrease, indicative of heightened relaxation, while FAA and TBR outcomes will be affected non-homogeneously across participants; (H3) after exposure to either the guided mindfulness meditation or vibroacoustic stimulation, participant FAA, TBR and BAR levels will sustain the Treatment-elicited effects for a short period of time. How, and from whom, these EEG measurements were recorded is now discussed before results are explained and explored.
2. Materials and Methods
2.1. Participant Recruitment
All participants were recruited through social media advertisements, with exclusion criteria including chronic pain and/or pre-existing mental health conditions. A single-blind study design circumvented participant priming and biases with the potential to affect results. From the applicants, a random sample of voluntary participants were invited to partake in the study. A total of 41 individuals between 27 and 55 years old (8 males/31 females/2 undisclosed) took part.
2.2. Experimental Procedure
The study design consisted of four stages: the before-treatment reading of a short text (Speech1); a control EEG baseline (Rest); exposure to one treatment only (Treatment) that constituted either a guided mindfulness meditation (Group1), vibroacoustic stimulation (Group2), or a no-stimuli control (Group3); and an after-treatment reading of the same short text as in Speech1, constituting the final study stage (Speech2), see Figure 1.
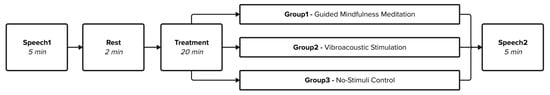
Figure 1.
Flow diagram of each study stage and respective duration.
The Speech1 and Speech2 stages involved a low-cognitive-load reading task (see Section 2.3 Speech1 and Speech2: Elicited speech). The same text was read aloud by participants in both stages to facilitate EEG comparisons between the two conditions. The Rest stage was used as a resting-state EEG control baseline for participants, in which they were required to do nothing but lie with their eyes closed. A between-subjects experimental design was used in which participants were split evenly into one of three treatment groups: Group1 (guided mindfulness meditation), Group2 (vibroacoustic stimulation), and Group3 (a no-stimuli control group). The study design allocated 5 min to the Speech1 and Speech2 stages, and each took approximately 2 min to complete. The Rest stage lasted for 2 min. All Treatments (Group1, Group2, and Group3) had a duration of 20 min. Verbal accounts detailing subjective experiences of each Treatment condition were recorded in the Semi-structured Interview study stage (as itemised in Table 2), which lasted for 5 min. The total required time for each session was 40 min.
Participants were assessed individually, with all sessions conducted at the National Institute of Public Health in Copenhagen, Denmark. The study environment was a calm setting with soft lighting. The experimental environment was lit using one BÖJA IKEA table lamp to create a calm ambience. As the study was conducted during daylight hours, natural light lit the rest of the room. Room temperature was centrally controlled by the building at 19 °C, and due to the nature of the intervention, participants were provided a blanket should they require additional comfort in this regard. The study was conducted in a quiet room that was away from the entrance hall and others using the National Institute of Public Health. To further ensure that external background noise was avoided, signs were hung both on the main door to the study, down the preceding corridor, and throughout the building, informing others that a quiet working environment was imperative. To reduce priming, a sight-blocking and sound-absorbing mobile wall panel was situated between the participant and researcher for all study stages. All participants gave their informed, signed consent prior to their participation, which included the choice to opt-in or opt-out of identifying imagery publication.
To collect and record EEG data, participants wore a Muse S (Gen 2) headset. This apparatus measures the electrical activity of the delta (δ), theta (θ), alpha (α), beta (β), and gamma (γ) frequency bands. The device has a single reference electrode in the middle of the frontal bone at FpZ, and four working dry electrodes located at TP9, AF7, AF8, TP10, as in Figure 2. Additionally, two amplified auxiliary channels can be used to record peripheral signals, which was unnecessary for the present study. The electrodes are positioned at two key locations: the anterior frontal region (AF), which is associated with the prefrontal cortex; and the temporal parietal region (TP), corresponding to the temporoparietal junction. Both brain areas are integral for cognitive functions associated with decision-making, attention, and sensory integration, and thus processes related to emotional processing and stimulus evaluation. The Muse S (Gen 2) device was chosen for multiple reasons: firstly, it provides an accurate and reliable measure of EEG, with electrode locations that measure brain regions directly concerning the research question; secondly, unlike other EEG technologies, the headset offers both the precision and mobility required for this specific study design; and thirdly the technology ensured participant comfort for the full 20 min duration of supine-position data collection (see Figure 3).
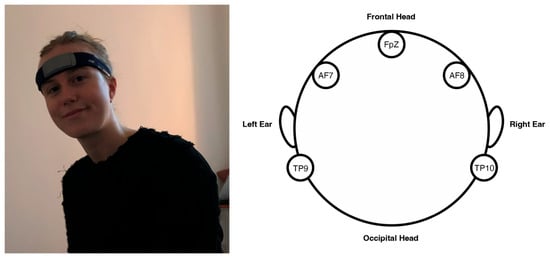
Figure 2.
Participant wearing the Muse S (Gen 2) electroencephalogram (EEG) and respective headband electrode locations.
Figure 3.
Participants during Treatment in the experimental setting.
To support electrode contact and accurate EEG data recording, participants were required to remain still during the 20 min Treatment exposure. The 4 electrodes recorded raw EEG data at 256 Hz, a sampling rate. The EEG device was connected via Bluetooth to Mind Monitor (MindMonitor Technical Manual, 2023), an iPad application which records signals in real time. Mind Monitor conducted a Fast Fourier Transform (FFT) spectral analysis on the raw EEG signals from all four electrodes. This spectral analysis broke the signals down into wavelength frequency components, before dividing them into five frequency bands: delta (<4 Hz), theta (4–7 Hz), alpha (8–15 Hz), beta (16–31 Hz), and gamma (>31 Hz) (Garcia-Moreno et al., 2020), which were visualised in the iPad app (see Figure 4). This data was then further processed before analysis, see Section 2.8 Processing EEG data.
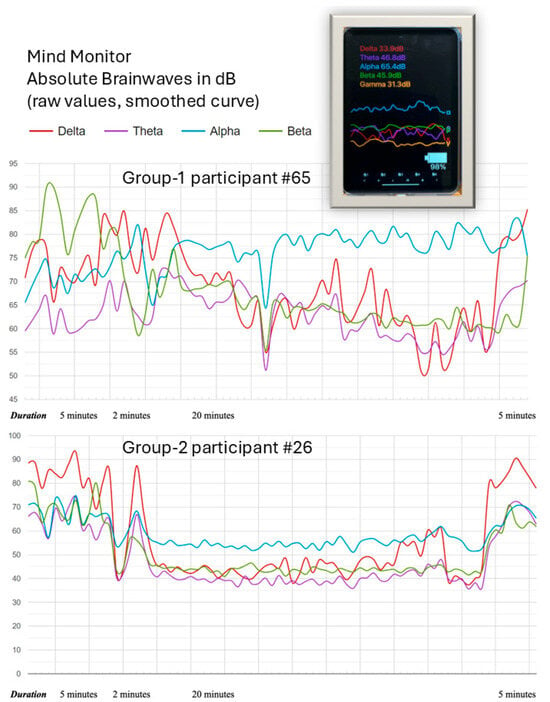
Figure 4.
Two examples of EEG plots (in terms of absolute raw signal amplitudes) for group-1 participant #65 and group-2 participant #26. The plots show the smoothed time courses of the four measured EEG bands, i.e., delta, theta, alpha, and beta. In addition, the Figure shows the Mind Monitor iPad application (top right) displaying live EEG data from the Muse S (Gen 2) as seen by the experimenter during a session.
2.3. Speech1 and Speech2: Elicited Speech
For both the Speech1 and Speech2 stages, participants read aloud “The Rainbow Passage”. This standardised text is widely recognised and frequently employed in speech and voice research, as well as in clinical practice. Its primary purpose is to facilitate the training and evaluation of the pronunciation of all sounds in the English language. Over and above such training and diagnostic purposes, the “Rainbow Passage” is also frequently used to elicit speech material from participants for in-depth phonetic and prosodic analyses. The text is known for eliciting consistent and comprehensive speech samples. Its descriptive nature, focusing on a natural phenomenon and its scientific and mythological interpretations, contributes to its emotional neutrality. This characteristic is vital for minimising emotional variability in speech elicitation, thereby ensuring that the collected speech signals primarily reflect physiological and articulatory aspects rather than variable degrees of affective states. The passage is a phonetically balanced, comprehensive English-language text which is designed to elicit fluent speech, constituting 108 content words (1096 phonemes). It measures speech characteristics of linguistic and emotional valence (Ben-David et al., 2016) that can be recorded by alterations in prosodic speech parameters (or the melody and rhythm of speech) (Xie et al., 2021). The results of this prosodic speech analysis can be retrieved in a separate paper by the same authors (Fooks & Niebuhr, 2025a). The Speech1 and Speech2 study stages are included in the research design as they are used as a comparative EEG metric of a low-cognitive-load task conducted before and after each Treatment.
2.4. Treatment Group1: Guided Mindfulness Meditation
A guided mindfulness meditation recited by well-being practitioner Shamash Alidina was used in the study (Alidina, 2024), which participants listened to with Bose QuietComfort 35 II noise-cancelling headphones. Participants heard the guided mindfulness meditation in full while lying in the supine position, as in Figure 3. The noise-cancelling headphones ensured external noise did not disturb the meditative practice, and an eye mask was provided to avoid extraneous light distraction.
The meditation consisted of a body scan requiring participants to cognitively conduct a slow scan of their body starting from the toes and working methodically up through the limbs and bodily sections towards, and culminating in, the head. During which, they were calmly audibly prompted to mentally project self-compassion and gratitude toward various aspects of themselves and their life. This practice included mustering compassion towards existential life experiences, including able-bodiedness, their employment situation, and relationships with the Self and Other, etc. The recording featured ambient low-level background music of 16 bars of looped string instrumentation and pitched percussion instruments, including bells. Each repetition was delimited by a triangle, after which it proceeded again until the recording’s end. The meditation recording can be retrieved upon request from the first author.
2.5. Treatment Group2: Vibroacoustic Stimulation
The vibroacoustic technology used in the study was hardware designed by the Danish startup vibroacoustics.dk (VibroAcoustics, 2024). This is a single mattress-sized wooden box with one centralised transducer speaker installed. To support participant comfort, it had a yoga mat, pillow, and blanket placed on top. Participants lay with their eyes closed on top of this during all three of the treatment conditions (Group1, Group2, and Group3). It was turned off when not in use. A vibroacoustic soundscape was designed specifically for the study, which participants listened to through Bose QuietComfort 35 II noise-cancelling headphones. This featured sounds of nature, such as bird song and running water (Buxton et al., 2021), instruments of Tibetan origin, including drums, chimes, bells, and gongs, and repetitive low-frequency bass between 40 and 80 Hz. These sonic elements are known to induce relaxation and improve the vibroacoustic experience (L. R. Bartel et al., 2017; Buxton et al., 2021; Mosabbir et al., 2020). Bass in the soundscape was both audible and tactile due to the participants’ physical contact with the vibrating vibroacoustic technology. Notably, it is this audible/tactile element which the study sought to assess, as compared to guided mindfulness meditation. The soundtrack had a set amplitude of 60 dB SPL for all participants. Due to the changing sonic nature of the track, vibroacoustic stimulation frequency occurred between 40 and 80 Hz.
To exemplify how the study assessed a tactile-sonic variable, a separate acoustic analysis was conducted to examine the different sonic characteristics of the guided mindfulness meditation (Group1) and vibroacoustic soundscape (Group2), averaged over their entire length. The main results are summarised in Figure 5, which illustrates that both soundscapes had a similar overall distribution of sound energy: most of it is concentrated in the lower frequency range below 1.5 kHz. However, the meditation (Group1) audio had increasingly less sound energy towards the higher frequency range compared to the vibroacoustic audio (Group2), which made it sound duller overall. This is also reflected in the frequency that was at the centre of the entire soundscape insofar as there were equal amounts of sound energy above and below it—also known as the ‘spectral centre of gravity’ (Kazazis et al., 2022). In the meditation soundtrack, this value was much lower (400.7 Hz) than in the vibroacoustic track (1093.6 Hz), meaning the Group1 audio contained less high-frequency detail and brightness. The Group2 audio also sounded clearer and more vibrant, which is supported by a higher value of the harmonic-to-noise ratio (10.3 dB in Group2, compared to just 1.1 dB in Group1). For all other voice-related features—such as the acoustic equivalents of pitch stability, roughness, and sonority—there were no meaningful differences between the two audio tracks.
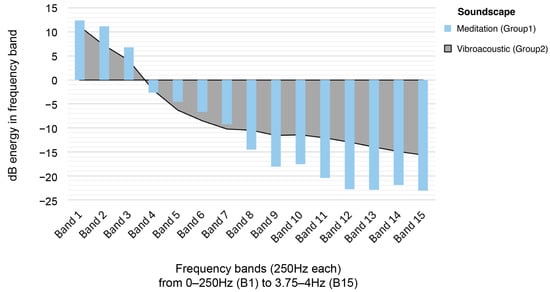
Figure 5.
Frequency band acoustic analysis of Group1 (Guided Mindfulness Meditation) and Group2 (Vibroacoustic Stimulation) soundscapes.
This comparative soundscape analysis suggests that sonic differences between the Group1 and Group2 conditions were too minor for any observed effects to be attributed to the sound alone. That is, the analysis shows that Treatment effects are not only a consequence of the audible soundscapes solely, and that the addition of low-frequency tactile vibration in the Group2 Treatment is an assessed variable in the study design. It is even harder to conclude that it is only the low-frequency audio stimulation that triggers potential, beneficial vibroacoustic effects, as the meditation soundscape is overall more focused on low-frequency sounds. This is in part due to the low-baritone bassy vocal quality of the meditation narrator, as well as the high-pitched sounds of nature in the vibroacoustic soundscape. This acoustic analysis demonstrates that the present study is an assessment of the EEG effects of guided mindfulness meditation compared to synchronised tactile and audible sound, as emitted by vibroacoustic technology.
2.6. Treatment Group3: No-Stimuli Control
Participants in Group3 were instructed to lie in the supine position with their eyes closed for the full 20 min duration of the Treatment. To reduce variables such as external sonic distractions that could cause data noise affecting results, participants wore Bose QuietComfort 35 II noise-cancelling headphones throughout.
2.7. Semi-Structured Interviews
Immediately after the Speech2 study stage, semi-structured interviews were conducted individually with participants to record their subjective first-person experiential accounts of the Treatment (see Table 2). Occurring immediately after the intervention to support memory recall, participants remained in the study setting with their answers transcribed by the experimenter in real-time.
2.8. Processing EEG Data
The collected data underwent a series of sequential signal processing and enhancement steps conducted by the Mind Monitor app: first, basic measurement errors and interferences were filtered out of the raw signals, and secondly, the cleaned data was converted into the delta (δ), theta (θ), alpha (α), beta (β), and gamma (γ) frequency bands. To analyse Treatment effects, the summed power of theta (θ), alpha (α), and beta (β) was analysed and compared (see Section 3: Results). The initial filtering included: a high-pass filter, which removed low-frequency drift; a low-pass filter that eliminated high-frequency noise caused by electronic components and Bluetooth transmission; and a notch filter to remove power supply-related noise, such as typical 50/60 Hz interference. After this, a FFT with 512 points broke the signal into individual spectral frequency components and their amplitudes. For the target frequency bands (theta, alpha, beta), the signal energy was integrated using a root-mean-square (RMS) and converted into decibel (dB) values, which served as the assessed measurement units. The Muse EEG hardware performed some bandpass filtering (0.5–50 Hz) before the data reached the Mind Monitor app. To ensure the data was adequately cleaned, the measurements were manually reviewed to remove zero values, with outliers identified through Q tests. Additional high-frequency noisy segments, such as electrical interference and that caused by artefacts like eye blinks and jaw clenches—detected by the Muse S (Gen 2) device—were excluded prior to analysis. The EEG measurements were recorded per second. With the device’s sampling rate at 256 Hz, mean values were integrated for all dB values over the time window of one second. This further reduced the influence of any unreliable individual values on the final measurements.
3. Results
EEG measurements are inherently noisy and as prior tests on variance homogeneity and normal distribution indicate this, a non-parametric statistical approach was applied instead of parametric tests, such as an analysis of variance. Specifically, a Friedman test for K-paired samples was conducted, i.e., the pendant of a repeated-measures ANOVA. The four levels of the variable Stage served as paired samples, while the three types of EEG measures were the dependent variables. The results of these Friedman tests are summarised in Table 1. A large Friedman test that incorporated all measures and conditions from Table 1 was also performed.

Table 1.
Results summary of the 3 × 3 Friedman tests, in total N = 41. Asterisks indicate p values: * → p < 0.05 (statistical significance at the 5% level), ** → p < 0.01 (statistical significance at the 1% level), *** → p < 0.001 (high statistical significance).
The latter holistic test came out highly significant (c2[11] = 83.799, p < 0.001 ***), indicating that EEG measurements, when expressed as mean ranks, were overall systematically influenced by the four stages of the experimental session (the Study Stages). In other words, EEG values were not stable across the four stages of an experimental session but showed significant increases and decreases between them. Figure 6, Figure 7 and Figure 8 further illustrate these variations by summarising the results for each treatment condition (see also Figure 4 above).
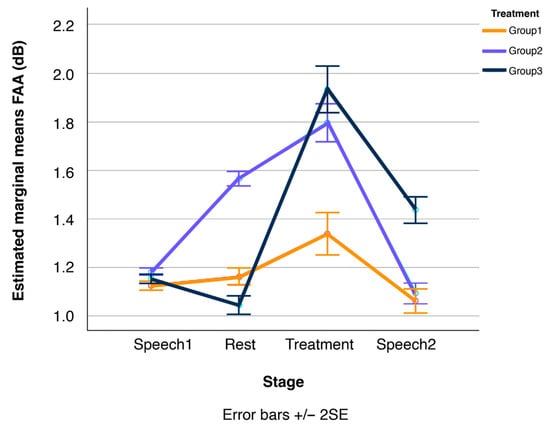
Figure 6.
Line plots of changes in participants’ Frontal/Alpha Asymmetry (FAA) over the four stages of the experimental session, displayed separately for the three treatment conditions, Group1, Group2, and Group3 (N = 41 in total). Error bars show standard errors.
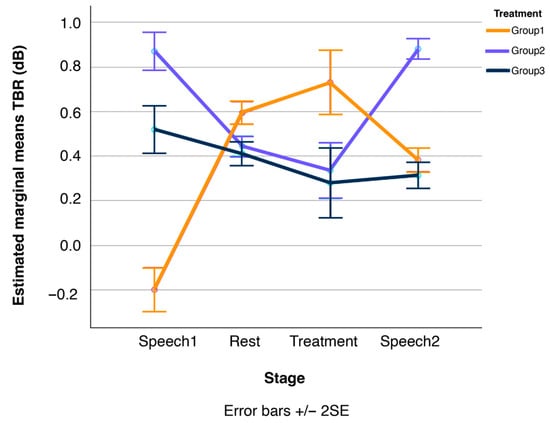
Figure 7.
Line plots of changes in participants’ Theta/Beta Ratio (TBR) over the four stages of the experimental session, displayed separately for the three treatment conditions, Group1, Group2, and Group3 (N = 41 in total). Error bars show standard errors.
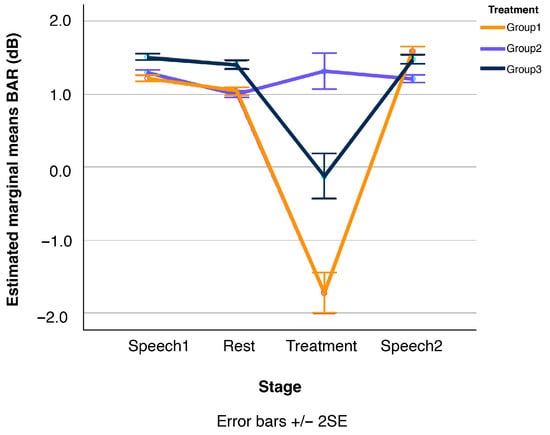
Figure 8.
Line plots of changes in participants’ Beta/Alpha Ratio (BAR) over the four stages of the experimental session, displayed separately for the three treatment conditions, Group1, Group2, and Group3 (N = 41 in total). Error bars show standard errors.
The first key result from Table 1 is that the meditation treatment (Group1) resulted in one significant effect, the vibroacoustic stimulation (Group2) yielded two significant effects, and the control treatment (Group3) produced no significant effects at all—an expected and desirable outcome for a control condition.
The second key result is that the one vs. two effects of the meditation (Group1) and vibroacoustic (Group2) treatments were associated with different EEG measures, i.e., BAR in the case of meditation, and FAA as well as TBR in the case of vibroacoustics. These findings imply that the way Group1 and Group2 stimuli were processed in the brain—and how they cognitively affected participants, particularly in relation to the surrounding Speech1 and Speech2 tasks—differed markedly between the two treatments. (Acknowledging the construct of mereological fallacy (Bennett & Hacker, 2003; Eyghen, 2023), it is imperative to note that the present study is an EEG assessment and thus, results apply solely to the domain of cognition alone. Integration of other quantification metrics (i.e., ECG, galvanic skin response, etc.) would support a comprehensive analysis of neurophysiological intervention effects. Indeed, the quantified EEG outcomes are not independent of embodied physiology, and it is highly likely that gestalt bodily functioning will have also affected outcomes.
Recall that FAA is positively correlated with well-being (or more generally with a positive evaluation of incoming stimuli), while TBR is negatively correlated with concentration. BAR, in turn, is positively correlated with arousal. Thus, these results suggest that participants in the vibroacoustic treatment (Group2) experienced increased cognitive well-being alongside enhanced concentration (i.e., reduced mind-wandering). Verbal accounts transcribed in the semi-structured interviews, as in Table 2, support the subjective participatory experience in this regard. Note that additional non-parametric pairwise comparisons (Mann–Whitney U tests) confirmed that these changes held true relative to both the Speech1 and Speech2 tasks and the Rest stage (at p ≤ 0.05, see Figure 6 and Figure 7).

Table 2.
First-person verbal accounts and EEG relevancy.
In contrast, the primary effect of the meditation (Group1) treatment was a reduction in participants’ arousal levels, or in other words, an increase in relaxation. Again, according to further Mann–Whitney U pairwise comparisons, this effect was evident not only in relation to the surrounding Speech1 and Speech2 tasks, but also in comparison to the Rest stage (at p ≤ 0.05). That is, while vibroacoustic stimulation induced a state of mental balance or ‘being in a flow’ (Csikszentmihalyi, 1990), of heightened well-being and concentration, the meditation treatment led to relaxation but did not enhance perceived well-being or reduce mind-wandering. The vibroacoustic treatment, in turn, had no measurable effect on arousal levels. Also, the corresponding measurement curve in Figure 8 remains largely flat, with a slight but non-significant increase from the Rest to the vibroacoustic stimulation (Group2) stage.
Finally, the third key result is that the effects of both Group1 and Group2 treatments were short-lived. The calming effect of the meditation treatment (Group1) in terms of lower BAR levels was entirely nullified once participants conducted the second speech task (Speech2), as shown in Figure 8. Additional Mann–Whitney U pairwise comparisons confirmed that the BAR levels during both speech tasks before and after the meditation treatment are statistically equivalent. The exact same pattern was observed for participants in the vibroacoustic condition (Group2): their FAA (Figure 6) and TBR levels (Figure 7) during the speech tasks before and after treatment (Speech1 and Speech2) were statistically indistinguishable, see also Figure 4.
Individual verbal accounts of subjective experiences were also recorded in the final Semi-structured Interview stage, as in Table 2. Collating the quantitative and qualitative data, these first-person accounts are consistent with experienced changes recorded in TBR, BAR, and FAA by the EEG. The pull quotes in Table 2 are itemised by their respective EEG relevancy to highlight the potential emotional valence of all three tools for inducing well-being, concentration, and relaxation.
4. Discussion
Through both qualitative and quantitative assessments, this study highlights the aptitude of Frontal/Alpha Asymmetry (FAA), Theta/Beta Ratio (TBR), and Beta/Alpha Ratio (BAR) as cognitive state markers of well-being, concentration, and relaxation.
Results partially support the first and main study hypotheses, that (H1) during vibroacoustic treatment, participant FAA and BAR would increase, whereas TBR would decrease. The EEG data provide positive evidence that during vibroacoustic stimulation (Group2), cognitive well-being and concentration both increased (higher FAA and lower TBR levels). The metric of relaxation (BAR) marginally increased during vibroacoustic treatment, which was indicative of greater cognitive arousal in response to vibroacoustic stimulation. This increase is in line with H1, but came out statistically as not significant.
Hypothesis two (H2) stated that, during the guided mindfulness meditation, participant BAR levels would decrease, while FAA and TBR outcomes would not yield significant effects, e.g., due to being non-homogeneously affected by the treatment across participants. This second hypothesis is fully supported, with results illustrating that during the meditation treatment (Group1), relaxation increased. Furthermore, results show that during this condition, FAA-related well-being increased only marginally (and insignificantly) compared to the vibroacoustic stimulation, while concentration actually decreased for the Group1 meditation participants, despite it being a guided mindfulness intervention.
Hypothesis three (H3) assumed that after exposure to meditation and vibroacoustic stimulation, participant FAA, TBR, and BAR levels would sustain the treatment-elicited effects during the Speech2 phase. Results do not support this third hypothesis, as after all Treatment conditions, the following speech stage EEG data (Speech2) were statistically equivalent to that recorded during the initial speech task prior to the treatment (Speech1). This contrasts with previous research, alike results from a study by the same authors that showed sustained ECG, HRV, and speech prosodic well-being effects after a 45 min vibroacoustic treatment (Fooks & Niebuhr, 2024b). The authors speculate that results differ in the present study due to the shorter Treatment exposure of 20 min, as compared to 45 min in the authors’ own previous study. The sustained well-being effects of meditation have been extensively assessed in longevity studies that indicate the value of repeated exposure to support incremental well-being improvement (Matko et al., 2021). An interesting observation from the present study is that for the no-stimuli control condition (Group3), there was a marginally significant trend of higher FAA values in the Treatment stage compared to the Rest stage of the same group (at p < 0.07). This pairwise comparison indicates that 20 min of stimuli-free respite is potentially beneficial for temporary well-being induction. This EEG data aligns with verbal accounts recorded during the Semi-structured Interviews stage.
4.1. Assumptions and Limitations
As with all research, there are methodological limitations. These are now thoroughly addressed. Firstly, the study’s small heterogeneous sample is ungeneralisable. This limitation is due to individual EEG variances and other differences determining the degree of effectiveness. In future research, a larger participant sample is required to cater for intra-individual variables and ensure result generalisability. Including extraneous variables such as socioeconomic data (i.e., ethnicity, education, and employment status) would support scientific validity and comprehensive insights as to how sample diversity may affect intervention outcomes. Nevertheless, note that the present sample was relevant to everyday life and externally valid. Rather than limiting it to a convenience sample and hence a specific age and/or educational group (such as university students), a random segment of the population that included many age and professional groups was sampled. Additionally, participants were randomly assigned to either the control or test conditions. Although the overall sample was not gender balanced due to the random recruitment principle, this random distribution ensured that the relative gender ratios remained comparable between the two groups. Secondly, a no-stimuli control group (Group3) was chosen in the study design as opposed to an active control to provide a comparison baseline for the effectiveness of the assessed interventions (Group1 and Group2). The study’s single-blinding method supported the methodological decision not to include a placebo, though future research could consider implementing this. Thirdly, the study assessed whether a single 20 min exposure to either a vibroacoustic intervention or guided mindfulness meditation evoked temporary changes in cognitive well-being, concentration, and relaxation; thus, long-term effects are not evaluated. In the future, longevity studies with repeated exposure and follow-ups could implement realist evaluation principles such as a Context-Mechanism-Outcome (CMO) configuration to support a deeper understanding of who the modality works for, and why. A robust study design of this nature would elicit a deeper understanding of the long-term effects of each intervention with respect to what works, how, for whom, to what extent, and in what contexts, to support feasibility and potential scalability. Fourthly, additional qualitative data, in addition to the conducted semi-structured interviews, such as in-depth interviews, would complement and further support EEG findings. Unfortunately, the conduction, coding, and subsequent analysis of these were not viable in the present study. Collecting additional qualitative data should be considered in future vibroacoustic research to support conclusions about interventions that are personal and subjective in nature, such as meditation. Fifthly, this study set out to explore the cognitive effects of the Treatments through empirical investigation using EEG, which it does succinctly, with results illustrating the differences in the modality outcomes. Naturally, additional data would further support these findings, and the study design could be improved with integrated measurement tools, such as heart rate variability (HRV), to assess physiological effects. HRV data were also recorded during this study, which will be published in a separate paper dedicated to the assessment of the physiological effects of the interventions. Prosodic speech data was also recorded and published accordingly (Fooks & Niebuhr, 2025a). In the future, these psychological and physiological metrics could be integrated into one succinct research paper, with cross-correlation conducted between data streams and their respective results to support outcomes and broader investigation. Additionally, in-depth EEG signal processing was beyond the scope of the present study due to the choice of equipment, which will be addressed in future research to support furthered cognitive understanding of these modalities.
4.2. Conceptual Implications of the Results
The observation is that vibroacoustic stimulation elicited a more pronounced increase in FAA than meditation, and that the FAA increase from meditation was only marginal, even compared to a simple Rest condition. This calls for a deeper consideration of the nature of the “well-being” induced by these interventions. If FAA is indeed a marker of well-being, then the differential response of the two treatment conditions suggests that the type or quality of well-being cultivated by a single 20 min meditation session might differ from that captured by FAA. Meditation’s effects on well-being may be more subtle, for example, in the sense that they focus on internal states such as emotional regulation or self-awareness (Fitzgerald, 2024).
As one participant outlines in a post-treatment interview: “Voice and music together bring emotion—this kind of music does it a lot—best way to shut down the brain a bit more.” These more profound, internally focused shifts may not immediately manifest as a strong left-frontal activation, which FAA primarily measures, or they might require longer or repeated exposure to become robustly observable via this specific metric. One participant explicitly stated that they “wanted it to last longer”, while another described the experience as ‘submersive’; “Submersed with the sound of somebodies’ voice…like a cuddle…nice to merge all sounds together.” Such qualitative perception points to a subjective shift that may not be fully captured by FAA alone. Moreover, FAA might be less sensitive to short-duration state changes induced by meditation compared to vibroacoustic stimulation, which could have a more direct, physiologically driven impact, leading to a clearer FAA shift. This highlights that well-being is not a monolithic construct and that its measurement via FAA may capture only specific facets—ones that respond differently to various interventions over different timescales.
The observed significant trend of an increase in TBR during guided mindfulness meditation, signifying decreased concentration, is another interesting finding that is worth reflecting upon separately. Research explicitly links higher TBR to states of mind-wandering (cf. Section 1: Introduction). It is plausible for participants in the meditation group—particularly if they were novices—that guided mindfulness meditation involved an increased awareness of their own internal distractions and mind-wandering. One participant framed it aptly: “Thinking about everything—‘oh I should stop thinking and focus on the activity!’” A similar example is evident in the stated benefits of a “long, deep, calm voice [that] helps to guide, especially if you’re not trained.” That is, the brain is actively processing and noting the guided internal body feelings and actions rather than maintaining a singular, externally directed focus. This interpretation suggests that the observed change is not necessarily a genuine inability to concentrate but rather a reorientation or recalibration of attentional processing during mindfulness practice. Furthermore, it suggests a re-evaluation of how “concentration” is operationally defined within the context of mindfulness. The “concentration” cultivated in mindfulness practices often encompasses both focused attention (e.g., on the breath) and open monitoring (a non-judgmental awareness of thoughts, feelings, and sensations as they arise). If the guided meditation encouraged a broader, less rigid focus, or if participants were engaging in open monitoring, this could lead to a different EEG signature that, when interpreted by a measure primarily designed for sustained, externally directed attention (like TBR in ADHD research, (Arns et al., 2013)), might be labelled as “decreased concentration.” This highlights the need for a more nuanced understanding of attentional processes in mindfulness and suggests that future research might benefit from employing a broader array of attentional measures or more sophisticated EEG analyses to differentiate between various types of attention.
Further worthy of discussion are the contrasting outcomes for BAR: meditation clearly induced relaxation (decreased BAR), while vibroacoustic treatment tended to induce arousal (increased BAR). This suggests that the two interventions might operate through distinct physiological or cognitive pathways. Mindfulness meditation, particularly guided forms, often involves a top-down cognitive process of self-regulation and attentional control, leading to a calm, relaxed state. Vibroacoustic stimulation, by contrast, is a more direct, bottom-up sensory input (auditory and vibrotactile), which may—depending on its specific parameters—stimulate the nervous system in a way that increases arousal and sensory processing (L. R. Bartel et al., 2017). A participant from the vibroacoustic (Group2) expressed this experience vividly: “I felt it in my cells…it goes so deep into the body, into every muscle, every nerve. I’m in awe—I have a really deep calmness. Compared to meditation it goes way deeper in the body—wow!” This illustrates how the somatic grounding provided by vibroacoustic interventions may contribute to a state of active relaxation or bodily immersion that differs in quality from the cognitive calm induced by mindfulness. This supports the idea that “relaxation” is not a single, undifferentiated state but can be achieved via different neural and physiological routes, and accompanied by varying levels of cognitive engagement or arousal.
To compare these result outcomes with existing research, Table 3 itemises relevant literature on the differential neurocognitive profiles of vibroacoustic stimulation and mindfulness meditation.

Table 3.
Comparison of guided mindfulness meditation and vibroacoustic research methodologies.
Table 3 extrapolates findings from comparable EEG research into guided mindfulness meditation and vibroacoustic stimulation to showcase how methodologies and thus outcomes differ. Vibroacoustic research that employs EEG to quantify effects is sparse. Notably, the field requires further investigation as methodological conformity currently lacks, from small to moderate sample sizes, to study duration, localised vs. generalised stimulation, and often state-dependent outcomes (i.e., low cognitive-load tasks, sitting position posture, etc.). As an emerging field, more research is required to understand the specific mechanisms at play and the psychological and physiological effects vibroacoustic interventions have. Evidence suggests the field demands further exploration, which the present work supports as a direct comparison of the two interventions within the same experimental protocol. Comparative studies like this one that assess the effects of known interventions with vibroacoustic stimulation are beneficial as they improve understanding of vibroacoustic effects in context, and in what ways differential outcomes occur.
4.3. Practical Implications
The study provides specific, objective data that can inform therapeutic practice. While bearing in mind its limitations outlined in 4.1 above, practitioners can carefully use these findings to recommend modalities based on a client’s immediate goals. For clients prioritising an immediate boost in concentration and cognitive well-being, vibroacoustic therapy may be more suitable for short sessions. For those primarily seeking relaxation, guided meditation appears effective. However, in regard to managing expectations on treatment duration, it is critical for guides to transparently communicate that 20 min sessions, while immediately impactful, do not appear to yield sustained changes in cognitive state markers post-session. This necessitates emphasising the importance of longer sessions or consistent, repeated practice over time to achieve more enduring benefits, aligning with the concept of incremental well-being improvement through repeated exposure. In addition, the observation that concentration decreased for some meditation participants, even in a guided setting, underscores the need for personalised approaches. Practitioners should be attuned to individual client responses and adapt their guidance accordingly, potentially integrating elements from both modalities or adjusting techniques to mitigate unintended effects.
Finally, but most importantly, the findings seem to hold the potential for a combined approach. The distinct immediate effects of vibroacoustic treatment (concentration/well-being) and (guided) meditation (relaxation) suggest a synergistic potential for combined or sequenced interventions. For instance, vibroacoustic treatment could be used to enhance focus before a meditation session, or meditation to induce relaxation after a stimulating vibroacoustic experience, depending on the desired outcome. Exploring such synergistic effects seems a fruitful point of departure for follow-up studies.
4.4. Conclusions
This study investigated the immediate effects of vibroacoustic stimulation and guided mindfulness meditation on three EEG-derived indices associated with cognitive well-being (FAA), concentration (TBR), and relaxation (BAR). The results suggest that even brief 20 min exposures to these interventions can elicit measurable neurocognitive effects, albeit with distinct profiles: Vibroacoustic stimulation significantly enhanced well-being and concentration but had a less pronounced impact on relaxation. In contrast, mindfulness meditation effectively induced relaxation while yielding more variable or marginal effects on concentration and well-being.
These outcomes underscore the importance of differentiating between cognitive states that are often grouped together under umbrella terms like “well-being.” They further highlight the value of EEG as a method for detecting subtle and temporally dynamic responses to interventions that operate via distinct neural pathways—top-down cognitive regulation in the case of meditation and bottom-up somatosensory stimulation in the case of vibroacoustic treatment. Notably, none of the observed EEG effects persisted into the subsequent speech task, emphasising the short-lived nature of these changes and pointing to the need for longer or repeated exposures to achieve sustained benefits.
In practical terms, the study offers a framework for tailoring interventions to specific therapeutic goals. Vibroacoustic stimulation may be recommended for enhancing cognitive performance and attentional readiness, while guided meditation appears more effective for inducing short-term relaxation. However, the decrease in concentration observed in some meditation participants also calls for personalised approaches, especially for novices, and more nuanced understandings of attentional processing in meditative contexts.
Looking ahead, several avenues merit further exploration. First, future research should investigate the longer-term and cumulative effects of repeated intervention exposure. Second, larger and more diverse samples would allow for testing individual and biographical factors—such as prior meditation experience, sensory sensitivity, or cognitive style—that may influence the strength and quality of intervention effects. Third, testing different soundscapes and voice types in both vibroacoustic and meditative protocols could illuminate how aesthetic or acoustic preferences modulate responsiveness. Fourth, combining EEG with additional biosignal modalities (e.g., HRV, speech acoustics) may yield a richer, more ecologically valid understanding of relaxation, focus, and well-being. Finally, hybrid intervention designs—e.g., priming focus with vibroacoustic input before mindfulness, or using meditation to consolidate somatosensory relaxation—represent an exciting direction for maximising treatment efficacy through sequencing and synergy.
Taken together, these findings and future directions contribute to a more differentiated and data-driven foundation for designing targeted cognitive well-being interventions that are both scientifically grounded and practically applicable.
Author Contributions
Conceptualisation, C.F.; methodology, C.F.; software, O.N.; validation, O.N.; formal analysis, O.N.; investigation, C.F.; resources, O.N.; data curation, C.F. and O.N.; writing—original draft preparation, C.F.; writing—review and editing, C.F. and O.N.; visualisation, C.F. and O.N.; supervision, O.N.; project administration, C.F.; funding acquisition, C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Danish Sound Cluster (DSC), project number 1022-00015B, CVR-40137319, see also https://danishsoundcluster.dk/en/comparing-vibroacoustics-sound-massage-vsm-mindfulness/.
Institutional Review Board Statement
Ethical review and approval were waived for this study, in accordance with Retsinformation Decree on Medical Devices, pursuant to §§1–6 of Act No. 1046 of 17 December 2002 on medical equipment, as amended by §109 of Act No. 1180 of 12 December 2005. As a quality control efficacy study, this waiver is also in accordance with guideline no. 11052 of 2 July 1999 on “Guidance on the introduction of new treatments in the healthcare system”. It is also in accordance with the National Videnskabsetisk Komité (NVK) health science and health data science research project guidelines. All personal information obtained was used in accordance with regulations in the Danish Data Protection Act and treated under Article 6, Paragraph 1, Litra A, and Article 9, Paragraph 2, Litra A.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study. All personal information obtained was used in accordance with regulations in the Danish Data Protection Act and treated under Article 6, Paragraph 1, Litra A, and Article 9, Paragraph 2, Litra A.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to signed confidentiality agreements with each participant. Data may be obtained from the first author upon request.
Acknowledgments
The University of Southern Denmark (SDU), Copenhagen, is thanked for the study location. Extensive gratitude is shared with Paul Fooks for preliminary data cleaning and Lily Baastrup for data collection assistance. Gratitude is given to all participants.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
References
- Ala-Ruona, E., Punkanen, M., & Campbell, E. (2015). Vibroacoustic therapy: Conception, development, and future directions. Musiikkiterapia, 30(1–2), 48–71. [Google Scholar]
- Alidina, S. (2024). New year meditation 2024. Available online: https://www.youtube.com/watch?v=ZjqKQgR-QBw&ab_channel=Mindfulness%7CShamashAlidina (accessed on 9 May 2024).
- Alyan, E., Saad, N. M., Kamel, N., Yusoff, M. Z., Zakariya, M. A., Rahman, M. A., Guillet, C., & Merienne, F. (2021). Frontal electroencephalogram alpha asymmetry during mental stress related to workplace noise. Sensors, 21(6), 1968. [Google Scholar] [CrossRef]
- Arns, M., Conners, C. K., & Kraemer, H. C. (2013). A decade of EEG theta/beta ratio research in ADHD: A meta-analysis. Journal of Attention Disorders, 17(5), 374–383. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-H., Ha, J.-Y., Choung, J. J., Cho, M.-W., Oh, B.-I., Lee, K. H., Youn, Y. C., Kim, S., Song, D.-K., & Shin, C.-H. (2025). Improvement of neuropsychological and cognitive functions in older adults through transcranial vibroacoustic stimulation: A double blind, randomized, comparative trial. Frontiers in Aging Neuroscience, 17, 1526088. [Google Scholar] [CrossRef]
- Bartel, L., & Mosabbir, A. (2021). Possible mechanisms for the effects of sound vibration on human health. Healthcare, 9(5), 597. [Google Scholar] [CrossRef] [PubMed]
- Bartel, L. R., Chen, R., Alain, C., & Ross, B. (2017). Vibroacoustic stimulation and brain oscillation: From basic research to clinical application. Music and Medicine, 9(3), 153. [Google Scholar] [CrossRef]
- Ben-David, B. M., Moral, M. I., Namasivayam, A. K., Erel, H., & van Lieshout, P. (2016). Linguistic and emotional-valence characteristics of reading passages for clinical use and research. Journal of Fluency Disorders, 49, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M. R., & Hacker, P. M. S. (2003). Philosophical foundations of neuroscience. John Wiley & Sons. [Google Scholar]
- Benson, H., Beary, J. F., & Carol, M. P. (1974). The relaxation response. Psychiatry, 37(1), 37–46. [Google Scholar] [CrossRef]
- Boyd-Brewer, C., & McCaffrey, R. (2004). Vibroacoustic sound therapy improves pain management and more. Holistic Nursing Practice, 18(3), 111–118. [Google Scholar] [CrossRef]
- Braun Janzen, T., Al Shirawi, M. I., Rotzinger, S., Kennedy, S. H., & Bartel, L. (2019). A pilot study investigating the effect of music-based intervention on depression and anhedonia. Frontiers in Psychology, 10, 1038. [Google Scholar] [CrossRef]
- Brewer, J. A., Worhunsky, P. D., Gray, J. R., Tang, Y. Y., Weber, J., & Kober, H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences, 108(50), 20254–20259. [Google Scholar] [CrossRef] [PubMed Central]
- Bunt, L., Hoskyns, S., & Swamy, S. (2024). The handbook of music therapy. Routledge. [Google Scholar]
- Burton, H., Sinclair, R. J., & McLaren, D. G. (2008). Cortical network for vibrotactile attention: A fMRI study. Human Brain Mapping, 29(2), 207–221. [Google Scholar] [CrossRef] [PubMed Central]
- Buxton, R. T., Pearson, A. L., Allou, C., Fristrup, K., & Wittemyer, G. (2021). A synthesis of health benefits of natural sounds and their distribution in national parks. Proceedings of the National Academy of Sciences, 118(14), e2013097118. [Google Scholar] [CrossRef]
- Cahn, B. R., & Polich, J. (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin, 132(2), 180–211. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E., Hynynen, J., & Ala-Ruona, E. (2017). Vibroacoustic treatment for chronic pain and mood disorders in a specialised healthcare setting. Music and Medicine, 9, 187–197. [Google Scholar] [CrossRef]
- Campbell, E. A., Hynynen, J., Burger, B., & Ala-Ruona, E. (2019). Exploring the use of vibroacoustic treatment for managing chronic pain and comorbid mood disorders: A mixed methods study. Nordic Journal of Music Therapy, 28(4), 291–314. [Google Scholar] [CrossRef]
- Campbell, E. A., Kantor, J., Kantorová, L., Svobodová, Z., & Wosch, T. (2022). Tactile low frequency vibration in dementia management: A scoping review. Frontiers in Psychology, 13, 854794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chopra, D. (2020). Total meditation: Stress free living starts here. Rider. [Google Scholar]
- Chopra, D., Stern, E., Bushell, W. C., & Castle, R. D. (2023). Yoga and pain: A mind-body complex system. Frontiers in Pain Research, 4, 1075866. [Google Scholar] [CrossRef] [PubMed Central]
- Csikszentmihalyi, M. (1990). Flow: The psychology of optimal experience. Harper & Row. [Google Scholar]
- Davidson, R. J., Schaffer, C. E., & Saron, C. (1985). Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed subjects. Psychophysiology, 22(3), 353–364. [Google Scholar] [CrossRef] [PubMed]
- de Witte, M., Spruit, A., van Hooren, S., Moonen, X., & Stams, G. J. (2020). Effects of music interventions on stress-related outcomes: A systematic review and two meta-analyses. Health Psychology Review, 14(2), 294–324. [Google Scholar] [CrossRef]
- Duda, A. T., Clarke, A. R., & Barry, R. J. (2024). Mindfulness meditation alters neural oscillations independently of arousal. International Journal of Psychophysiology, 205, 112439. [Google Scholar] [CrossRef]
- Duda, A. T., Clarke, A. R., & Barry, R. J. (2025). Differential effects of mindfulness meditation and paced breathing on neural oscillations and arousal. Mindfulness. [Google Scholar] [CrossRef]
- ElDeeb, A. M., & Abdel-Aziem, A. A. (2020). Effect of whole-body vibration exercise on power profile and bone mineral density in postmenopausal women with osteoporosis: A randomized controlled trial. Journal of Manipulative and Physiological Therapeutics, 43(4), 384–393. [Google Scholar] [CrossRef]
- Engel, A., Hoefle, S., Monteiro, M. C., Moll, J., & Keller, P. E. (2022). Neural correlates of listening to varying synchrony between beats in samba percussion and relations to feeling the groove. Frontiers in Neuroscience, 16, 779964. [Google Scholar] [CrossRef] [PubMed Central]
- Erkkilä, J., Punkanen, M., Fachner, J., Ala-Ruona, E., Pöntiö, I., Tervaniemi, M., Vanhala, M., & Gold, C. (2011). Individual music therapy for depression: Randomised controlled trial. The British Journal of Psychiatry, 199(2), 132–139. [Google Scholar] [CrossRef]
- Eyghen, H. V. (2023). The brain perceives/infers. In R. Vinten (Ed.), Wittgenstein and the cognitive science of religion: Interpreting human nature and the mind (pp. 53–71). Bloomsbury Academic. [Google Scholar]
- Feneberg, A. C., & Nater, U. M. (2022). An ecological momentary music intervention for the reduction of acute stress in daily life: A mixed methods feasibility study. Frontiers in Psychology, 13, 927705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feneberg, A. C., Stijovic, A., Forbes, P. A. G., Lamm, C., Piperno, G., Pronizius, E., Silani, G., & Nater, U. M. (2023). Perceptions of stress and mood associated with listening to music in daily life during the COVID-19 lockdown. JAMA Network Open, 6(1), e2250382. [Google Scholar] [CrossRef] [PubMed Central]
- Fernandez, M. (1997). Acoustics and universal movement. In T. Wigram, & C. Dileo (Eds.), Music vibration and health (pp. 11–26). Jeffrey Books. [Google Scholar]
- Fitzgerald, P. J. (2024). Frontal alpha asymmetry and its modulation by monoaminergic neurotransmitters in depression. Clinical Psychopharmacology and Neuroscience, 22(3), 405–415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fooks, C., & Niebuhr, O. (2024a, April 14–19). Assessing vibroacoustic sound massage through the biosignal of human speech: Evidence of improved wellbeing. ICASSP 2024—2024 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Seoul, Republic of Korea. [Google Scholar]
- Fooks, C., & Niebuhr, O. (2024b). Effects of vibroacoustic stimulation on psychological, physiological, and cognitive stress. Sensors, 24(18), 5924. [Google Scholar] [CrossRef] [PubMed]
- Fooks, C., & Niebuhr, O. (2025a). Assessing effects of vibroacoustic stimulation compared to a guided mindfulness meditation using the biosignal of human speech [manuscript under peer review]. Centre for Industrial Electronics, University of Southern Denmark; Frontiers in Network Physiology. [Google Scholar]
- Fooks, C., & Niebuhr, O. (2025b). Comparing the psychological, physiological, and cognitive effects of vibroacoustic stimulation with a guided mindfulness meditation [manuscript in preparation]. Centre for Industrial Electronics, University of Southern Denmark. [Google Scholar]
- Gale, N. (2014). The sociology of traditional, complementary and alternative medicine. Sociology Compass, 8(6), 805–822. [Google Scholar] [CrossRef] [PubMed Central]
- Gangadhar, B., & Porandla, K. (2015). Yoga and mental health services. Indian Journal of Psychiatry, 57(4), 338–340. [Google Scholar] [CrossRef]
- Garcia-Moreno, F. M., Bermudez-Edo, M., Garrido, J. L., & Rodríguez-Fórtiz, M. J. (2020). Reducing response time in motor imagery using a headband and deep learning. Sensors, 20(23), 6730. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves de Oliveira, R., Coutinho, H., Martins, M. N. M., Bernardo-Filho, M., de Sá-Caputo, D. D. C., Campos de Oliveira, L., & Taiar, R. (2023). Impacts of whole-body vibration on muscle strength, power, and endurance in older adults: A systematic review and meta-analysis. Journal of Clinical Medicine, 12(13), 4467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goyal, M., Singh, S., Sibinga, E. M., Gould, N. F., Rowland-Seymour, A., Sharma, R., Berger, Z., Sleicher, D., Maron, D. D., Shihab, H. M., Ranasinghe, P. D., Linn, S., Saha, S., Bass, E. B., & Haythornthwaite, J. A. (2014). Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Internal Medicine, 174(3), 357–368. [Google Scholar] [CrossRef] [PubMed Central]
- Hinterberger, T., Schmidt, S., Kamei, T., & Walach, H. (2014). Decreased electrophysiological activity represents the conscious state of emptiness in meditation. Frontiers in Psychology, 5, 99. [Google Scholar] [CrossRef] [PubMed Central]
- Hoffmann, A., & Gill, H. (2012a). Diastolic timed Vibro-Percussion at 50 Hz delivered across a chest wall sized meat barrier enhances clot dissolution and remotely administered Streptokinase effectiveness in an in-vitro model of acute coronary thrombosis. Thrombosis Journal, 10(1), 23. [Google Scholar] [CrossRef] [PubMed Central]
- Hoffmann, A., & Gill, H. (2012b). Externally applied vibration at 50 Hz facilitates dissolution of blood clots in-vitro. American Journal of Biomedical Sciences, 4(4), 274–284. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. (1982). An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry, 4(1), 33–47. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. (2018). Meditation is not what you think: Mindfulness and why it is so important. Little, Brown Book Group. [Google Scholar]
- Kantor, J., Campbell, E. A., Kantorová, L., Marečková, J., Regec, V., Karasová, K., Sedláčková, D., & Klugar, M. (2022). Exploring vibroacoustic therapy in adults experiencing pain: A scoping review. BMJ Open, 12(4), e046591. [Google Scholar] [CrossRef]
- Katusic, A., Alimovic, S., & Mejaski-Bosnjak, V. (2013). The effect of vibration therapy on spasticity and motor function in children with cerebral palsy: A randomized controlled trial. NeuroRehabilitation, 32(1), 1–8. [Google Scholar] [CrossRef]
- Kazazis, S., Depalle, P., & McAdams, S. (2022). Interval and ratio scaling of spectral audio descriptors. Frontiers in Psychology, 13, 835401. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J., & Kim, M.-J. (2024). How artificial intelligence-induced job insecurity shapes knowledge dynamics: The mitigating role of artificial intelligence self-efficacy. Journal of Innovation & Knowledge, 9(4), 100590. [Google Scholar] [CrossRef]
- Kislov, A., Gorin, A., Konstantinovsky, N., Klyuchnikov, V., Bazanov, B., & Klucharev, V. (2023). Central EEG beta/alpha ratio predicts the population-wide efficiency of advertisements. Brain Sciences, 13(1), 57. [Google Scholar] [CrossRef] [PubMed]
- Köpruner, V., Pfurtscheller, G., & Auer, L. M. (1984). Quantitative EEG in normals and in patients with cerebral ischemia. Progress in Brain Research, 62, 29–50. [Google Scholar] [CrossRef]
- Lee, H. Y., Jamieson, J. P., Reis, H. T., Beevers, C. G., Josephs, R. A., Mullarkey, M. C., O’Brien, J. M., & Yeager, D. S. (2020). Getting fewer “likes” than others on social media elicits emotional distress among victimized adolescents. Child Development, 91(6), 2141–2159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehikoinen, P. (1997). The physioacoustic method. In T. Wigram, & C. Dileo (Eds.), Music vibration and health (pp. 206–216). Jeffrey Books. [Google Scholar]
- Lomas, T., Ivtzan, I., & Fu, C. H. (2015). A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neuroscience & Biobehavioral Reviews, 57, 401–410. [Google Scholar] [CrossRef]
- Mao, N. (2022). The role of music therapy in the emotional regulation and psychological stress relief of employees in the workplace. Journal of Healthcare Engineering, 2022, 4260904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matko, K., Sedlmeier, P., & Bringmann, H. C. (2021). Differential effects of ethical education, physical hatha yoga, and mantra meditation on well-being and stress in healthy participants-an experimental single-case study. Frontiers in Psychology, 12, 672301. [Google Scholar] [CrossRef] [PubMed Central]
- MindMonitor Technical Manual. (2023). Available online: https://mind-monitor.com/Technical_Manual.php (accessed on 5 April 2023).
- Mosabbir, A., Almeida, Q. J., & Ahonen, H. (2020). The effects of long-term 40-hz physioacoustic vibrations on motor impairments in parkinson’s disease: A double-blinded randomized control trial. Healthcare, 8(2), 113. [Google Scholar] [CrossRef] [PubMed Central]
- Naghdi, L., Ahonen, H., Macario, P., & Bartel, L. (2015). The effect of low-frequency sound stimulation on patients with fibromyalgia: A clinical study. Pain Research & Management, 20(1), 21–27. [Google Scholar] [CrossRef]
- Ng, J. Y., Dhawan, T., Fajardo, R.-G., Masood, H. A., Sunderji, S., Wieland, L. S., & Moher, D. (2023). The brief history of complementary, alternative, and integrative medicine terminology and the development and creation of an operational definition. Integrative Medicine Research, 12(4), 100978. [Google Scholar] [CrossRef]
- Patrick, G. (1999). The effects of vibroacoustic music on symptom reduction. IEEE Engineering in Medicine and Biology Magazine, 18, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Precedence Research. (2023). Health and wellness market (By sector: Personal care & beauty & anti-aging, nutrition & weight loss, physical activity, wellness tourism, preventive & personalized medicine, spa economy, and others)—Global industry analysis, size, share, growth, trends, regional outlook, and forecast 2023–2032. Available online: https://www.precedenceresearch.com/health-and-wellness-market (accessed on 11 April 2024).
- Punkanen, M., & Ala-Ruona, E. (2012). Contemporary vibroacoustic therapy: Perspectives on clinical practice, research and training. Music and Medicine, 4, 128–135. [Google Scholar] [CrossRef]
- Raufi, B., & Longo, L. (2022). An evaluation of the Egg alpha-to-theta and theta-to-alpha band ratios as indexes of mental workload. Frontiers in Neuroscience, 16, 861967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Larios, J., Foong Wong, K., & Lim, J. (2024). Assessing the effects of an 8-week mindfulness training program on neural oscillations and self-reports during meditation practice. PLoS ONE, 19(6), e0299275. [Google Scholar] [CrossRef]
- Rüütel, E., Ratnik, M., Tamm, E., & Zilensk, H. (2004). The experience of vibroacoustic therapy in the therapeutic intervention of adolescent girls. Nordic Journal of Music Therapy, 13(1), 33–46. [Google Scholar] [CrossRef]
- Sandler, H., Tamm, S., Fendel, U., Rose, M., Klapp, B. F., & Bösel, R. (2016). Positive emotional experience: Induced by vibroacoustic stimulation using a body monochord in patients with psychosomatic disorders: Is associated with an increase in EEG-theta and a decrease in EEG-alpha power. Brain Topography, 29(4), 524–538. [Google Scholar] [CrossRef]
- Singphow, C., Purohit, S., Tekur, P., Bista, S., Panigrahy, S. N., Raghuram, N., & Nagendra, H. R. (2022). Effect of yoga on stress, anxiety, depression, and spinal mobility in computer users with chronic low back pain. International Journal of Yoga, 15(2), 114–121. [Google Scholar] [CrossRef] [PubMed Central]
- Skille, O. (1989). VibroAcoustic therapy. Music Therapy, 8(1), 61–77. [Google Scholar] [CrossRef]
- Smart, C. M., & Smart, C. M. (2021). 167 meditation practice scripts for audio recordings. In Wisdom mind: Mindfulness for cognitively healthy older adults and those with subjective cognitive decline, facilitator guide. Oxford University Press. [Google Scholar]
- Stewart, J. L., Bismark, A. W., Towers, D. N., Coan, J. A., & Allen, J. J. (2010). Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology, 119(3), 502–512. [Google Scholar] [CrossRef] [PubMed Central]
- Swartwood, J. N., Swartwood, M. O., Lubar, J. F., & Timmermann, D. L. (2003). EEG differences in ADHD-combined type during baseline and cognitive tasks. Pediatric Neurology, 28(3), 199–204. [Google Scholar] [CrossRef]
- Tang, Y.-Y., Ma, Y., Wang, J., Fan, Y., Feng, S., Lu, Q., Yu, Q., Sui, D., Rothbart, M. K., Fan, M., & Posner, M. I. (2007). Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences, 104(43), 17152–17156. [Google Scholar] [CrossRef]
- Taren, A. A., Gianaros, P. J., Greco, C. M., Lindsay, E. K., Fairgrieve, A., Brown, K. W., Rosen, R. K., Ferris, J. L., Julson, E., Marsland, A. L., Bursley, J. K., Ramsburg, J., & Creswell, J. D. (2015). Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: A randomized controlled trial. Social Cognitive and Affective Neuroscience, 10(12), 1758–1768. [Google Scholar] [CrossRef] [PubMed Central]
- Tee, Y. W., & Mohd Aris, S. A. (2020). Electroencephalogram (EEG) stress analysis on alpha/beta ratio and theta/beta ratio. Indonesian Journal of Electrical Engineering and Computer Science, 17, 175. [Google Scholar] [CrossRef]
- Thaut, M. H., McIntosh, G. C., & Rice, R. R. (1997). Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. Journal of the Neurological Sciences, 151(2), 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tisdell, E. J., Lukic, B., Banerjee, R., Liao, D., & Palmer, C. (2024). The effects of heart rhythm meditation on vagal tone and well-being: A mixed methods research study. Applied Psychophysiology and Biofeedback, 49(3), 439–455. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, S., Singh, D., & Chaudhary, S. (2023). Impact of social media usage on inferiority complex in youth. Journal of Kavikulaguru Kalidas Sanskrit University, 10, 62–68. [Google Scholar]
- Valosek, L., Wendt, S., Link, J., Abrams, A., Hipps, J., Grant, J., Nidich, R., Loiselle, M., & Nidich, S. (2021). Meditation effective in reducing teacher burnout and improving resilience: A randomized controlled study. Frontiers in Education, 6, 627923. [Google Scholar] [CrossRef]
- van der Zwan, J. E., de Vente, W., Huizink, A. C., Bögels, S. M., & de Bruin, E. I. (2015). Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: A randomized controlled trial. Applied Psychophysiology and Biofeedback, 40(4), 257–268. [Google Scholar] [CrossRef] [PubMed Central]
- van Son, D., de Rover, M., De Blasio, F. M., van der Does, W., Barry, R. J., & Putman, P. (2019). Electroencephalography theta/beta ratio covaries with mind wandering and functional connectivity in the executive control network. Annals of the New York Academy of Sciences, 1452(1), 52–64. [Google Scholar] [CrossRef] [PubMed Central]
- VibroAcoustics. (2024). The viboard 3.0. Available online: https://vibroacoustics.dk/product (accessed on 28 November 2024).
- Vilímek, Z., Kantor, J., Krejčí, J., Janečka, Z., Jedličková, Z., Nekardová, A., Botek, M., Bucharová, M., & Campbell, E. A. (2022). The effect of low frequency sound on heart rate variability and subjective perception: A randomized crossover study. Healthcare, 10(6), 1024. [Google Scholar] [CrossRef]
- Vuust, P., & Kringelbach, M. L. (2010). The pleasure of music. In Pleasures of the brain (pp. 255–269). Oxford University Press. [Google Scholar]
- Welch, D., Reybrouck, M., & Podlipniak, P. (2022). Meaning in music is intentional, but in soundscape it is not—A naturalistic approach to the qualia of sounds. International Journal of Environmental Research and Public Health, 20(1), 269. [Google Scholar] [CrossRef] [PubMed Central]
- Wigram, A. L., Pedersen, I. N., & Bonde, L. O. (2002). A comprehensive guide to music therapy: Theory, clinical practice, research and training. Jessica Kingsley Publishers. [Google Scholar]
- World Health Organisation. (2021). Comprehensive mental health action plan 2013–2030. Available online: https://www.who.int/publications/i/item/9789240031029 (accessed on 26 November 2024).
- Wuestefeld, A., Fuermaier, A. B. M., Bernardo-Filho, M., da Cunha de Sá-Caputo, D., Rittweger, J., Schoenau, E., Stark, C., Marin, P. J., Seixas, A., Judex, S., Taiar, R., Nyakas, C., van der Zee, E. A., van Heuvelen, M. J. G., & Tucha, O. (2020). Towards reporting guidelines of research using whole-body vibration as training or treatment regimen in human subjects—A Delphi consensus study. PLoS ONE, 15(7), e0235905. [Google Scholar] [CrossRef] [PubMed Central]
- Wutzl, B., Leibnitz, K., Kominami, D., Ohsita, Y., Kaihotsu, M., & Murata, M. (2023). Analysis of the correlation between frontal alpha asymmetry of electroencephalography and short-term subjective well-being changes. Sensors, 23(15), 7006. [Google Scholar] [CrossRef] [PubMed]
- Xie, X., Buxó-Lugo, A., & Kurumada, C. (2021). Encoding and decoding of meaning through structured variability in intonational speech prosody. Cognition, 211, 104619. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, F., Johnson, S. K., Diamond, B. J., David, Z., & Goolkasian, P. (2010). Mindfulness meditation improves cognition: Evidence of brief mental training. Consciousness and Cognition, 19(2), 597–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L., Weng, C., Liu, M., Wang, Q., Liu, L., & He, Y. (2014). Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: A pilot randomized controlled trial. Clinical Rehabilitation, 28(1), 59–68. [Google Scholar] [CrossRef]
- Zollars, I., Poirier, T. I., & Pailden, J. (2019). Effects of mindfulness meditation on mindfulness, mental well-being, and perceived stress. Currents in Pharmacy Teaching and Learning, 11(10), 1022–1028. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).