Abstract
The growing threat of Antimicrobial Resistance (AMR) demands innovative drug discovery, yet conventional 2D cell cultures fail to accurately mimic in vivo conditions, leading to high failure rates in preclinical studies. This review addresses the critical need for more physiologically relevant platforms by exploring recent advancements in bioengineered 3D tissue models for studying bacterial pathogenesis and antimicrobial drug discovery. We conducted a systematic search of peer-reviewed articles from 2015 to 2025 across PubMed, Scopus, and Web of Science, focusing on studies that used 3D models to investigate host–pathogen interactions or antimicrobial screening. Data on model types, biomaterials, fabrication techniques, and key findings were systematically charted to provide a comprehensive overview. Our findings reveal that a diverse range of biomaterials, including biopolymers and synthetic polymers, combined with advanced techniques like 3D bioprinting, are effectively used to create sophisticated tissue scaffolds. While these 3D models demonstrate clear superiority in mimicking biofilm properties and complex host–pathogen dynamics, our analysis identified a significant research gap: very few studies directly integrate these advanced bioengineered 3D models for high-throughput antimicrobial drug discovery. In conclusion, this review highlights the urgent need to bridge this disparity through increased research, standardization, and scalability in this critical interdisciplinary field, with the ultimate goal of accelerating the development of new therapeutics to combat AMR.
1. Introduction
Infectious diseases remain a leading cause of global morbidity and mortality [1,2,3], driving the urgent need for continuous development of novel therapeutic and preventive strategies [4,5]. The escalating concern surrounding antimicrobial resistance (AMR), which is transforming treatable infections into life-threatening challenges [6,7,8], further exacerbates this scenario, making the discovery and development of new antimicrobial drugs a complex endeavor fraught with high failure rates in clinical phases [9,10,11].
Traditionally, in vitro research has predominantly relied on two-dimensional (2D) cell culture models [12]. While these models are cost-effective and relatively easy to manipulate [13], they fundamentally fail to accurately replicate the intricate in vivo physiological environment [14,15]. The absence of three-dimensional (3D) tissue architecture [16], the precise composition and organization of the extracellular matrix (ECM) [17], proper cellular polarity, and the intricate cell–cell and cell-ECM interactions are severely compromised in 2D systems [18,19,20,21]. For instance, in a 2D culture, bacterial cells might exhibit gene expression profiles and biofilm formation patterns that are starkly distinct from those observed in complex tissue environments [22,23,24], leading to low predictability of preclinical results and hindering effective translation to the clinical setting, thus contributing to the high failure rates in drug development [25,26].
In this critical context, bioengineered 3D tissue models emerge as a groundbreaking platform, offering a more physiologically relevant environment for in-depth studies of host–pathogen interactions and for screening novel antimicrobial compounds [27,28,29,30]. At the core of many of these advanced models lies the strategic use of scaffolds [31,32,33]. These are porous, biocompatible structures meticulously designed to provide the necessary physical support for cell adhesion, proliferation, and differentiation in three dimensions, acting as the architectural framework upon which a miniature tissue is built [34,35,36,37,38]. The meticulous design of these scaffolds, utilizing a diverse array of biomaterials from materials science, both natural (proteins, polysaccharides) and synthetic (biocompatible polymers) [39,40,41], is critical as they mimic the native ECM, guiding cellular organization and function [42,43,44]. This allows tissue engineering, by integrating principles from cell biology, materials science, and engineering, to create structures that truly mimic the complexity of human tissues [45,46].
The development of these 3D models leverages various sophisticated techniques and approaches. These include top-down fabrication methods such as 3D bioprinting [47,48,49], which offers unparalleled spatial control over cell and material placement to engineer precise tissue architectures [50,51,52], and electrospinning, which generates fibrous scaffolds mimicking the fibrous nature of native ECM for enhanced cell infiltration and nutrient exchange [53,54,55].
On the other hand, bottom-up approaches like cell encapsulation in hydrogels enable the formation of controlled microenvironments [56,57,58], while spheroids and organoids, which are self-assembling cellular aggregates derived from stem cells or primary cells, represent another powerful strategy [59,60,61]. These organoid models, a significant advancement influenced by stem cell biology, can spontaneously organize into structures that recapitulate key aspects of organ physiology and complexity (intestinal villi, lung alveoli), providing advanced platforms for infection studies [62,63,64,65,66].
Furthermore, the integration of these 3D constructs with microfluidic systems and “organ-on-a-chip” technology, originating from microelectronics [67,68,69] and biomedical engineering, allows for dynamic culture conditions, precise control over flow and chemical gradients, and the simulation of multi-organ interactions, pushing the boundaries of in vitro relevance [70,71,72]. These meticulously designed 3D models provide a tridimensional niche that fosters cell differentiation, promotes more realistic biofilm formation, enables the simulation of nuanced host immune responses, and allows for the accurate expression of genes relevant to both microbial virulence and host defense, all under conditions that more closely resemble the human body [73,74].
This literature review’s primary objective is to explore recent advancements in the development and application of these sophisticated bioengineered 3D tissue models for two crucial areas: early antimicrobial drug discovery and a deeper understanding of bacterial pathogenesis. Specifically, we will address the main biomaterials and innovative techniques employed in constructing these models, with a particular focus on scaffold design and fabrication (including breakthroughs in 3D bioprinting and the development of organoids); their specific applications in microbiology research, such as the evaluation of new antibacterial molecules and the intricate study of resistance mechanisms; current challenges in their standardization and scalability; and future perspectives.
Importantly, our systematic search strategy, as detailed in the results, revealed a strikingly low number of studies at the precise intersection of bioengineered 3D tissue models and antimicrobial drug discovery. This review is distinctly significant because, unlike broader reviews that simply summarize advancements in 3D models or antimicrobial research separately, it critically highlights and underscores this specific, underserved research gap. A scoping review approach was specifically chosen to systematically map the breadth and nature of the existing literature in this interdisciplinary and emerging field, allowing us to identify key concepts, main sources of evidence, and crucial research gaps, rather than to synthesize findings from specific intervention studies. By consolidating current knowledge and pinpointing directions for future research, this review aims to be a pivotal resource for overcoming the inherent limitations of 2D models and accelerating the development of much-needed antimicrobials. While several recent reviews have addressed organoid systems for infection biology [30] or organ-on-chip platforms in microbiology [32,73], these studies primarily focus on technological innovations in microfluidics and stem-cell derived organoids. By contrast, this review emphasizes the role of biopolymer scaffolds and engineered 3D constructs in bridging infection models with antimicrobial drug discovery pipelines. This distinction highlights our contribution as a complementary and scaffold-centered synthesis.
2. Materials and Methods
To prepare this review, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [75] to ensure transparency and comprehensiveness in our methodology (Table 1). The protocol was registered prospectively in the PROSPERO database (Registration ID: CRD4201126629).

Table 1.
PRISMA Checklist.
2.1. Eligibility Criteria
Our inclusion criteria for selecting articles were stringent, aligning with PRISMA’s emphasis on clear eligibility criteria. Only original research articles with full-text availability were included. ‘Free full original research’ indicates that articles were accessible either via open-access publication or institutional subscriptions. No studies were excluded based on journal access policies or paywalls. We restricted our search to publications available in English. Crucially, selected articles had to explicitly address the application of 3D models in the investigation of host–pathogen interactions or for the screening of antimicrobial compounds. To ensure relevance and currency, we primarily focused on articles published within the last 10 years (from 2015 to 2025), making exceptions only for seminal studies that introduced foundational concepts to the field. The 2015–2025 timeframe was chosen because this period marks the emergence and consolidation of advanced 3D tissue engineering methods, such as organoids, spheroids, and bioprinting, which are central to infection models and antimicrobial testing. Studies prior to 2015 were included only if they were seminal and provided foundational concepts still relevant today. Conversely, our exclusion criteria filtered out several types of publications, contributing to the specificity of our review. We omitted articles that solely concentrated on 3D models for cancer research, regenerative tissue engineering without a specific emphasis on infectious diseases, or drug delivery systems without direct antimicrobial testing. Furthermore, conference reports, editorials, and book chapters that did not present original research data or a consolidated review were also excluded from our analysis.
2.2. Information Sources and Search Strategy
We conducted a systematic search across prominent scientific literature databases, including PubMed, Scopus, and Web of Science. The most recent search was last updated on 1 July 2025. No additional sources were identified through contact with authors or grey literature searches. Our comprehensive search strategy involved using a set of keywords, either individually or in various combinations, to maximize sensitivity. Below is the full electronic search strategy used for PubMed, which can be repeated: (“drug screening” [MeSH Terms] OR “drug screening” [tiab] OR “antimicrobial drug discovery” [tiab]) AND (“3D tissue model” [MeSH Terms] OR “3D tissue model” [tiab] OR “bioengineered models” [tiab]) AND (“bacterial pathogenesis” [MeSH Terms] OR “bacterial pathogenesis” [tiab] OR “host–pathogen interaction” [tiab]) AND (“2015” [pdat]: “2025” [pdat]) AND (English[la]). These databases were selected due to their comprehensive coverage of biomedical, life sciences, and engineering fields, ensuring broad retrieval of relevant literature.
2.3. Selection of Sources of Evidence
The article selection process was executed in two distinct phases, consistent with PRISMA recommendations for study selection. Initially, potential articles were identified through an initial screening based on titles and abstracts to ascertain content relevance. Subsequently, a thorough full-text review of the shortlisted articles was performed to confirm their complete alignment with the review’s scope and eligibility criteria. Any discrepancies or uncertainties during the selection process were resolved through discussion and consensus among the authors.
The detailed flow of article selection, from initial identification to final inclusion, is presented in the PRISMA-ScR Flow Diagram (Figure 1).
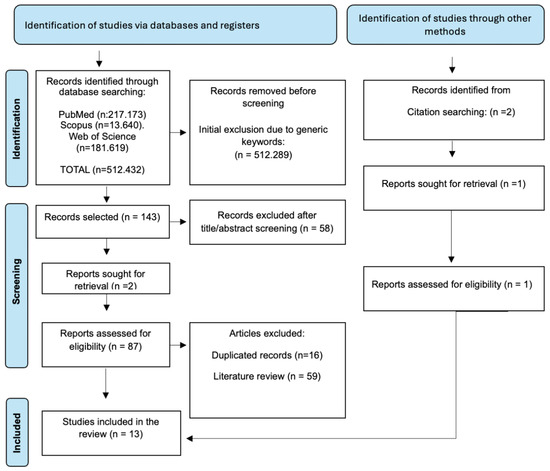
Figure 1.
PRISMA-ScR Flow Diagram detailing the article selection process, from initial identification to final inclusion.
This diagram illustrates the numbers of records identified, screened, and assessed for eligibility, along with reasons for exclusion at each stage.
2.4. Data Charting Process
The collection and analysis of data from the included articles centered on key aspects relevant to the review’s objectives. Data on model types, biomaterials, fabrication techniques, pathogens studied, primary applications, and key findings were systematically charted. A standardized data charting form was developed in advance and piloted with a subset of articles to ensure consistency. Data charting was performed by one reviewer and verified by another. No data confirmation from original investigators was attempted.
2.5. Data Items
For each included article, data were extracted on the following specific variables: Model Type: Describes the nature of the bioengineered model (organoid, scaffold-based, spheroid, among others); Biomaterials: The fundamental materials used in constructing these models (natural polymers like collagen, silk fibroin; synthetic polymers like PCL, PLA); Main Application: The central focus of the study within the defined scope (antimicrobial testing, host–pathogen interaction, drug delivery); Pathogen/Microbiota: The specific microorganisms investigated (E. coli, S. aureus, polymicrobial wound biofilms); Key Findings/Contribution: The most relevant conclusions and contributions from each research paper regarding 3D models in infection and antimicrobial discovery.
2.6. Critical Appraisal of Individual Sources of Evidence
Consistent with the methodology of scoping reviews, a formal critical appraisal of individual sources of evidence was not performed, as the primary objective was to map the breadth and nature of the literature rather than to assess the methodological quality or risk of bias of individual studies.
2.7. Synthesis of Results
Data were summarized using descriptive statistics (number and frequency of studies per database, per keyword combination, and per methodological approach). No inferential statistics were applied, as the objective of this scoping review was to map the breadth of the literature. Thematic synthesis was then used to identify recurring concepts, methodological patterns, and research gaps.
3. Results
The systematic literature search, conducted across PubMed, Scopus, and Web of Science, yielded a substantial number of publications, reflecting the broad interest in the fields relevant to this review. Initial broad keyword searches, such as “drug screening,” generated a high volume of results, as detailed in Table 2.

Table 2.
Number of articles found in each database using the search keywords.
As expected, the number of retrieved articles significantly narrowed when combining keywords to target the specific scope of this review. For instance, the intersection of “3D tissue model” with “drug screening” resulted in over a thousand articles across databases, while highly specific combinations like “3D tissue model AND bioengineered models AND antimicrobial drug discovery” yielded a strikingly low number of studies (as few as 0–2 per database). These progressively smaller numbers underscore the novelty and focused nature of research at the precise intersection of bioengineered 3D tissue models and antimicrobial drug discovery. Consistent with the methodology of scoping reviews, a formal critical appraisal of individual sources of evidence was not conducted.
This section provides a detailed analysis of the selected articles, organized into thematic categories that guide the understanding and synthesis of advancements in bioengineered 3D models for infection studies and antimicrobial drug discovery (Table 3).

Table 3.
Summary of selected articles on bioengineered 3D models for infection and antimicrobial drug discovery.
This table summarizes the key aspects of the articles included in this review. The “3D Tissue Model Type” column describes the nature of the bioengineered model. “Key Biomaterials/Scaffolds” details the fundamental materials used in constructing these models. The “Main Application in Review” categorizes the central focus of the study within the defined scope. “Pathogen/Microbiota Studied” indicates the microorganisms investigated. Finally, “Key Findings/Contribution” summarizes the most relevant conclusions from each research paper.
3.1. Limitations of Conventional (2D) Models for Infection Studies and Drug Discovery
Traditional in vitro research, predominantly based on 2D cell culture models, exhibits significant deficiencies in replicating the in vivo physiological complexity, which directly impacts our understanding of infections and the effectiveness of new drug discovery. These two-dimensional systems lack the three-dimensional tissue architecture, the precise composition and organization of the extracellular matrix (ECM), proper cellular polarity, and the intricate cell–cell and cell-ECM interactions [83,86]. This oversimplification often leads to low predictability for clinical translation, as in vitro test results frequently do not correlate with in vivo drug performance, contributing to high failure rates in clinical trials. A critical example is the study of bacterial biofilms, inherently 3D complex structures that exhibit significantly higher antimicrobial resistance. Two-dimensional models fail to recapitulate this complexity, underestimating the microbial resistance and persistence observed in real biological environments [76]. Additionally, the host’s immune response to infection is drastically simplified or absent in 2D cultures, preventing robust analysis of host–pathogen interactions and the modulation of essential signaling pathways.
Another critical limitation of 2D culture platforms lies in the evaluation of drug delivery and antibacterial efficacy. In 2D monolayers, the diffusion of antimicrobials is almost unrestricted, often leading to artificially elevated drug efficacy. These systems lack the extracellular matrix, variable porosity, and gradients of oxygen, nutrients, and metabolites that exist in vivo. As a result, drug penetration is uniform and does not mimic the barriers encountered in human tissues or within microbial biofilms.
In contrast, 3D models recreate the heterogeneous tissue microenvironment, allowing controlled drug diffusion and the assessment of sustained-release strategies. For example, 3D scaffolds fabricated with PCL/alginate composites or antibiotic-loaded PMMA beads provide a realistic testbed for dual drug release, depot-based therapy, and penetration depth. Similarly, 3D bioprinted bacterial biofilms display resistance levels far greater than their 2D counterparts, mirroring the clinical challenge of eradicating chronic infections [83]. Moreover, 3D systems enable the study of host responses, such as cytokine release and tissue inflammation, which modulate antimicrobial effectiveness but are absent in 2D conditions.
Thus, 3D culture systems not only improve the predictive value of preclinical antibacterial testing but also provide insights into pharmacokinetics, pharmacodynamics, and drug–host–pathogen interactions that cannot be captured in simplified 2D models.
A direct comparison of the main differences between 2D and 3D models in the context of antimicrobial testing and drug delivery is summarized in Table 4.

Table 4.
Key differences between 2D and 3D models in antimicrobial testing and drug delivery studies.
Overall, while 2D systems are still valuable for preliminary screening due to their low cost and simplicity, they consistently overestimate drug efficacy by failing to replicate biofilm barriers and host tissue responses. Three-dimensional models, in contrast, enable evaluation of pharmacokinetics and pharmacodynamics in tissue-like environments, integrating drug penetration, immune modulation, and antimicrobial resistance. This distinction is further illustrated in Table 4 and Figure 2, which summarize the major methodological and translational differences between 2D and 3D platforms for infection studies.
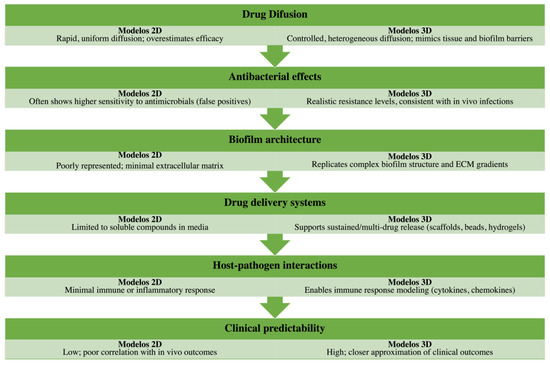
Figure 2.
Graphical comparison of 2D versus 3D infection models in antimicrobial testing. Two-dimensional culture systems, while simple, reproducible, and cost-effective, often fail to capture the complexity of tissue microenvironments, resulting in overestimated drug efficacy, poor modeling of biofilm structure, and limited insight into immune interactions. Conversely, 3D bioengineered models recreate key aspects of tissue organization, biofilm heterogeneity, and drug penetration barriers, supporting advanced drug delivery studies and producing data that align more closely with in vivo outcomes. This figure visually summarizes the methodological and translational differences, highlighting the growing relevance of 3D approaches for infection biology and antimicrobial screening.
3.2. Fundamentals of Tissue Bioengineering for 3D Infection Models
The development of bioengineered 3D infection models represents a fundamental advancement, enabling the creation of more physiologically relevant environments. The foundation of these models lies in the strategic selection and manipulation of biocompatible materials, as well as the application of innovative three-dimensional construction techniques.
3.2.1. Biocompatible Materials and Their Properties
The choice of biomaterials is crucial for mimicking ECM complexity and providing structural and bioactive support for cells. Natural polymers are widely used due to their inherent biocompatibility and biodegradability. Collagen, for instance, a primary ECM component, is employed in skin epidermal models [83,86] and as a basal layer for cervicovaginal epithelial models [85], providing mechanical support and cell adhesion. Silk fibroin, another natural biopolymer, stands out for its versatility and ability to form porous scaffolds, being used in intestinal tissue engineering [80] and anatomical gingival models [81]. Alginate, a polysaccharide derived from algae, is a popular hydrogel for 3D bioprinting bacterial biofilms [83] and as a component of dual drug delivery systems [86], valued for its cell encapsulation capabilities and crosslinking properties. Gelatin, a collagen derivative, is utilized in electrospun structures for gut microbiota culture, mimicking the ECM and supporting microbial growth [82].
Beyond natural polymers, synthetic polymers offer the advantage of tunable mechanical properties and degradation rates. Polycaprolactone (PCL), a biodegradable polyester, is employed in 3D-printed scaffolds for drug delivery in osteomyelitis treatment [77]. Poly(methyl methacrylate) (PMMA) and Poly(lactic acid) (PLA) are used in 3D printing antibiotic-doped beads and filaments, aiming to combat implant-related infections [78]. Biomaterial functionalization is an essential strategy to incorporate bioactive molecules, such as cell adhesion peptides or targeting ligands, to enhance the mimicry of the in vivo environment. A notable example is the use of photoresponsive protein hydrogels whose properties can be modified in real-time to dynamically control bacterial microenvironments [87].
3.2.2. Techniques for 3D Model Construction
Various tissue engineering techniques are employed to construct 3D models that overcome the limitations of 2D systems. The formation of spheroids and organoids represents a powerful approach. While not all articles explicitly detail de novo scaffold fabrication for these systems, a study demonstrates the use of human enteroid-derived organoids to generate a functional three-dimensional intestinal epithelium, mimicking complex tissue organization [80].
The creation of porous scaffolds is fundamental for ensuring proper nutrient diffusion, waste removal, and 3D cell growth. Fabrication methods range from casting replica molds of anatomical structures, as seen in the gingival pocket model that replicates the tooth-gingiva interface [81], to electrospinning gelatin to create intricate porous structures for microbial growth [82]. These methods are crucial for allowing proper cell infiltration, nutrient exchange, and waste removal.
3D bioprinting emerges as a transformative technology, offering unparalleled control over architectural design and material placement. A study pioneered the 3D bioprinting of mature bacterial biofilms using alginate bioinks, demonstrating the ability to create thick structures that replicate in vivo antimicrobial resistance [76]. Complementarily, another investigation printed bacteria directly into functional materials, opening doors for creating “living materials” with controlled biological properties [79]. Bioprinting is also applied in constructing drug delivery scaffolds, such as PCL/alginate systems for osteomyelitis [77] and PMMA/PLA antibiotic-doped beads/filaments [78], illustrating the versatility of this technique in optimizing antimicrobial delivery. Furthermore, a study explores the μ3D printing of photoresponsive hydrogels to dynamically control bacterial microenvironments, highlighting the potential for active manipulation of these platforms [87].
Cell encapsulation in hydrogels is another prevalent approach, where cells or microorganisms are suspended and cultured within crosslinked hydrogel matrices. This is evident in the polymicrobial wound biofilm model, where a cellulose matrix is supported by hydrogel [83], or the inclusion of fibroblasts in collagen gels for gingival tissue models [81], allowing control over matrix stiffness and composition to optimize the cellular environment.
Critically, while multiple fabrication strategies exist, scalability and reproducibility remain uneven. Natural polymers such as collagen and silk fibroin stand out for their biocompatibility and ease of regulatory acceptance, whereas alginate and gelatin, though effective for microbial encapsulation, suffer from batch-to-batch variability and limited mechanical stability. Electrospinning and bioprinting demonstrate superior architectural precision and scalability, but their cost and technical requirements still limit widespread adoption in high-throughput pipelines. A lack of standardized protocols for scaffold fabrication and characterization contributes to inconsistencies across studies, highlighting the urgent need for harmonized bioengineering practice.
3.3. Applications of 3D Models in Bacterial Pathogenesis Studies
The enhanced physiological relevance of 3D models provides superior platforms for dissecting bacterial pathogenesis in a more realistic manner.
The ability to study biofilm formation and persistence is one of the greatest advantages. Three-dimensional models prove superior in mimicking the complex architecture of biofilms and their intrinsic antimicrobial resistance. A study investigated polymicrobial wound biofilms in a 3D skin wound model [83], while other successfully demonstrated the 3D bioprinting of mature bacterial biofilms that exhibited significantly higher antimicrobial resistance compared to 2D cultures [76]. Similarly, other authors observed biofilm formation in their 3D human pleural organotypic model infected with S. aureus, showcasing the ability of these systems to recapitulate complex microbial structure formation in a tissue context [88].
Polymicrobial infection modeling remains a frontier in 3D bioengineering. Recent innovations include co-culture scaffolds, layered hydrogel matrices, and compartmentalized chip-based models that replicate inter-species signaling, competition, and spatial distribution, advancing beyond single-pathogen approaches.
The modulation of the host immune response is another strength of 3D models. Studies demonstrate that these models can induce and allow for the analysis of complex inflammatory and immune responses. The induction of an inflammatory response was observed in a 3D wounded human skin model upon bacterial infection [86], while other authors performed detailed transcriptional and proteomic profiling of inflammation in response to wound biofilms [83]. A significant antibacterial response was reported in a 3D intestinal model infected with E. coli, with the upregulation of innate immune genes [80]. The measurement of cytokine levels (TNF-α, MCP-1, IL-1β) in S. aureus-infected human pleural models [88] and the evaluation of macrophage polarization in a gut mucosal model [84] reinforce these systems’ capacity to mimic host–pathogen interactions more comprehensively.
While not always the primary focus, the three-dimensional structure and ECM composition in these models provide a more authentic environment for studying bacterial adhesion and invasion. A 3D cervicovaginal model, for example, supported infection by Chlamydia trachomatis and Neisseria gonorrhoeae, demonstrating pathogen interaction with multi-layered epithelial cells [85].
From a critical perspective, 3D models have significantly advanced our understanding of biofilm persistence and host immune responses, yet inconsistencies remain. Some studies demonstrate robust immune-like cytokine induction, while others show variable or attenuated responses, often due to differences in scaffold composition, cell type, or biofilm quantification methods. Collagen- and silk-based systems tend to provide more reliable results, whereas hydrogel-only constructs may lack sufficient tissue complexity to elicit reproducible responses. Without standardized readouts, cross-study comparisons remain challenging, which limits the translational impact of these infection models.
3.4. Three-Dimensional Models for Antimicrobial Drug Discovery and Screening
The application of 3D models in antimicrobial drug discovery significantly accelerates the translation of research to clinical practice by providing more accurate efficacy and toxicity assessments.
Efficacy and toxicity assessment in 3D models surpasses the limitations of 2D models, yielding data with higher in vivo correlation [76] demonstrated that 3D bioprinted S. aureus (MSSA and MRSA) biofilms exhibited greater resistance to antimicrobials compared to 2D cultures, highlighting the clinical relevance of these models for resistance testing. A study evaluated the efficacy of wound washes against polymicrobial biofilms in 3D skin models, noting that tissue response, not just bacterial killing, influenced treatment choice [83]. Similarly, it was used a standardized wound model to test antimicrobial wound dressings, monitoring both bacterial load and the reduction in inflammatory reaction as indicators of success [86]. These results suggest that 3D models can reduce failure rates in later stages of drug development.
3D models also drive the development of novel therapeutic approaches. A study designed a 3D-printed dual-drug delivery system for osteomyelitis, combining a PCL scaffold containing cefazolin with an alginate hydrogel loaded with rifampicin, demonstrating a synergistic effect against biofilm formation and bacterial activity [77]. Another investigation explored the incorporation of antibiotics into 3D-printed PMMA beads and filaments for local delivery, specifically targeting biofilms on implants. These innovations underscore the role of 3D engineering in creating targeted and controlled-release antimicrobial solutions [78].
In terms of antimicrobial discovery, 3D models offer more predictive and clinically relevant data than 2D cultures, particularly for testing biofilm-associated resistance and advanced drug delivery systems. However, their translational adoption is hampered by technical and economic challenges. Bioprinted constructs and synthetic polymer-based scaffolds (e.g., PCL, PMMA) show strong potential for scalable drug delivery platforms, yet remain costly and not fully compatible with standard pharmaceutical readouts such as MIC assays and high-content imaging. Furthermore, variability in antimicrobial penetration and assay reproducibility across models underscores the pressing need for validation frameworks to facilitate regulatory acceptance and industrial uptake.
3.5. Challenges and Future Perspectives
Despite promising advancements, the field of 3D models for infection studies still faces significant challenges and offers vast future perspectives.
Complexity and standardization remain ongoing hurdles. Replicating the full complexity of human organs, including vascularization, innervation, and the myriad of immune cell types, is a continuous challenge. While models of the intestine [80,84], skin [83,86], and gingiva [81] are advanced, they still simplify the in vivo environment. Incorporating vascularization within 3D constructs would improve antimicrobial testing by mimicking drug pharmacokinetics and immune cell infiltration, factors critical to clinical translation. Microvascular networks enhance nutrient and drug gradients, making models more predictive of tissue penetration barriers. Thus, the need for robust standardization of model preparation and assessment is crucial to ensure inter-laboratory reproducibility and comparability of results.
Scalability and cost are barriers to widespread adoption. The large-scale production of complex 3D models, especially those involving primary cells and intricate bioprinting, is currently resource-intensive and expensive, limiting their application in high-throughput screening for the pharmaceutical industry.
However, future perspectives are bright, driven by the integration with advanced technologies. Combining 3D models with microfluidics in “organ-on-a-chip” or “human-on-a-chip” systems will enable the simulation of complex physiological functions and inter-organ interactions, enhancing model relevance. The application of artificial intelligence and machine learning will be instrumental for complex data analysis, optimizing 3D model design, and predicting drug efficacy, thereby accelerating discovery. The industrial translation of these models for pharmaceutical research and development is the goal, requiring rigorous validation and regulatory considerations for their widespread acceptance and use.
Looking forward, future directions should incorporate the integration of AI-driven data analysis and drug discovery pipelines, which can enable predictive modeling of antimicrobial efficacy within complex 3D environments. Parallel efforts by regulatory agencies such as the OECD and FDA to establish standardized testing frameworks will be critical for facilitating wider adoption. Another promising strategy is the development of open-source biomaterial libraries and scaffold design platforms, which can reduce costs, democratize access, and foster reproducibility across laboratories. Collectively, these approaches will be essential to transform 3D models from experimental tools into validated, industry-ready platforms for antimicrobial research.
Development of inter-laboratory standard operating procedures (SOPs), reference scaffolds, and validated readouts will be key to achieving reproducibility in 3D infection models, similar to Good Laboratory Practice standards. Future research should prioritize vascularized models, using microfluidics or bioprinting, to replicate tissue perfusion and drug kinetics
4. Discussion
This review provides a comprehensive overview of the current state of bioengineered 3D tissue models for studying infections and discovering new antimicrobial drugs. Our analysis highlights a field rich with innovative approaches, yet one that faces a critical bottleneck: the limited integration of sophisticated 3D models directly into high-throughput antimicrobial drug discovery pipelines. While individual research areas, 3D modeling, bioengineering, and antimicrobial discovery, are vibrant, their synergistic application is profoundly underdeveloped.
4.1. Evaluating the Efficacy and Potential of 3D Models
The articles reviewed demonstrate a remarkable diversity in biomaterial selection and fabrication techniques. Natural polymers like collagen and silk fibroin are frequently employed for their biocompatibility and ability to create structured matrices that mimic specific tissues, such as skin [83,86] and the intestine [78]. The use of silk, for instance, allows for the precise control of porosity and mechanical properties necessary to foster physiological conditions for complex host–pathogen interactions within models like the gingival pocket [81]. Other materials, like alginate and gelatin, prove invaluable for direct cell or microbial encapsulation, enabling the precise architectural control seen in 3D bioprinted biofilms [76] and electrospun matrices for gut microbiota [82].
Beyond natural options, synthetic polymers like PCL, PMMA, and PLA offer tunable mechanical properties and slower degradation rates, making them ideal for applications such as sustained drug release from antibiotic-loaded implants [77,78]. This broad and versatile palette of materials, combined with advanced techniques like 3D bioprinting, electrospinning, and hydrogel encapsulation, allows for the creation of specialized 3D tissue models that capture specific aspects of the infection microenvironment.
These advanced models are a significant leap beyond conventional 2D cultures. They enable more realistic biofilm formation, recapitulate complex host–pathogen interactions, and provide more accurate assessments of antimicrobial efficacy. For example, 3D bioprinted S. aureus biofilms demonstrate significantly higher resistance to antimicrobials compared to their 2D counterparts [76], more accurately reflecting the clinical reality of persistent infections. Similarly, the ability of these models to elicit nuanced host immune responses, including cytokine and chemokine production [86,88], allows for a more holistic evaluation of a drug’s efficacy, considering not just bacterial killing but also host tissue protection and immunomodulation.
4.2. A Critical Research Gap in Antimicrobial Drug Discovery
Despite this clear superiority, our systematic literature search revealed a striking disparity. A broad search for “drug screening” or “bacterial pathogenesis” yielded tens of thousands of articles, while searches combining these terms with “3D tissue model” and “antimicrobial drug discovery” yielded a dramatically lower number, often in the single digits. Of over 5000 combined studies retrieved on infection models and drug discovery, only 8 papers (<5%) explicitly integrated bioengineered 3D models for antimicrobial screening. This stark contrast highlights that while the tools exist, their direct, synergistic application for high-throughput antimicrobial drug screening is profoundly underdeveloped and in its infancy.
This critical research gap is a significant bottleneck in addressing the urgent global challenge of antimicrobial resistance. The limited number of integrated studies points to several underlying factors such as interdisciplinary complexity, the field requires a unique combination of expertise across materials science, tissue engineering, microbiology, and pharmacology, which can be challenging to assemble; technical challenges, creating standardized, reproducible, and scalable 3D models suitable for drug screening remains a technical hurdle; cost and scalability, the high cost of developing and maintaining these advanced models, especially for large-scale production, limits their adoption in industrial high-throughput screening; and regulatory uncertainty, the lack of established regulatory guidelines for in vitro efficacy testing using such complex systems may also hinder their widespread use in drug development pipelines.
This gap persists largely due to translational bottlenecks. First, there is a lack of standardized validation frameworks that can align 3D infection models with regulatory requirements for preclinical testing. Second, industrial uptake remains limited because of high costs, scalability challenges, and limited compatibility with automated high-throughput pipelines. Third, while 3D models are biologically more predictive, their integration into drug discovery workflows requires adaptation to standard readouts such as MIC assays, high-content imaging, and transcriptomic profiling. Without harmonization, pharmaceutical adoption remains limited despite the clear biological advantages.
A critical methodological reason for the limited use of 3D models in antimicrobial screening is their partial incompatibility with standardized assays. For instance, thick scaffolds often hinder optical readouts in high-content imaging and fluorescence microscopy, while hydrogel variability can compromise reproducibility in MIC assays. Moreover, transcriptomic and proteomic readouts require optimized protocols for 3D matrices, which are not yet universally available. These technical barriers, combined with the higher costs of 3D culture systems, currently limit their large-scale integration into pharmaceutical screening pipelines.
4.3. The Way Forward: Bridging the Gap
The findings of this review serve as a crucial call to action, highlighting the immense, largely untapped potential of bioengineered 3D infection models. The imperative for accelerated research in this interdisciplinary domain cannot be overstated. We must bridge this research gap by encouraging the development of more standardized, scalable, and physiologically relevant platforms.
Advancing research in this critical area will provide more predictive, ethical, and effective in vitro tools, revolutionizing antimicrobial drug discovery and leading to desperately needed new treatments against infectious diseases.
Organ-on-a-chip platforms offer a unique opportunity to overcome limitations in immune modeling by allowing immune cell migration, vascular perfusion, and cytokine exchange in real-time. These systems bridge the gap between traditional 3D scaffolds and in vivo models, enabling dynamic host–pathogen studies.
The integration of patient-derived cells enhances predictive accuracy but creates variability across studies. Standardization will require pooled organoid repositories, harmonized protocols, and shared quality-control benchmarks.
To better illustrate the translational trajectory of 3D bioengineered models, we propose a conceptual framework that links biomaterial selection → 3D model fabrication → host–pathogen interaction studies → antimicrobial screening and validation (Figure 3). This schematic highlights how scaffold design choices directly impact infection modeling outcomes and ultimately the predictive value of drug discovery pipelines.
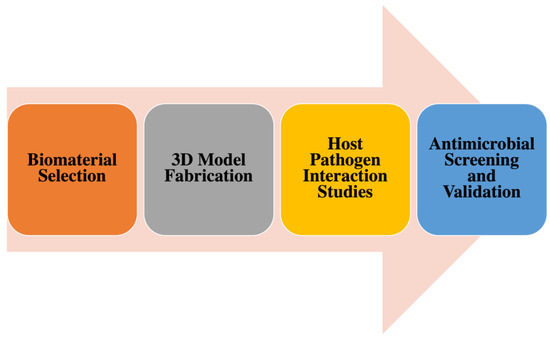
Figure 3.
Conceptual framework for the translational trajectory of 3D bioengineered models. The pipeline begins with biomaterial selection (focused on physicochemical properties, biocompatibility, and cost), progresses to 3D model fabrication (through scaffold design, additive manufacturing, or assembly techniques), and advances to host–pathogen interaction studies (enabling detailed analysis of infection dynamics, immune responses, and biofilm formation). Finally, the models support antimicrobial screening and validation, integrating standard assays (e.g., MIC testing, high-content imaging) and regulatory evaluation. Scaffold design decisions at the early stages directly influence downstream outcomes, improving the predictive power of drug discovery workflows.
In addition to these translational priorities, broader innovations will shape the future of 3D infection models. The integration of artificial intelligence and machine learning into drug discovery pipelines offers the possibility of screening vast chemical libraries and predicting antimicrobial performance in silico before validation in 3D constructs. At the regulatory level, organizations such as the OECD and FDA are moving toward harmonized testing guidelines, and incorporating 3D infection models into these frameworks will accelerate their acceptance in pharmaceutical development. Furthermore, the creation of open-source repositories of biomaterials, scaffold designs, and standardized protocols could dramatically reduce costs while enhancing reproducibility and transparency. Together, these strategies provide a clear roadmap for transitioning 3D models from promising research platforms into globally recognized tools for combating antimicrobial resistance.
Cost reductions can be achieved by standardizing scaffolds, using modular systems, and adopting automation, making high-throughput infection testing economically viable. Developing quantifiable metrics, including drug penetration profiling, biofilm viability scoring, and cytokine readouts, will validate 3D models as more predictive tools than 2D cultures. Automation and robotics, combined with high-throughput imaging and machine learning pipelines, represent the most practical route to scale 3D infection models for drug discovery.
Finally, policy and funding initiatives, including multi-center consortia, validation grants, and early regulatory incentives, will be essential to accelerate adoption of 3D infection models in antimicrobial discovery.
4.4. Limitations of This Scoping Review
This scoping review, while comprehensive in its goal to map the field, has several inherent limitations. Our search was restricted to specific databases (PubMed, Scopus, and Web of Science) and limited to English-only publications, which may have excluded relevant research from other sources or languages. The timeframe (2015–2025) was chosen for currency, but it may have omitted foundational studies from earlier periods. Consistent with scoping review methodology, a formal critical appraisal of individual studies was not performed, as the primary objective was to map the literature rather than assess the methodological quality or risk of bias.
5. Conclusions
This comprehensive review has elucidated the pivotal advancements in the field of bioengineered 3D tissue models as superior alternatives to conventional 2D systems for the study of bacterial infections and antimicrobial drug discovery. We have demonstrated how the judicious selection and sophisticated fabrication of diverse biomaterials, encompassing both natural polymers like collagen, silk fibroin, alginate, and gelatin, and synthetic counterparts such as PCL, PMMA, and PLA, enable the creation of physiologically relevant microenvironments. Techniques ranging from 3D bioprinting and electrospinning to advanced hydrogel encapsulation have proven instrumental in constructing models that accurately recapitulate complex tissue architectures, host–pathogen interactions, and the intricate dynamics of biofilm formation, which are often inadequately represented in simplified 2D cultures.
The application of these advanced 3D models has significantly enhanced our understanding of bacterial pathogenesis, allowing for more realistic assessments of biofilm resistance and host immune responses, including cytokine profiles and tissue damage. Crucially, these models have begun to demonstrate their potential in antimicrobial drug discovery, offering platforms for evaluating novel therapeutics and drug delivery systems with higher predictive validity for in vivo outcomes.
Despite these promising developments and the undeniable advantages of 3D models, our systematic literature search revealed a critical and striking gap: a remarkably low number of studies specifically focusing on the intersection of 3D bioengineered models and antimicrobial drug discovery. The stark contrast between the vast number of general publications in related fields and the minimal count at this precise nexus underscores that the full potential of these sophisticated systems for combating antimicrobial resistance remains largely unexplored. This highlights a significant bottleneck in translating cutting-edge tissue engineering into impactful solutions for a global health crisis.
In conclusion, while the foundational science and technological capabilities for developing highly representative 3D infection models are rapidly evolving, there is an urgent and compelling need for dedicated research efforts to bridge the existing gap in their direct application to antimicrobial drug discovery. Future endeavors must prioritize standardization, scalability, and cost-effectiveness to facilitate high-throughput screening. Furthermore, the integration of these models with advanced technologies such as organ-on-a-chip platforms and artificial intelligence will be paramount to unlock their full potential. By accelerating research in this interdisciplinary frontier, bioengineered 3D tissue models promise to revolutionize the development of new, effective antimicrobial agents, ultimately offering a more predictive, ethical, and powerful approach to safeguarding human health against infectious diseases.
Author Contributions
Conceptualization, J.d.A.S. and A.d.A.C.; methodology, J.d.A.S. and A.d.A.C.; validation, J.d.A.S. and A.d.A.C.; formal analysis, J.d.A.S. and A.d.A.C.; resources, J.d.A.S. and A.d.A.C.; data curation, J.d.A.S. and A.d.A.C.; writing—original draft preparation, J.d.A.S. and A.d.A.C.; writing—review and editing, J.d.A.S. and A.d.A.C.; visualization, J.d.A.S. and A.d.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to acknowledge the Federal University of Piauí for providing access to the databases on the Portal de Periódicos da CAPES.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| AMR | Antimicrobial Resistance |
| CC BY | Creative Commons Attribution |
| ECM | Extracellular Matrix |
| E. coli | Escherichia coli |
| LIMAV | Interdisciplinary Laboratory of Advanced Materials |
| MeSH | Medical Subject Headings |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| PCL | Polycaprolactone |
| PET | Polyethylene terephthalate |
| PLA | Poly(lactic acid) |
| PMMA | Poly(methyl methacrylate) |
| PPGTAIR | Graduate Program in Technologies Applied to Animals of Regional Interest |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| S. aureus | Staphylococcus aureus |
| TNF-α | Tumor Necrosis Factor-alpha |
| UFPI | Federal University of Piauí |
References
- Naghavi, M.; Mestrovic, T.; Gray, A.; Hayoon, A.G.; Swetschinski, L.R.; Aguilar, G.R.; Weaver, N.D.; Ikuta, K.S.; Chung, E.; Wool, E.E.; et al. Global burden associated with 85 pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2024, 24, 868–895. [Google Scholar] [CrossRef]
- Li, X.-C.; Zhang, Y.-Y.; Zhang, Q.-Y.; Liu, J.-S.; Ran, J.-J.; Han, L.-F.; Zhang, X.-X. Global burden of viral infectious diseases of poverty based on Global Burden of Diseases Study 2021. Infect. Dis. Poverty 2024, 13, 53–67. [Google Scholar] [CrossRef]
- Antabe, R.; Ziegler, B.R. Diseases, Emerging and Infectious; Elsevier eBooks: Amsterdam, The Netherlands, 2019; pp. 389–391. [Google Scholar]
- Omar, R.F.; Trottier, S.; Sato, S.; Ouellette, M.; Bergeron, M.G. Advances in the management of infectious diseases. Infect. Dis. Rep. 2025, 17, 26. [Google Scholar] [CrossRef]
- Falodun, M.O.; Olorunfemi, O.; Irinoye, O.O. Infectious diseases: Addressing global challenges and prevention strategies for national health improvement. Community Acquir. Infect. 2025, 12, 1–9. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Ferraz, M.P. Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies 2024, 14, 187. [Google Scholar] [CrossRef]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Da Silva, D.D. An overview of the recent advances in antimicrobial resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief overview of approaches and challenges in new antibiotic development: A focus on drug repurposing. Front. Cell. Infect. Microbiol. 2021, 11, 684515. [Google Scholar] [CrossRef]
- Shinu, P.; Mouslem, A.K.A.; Nair, A.B.; Venugopala, K.N.; Attimarad, M.; Singh, V.A.; Nagaraja, S.; Alotaibi, G.; Deb, P.K. Progress Report: Antimicrobial drug discovery in the Resistance Era. Pharmaceuticals 2022, 15, 413. [Google Scholar] [CrossRef]
- Chunduri, V.; Maddi, S. Role of in vitro two-dimensional (2D) and three-dimensional (3D) cell culture systems for ADME-Tox screening in drug discovery and development: A comprehensive review. ADMET & DMPK 2022, 11, 1–32. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative human Three-Dimensional Tissue-Engineered models as an alternative to animal testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Ferraresi, A.; Vallino, L.; Garavaglia, B.; Dhanasekaran, D.N.; Isidoro, C. Three-Dimensional in vitro cell cultures as a feasible and promising alternative to Two-Dimensional and animal models in cancer research. Int. J. Biol. Sci. 2024, 20, 5293–5311. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.S.; Mendes, M.; Cordeiro, M.A.; Sousa, J.J.; Pais, A.C.; Mihăilă, S.M.; Vitorino, C. Organ-on-a-Chip: Ubi sumus? Fundamentals and Design Aspects. Pharmaceutics 2024, 16, 615. [Google Scholar] [CrossRef] [PubMed]
- Mangani, S.; Vetoulas, M.; Mineschou, K.; Spanopoulos, K.; Vivanco, M.M.; Piperigkou, Z.; Karamanos, N.K. Design and applications of extracellular matrix scaffolds in tissue engineering and regeneration. Cells 2025, 14, 1076. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Joseph, J.S.; Malindisa, S.T.; Ntwasa, M. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culturing in Drug Discovery; IntechOpen eBooks: London, UK, 2019. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; Santos, F.G.D.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2019, 107, 110264. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016, 14, 910–919. [Google Scholar] [CrossRef]
- Bottaro, A.; Nasso, M.E.; Stagno, F.; Fazio, M.; Allegra, A. Modeling the bone marrow niche in multiple myeloma: From 2D cultures to 3D systems. Int. J. Mol. Sci. 2025, 26, 6229. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Mao, S.; Yan, J.; Alert, R.; Stone, H.A.; Bassler, B.L.; Wingreen, N.S.; Košmrlj, A. Nonuniform growth and surface friction determine bacterial biofilm morphology on soft substrates. Proc. Natl. Acad. Sci. USA 2020, 117, 7622–7632. [Google Scholar] [CrossRef] [PubMed]
- Law, A.M.K.; De La Fuente, L.R.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D cell culture systems for personalizing Anti-Cancer therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-Dimensional in vitro cell culture models for efficient drug discovery: Progress so far and future prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef]
- Moysidou, C.-M.; Barberio, C.; Owens, R.M. Advances in engineering human tissue models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef]
- Somana, S.S.; Kuchipudic, S.V.; Kumarab, S. Advances in Infectious Disease Modeling: A perspective on 3D-Bioprinted Tissue Models to Study Host-Pathogen Interactions. J. Vet. Anim. Sci. 2025, 56, 1–7. [Google Scholar] [CrossRef]
- Han, X.; Cai, C.; Deng, W.; Shi, Y.; Li, L.; Wang, C.; Zhang, J.; Rong, M.; Liu, J.; Fang, B.; et al. Landscape of human organoids: Ideal model in clinics and research. The Innovation 2024, 5, 100620. [Google Scholar] [CrossRef]
- Blutt, S.E.; Estes, M.K. Organoid models for infectious disease. Annu. Rev. Med. 2021, 73, 167–182. [Google Scholar] [CrossRef]
- Trifan, A.; Liciu, E.; Busuioc, C.; Stancu, I.-C.; Banciu, A.; Nicolae, C.; Dragomir, M.; Cristea, D.-D.; Sabău, R.-E.; Nițulescu, D.-A.; et al. Developing Bioengineered 3D-Printed Composite Scaffolds with Antimicrobial Potential for Bone Tissue Regeneration. J. Funct. Biomater. 2025, 16, 227. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Li, C. Advances in 3D printing technology for preparing bone tissue engineering scaffolds from biodegradable materials. Front. Bioeng. Biotechnol. 2024, 12, 1483547. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Li, G.; Zhou, F.; Wang, G.; Ren, X.; Su, J. Biomimetic structural design in 3D-Printed scaffolds for bone tissue engineering. Mater. Today Bio 2025, 32, 101664. [Google Scholar] [CrossRef]
- Lu, J.; Gao, Y.; Cao, C.; Wang, H.; Ruan, Y.; Qin, K.; Liu, H.; Wang, Y.; Yang, P.; Liu, Y.; et al. 3D bioprinted scaffolds for osteochondral regeneration: Advancements and applications. Mater. Today Bio 2025, 32, 101834. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A path towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Qiang, H.; Leng, D.; Yang, L.; Hu, X.; Chen, F.; Zhang, T.; Gao, J.; Yu, Z. Exploring the frontiers: The potential and challenges of bioactive scaffolds in osteosarcoma treatment and bone regeneration. Mater. Today Bio 2024, 29, 101276. [Google Scholar] [CrossRef] [PubMed]
- Tupe, A.; Patole, V.; Ingavle, G.; Kavitkar, G.; Tiwari, R.M.; Kapare, H.; Baheti, R.; Jadhav, P. Recent advances in Biomaterial-Based scaffolds for guided bone tissue Engineering: Challenges and future directions. Polym. Adv. Technol. 2024, 35, e6619. [Google Scholar] [CrossRef]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The role of biomaterials-based scaffolds in advancing skin tissue construct. J. Tissue Viability 2025, 34, 100858. [Google Scholar] [CrossRef]
- Angolkar, M.; Paramshetti, S.; Gahtani, R.M.; Shahrani, M.A.; Hani, U.; Talath, S.; Osmani, R.A.M.; Spandana, A.; Gangadharappa, H.V.; Gundawar, R. Pioneering a paradigm shift in tissue engineering and regeneration with polysaccharides and proteins-based scaffolds: A comprehensive review. Int. J. Biol. Macromol. 2024, 265, 130643. [Google Scholar] [CrossRef]
- Sousa, H.C.; Ruben, R.B.; Viana, J.C. On the Fused Deposition Modelling of Personalised Bio-Scaffolds: Materials, Design, and Manufacturing Aspects. Bioengineering 2024, 11, 769. [Google Scholar] [CrossRef]
- Salerno, A.; Netti, P.A. Review on bioinspired design of ECM-Mimicking scaffolds by Computer-Aided assembly of Cell-Free and cell laden Micro-Modules. J. Funct. Biomater. 2023, 14, 101. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, D.H.; Leach, J.K. Decellularized Cell-Secreted extracellular matrices as biomaterials for tissue engineering. Small Sci. 2024, 5, 202400335. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Lin, X.; Xu, R.; Liu, L.; Zhang, Y.; Tian, F.; Li, J.J.; Xue, J. Advances in the development of gradient scaffolds made of Nano-Micromaterials for musculoskeletal tissue regeneration. Nano-Micro Lett. 2024, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Katu Amina, H. Tissue Engineering: Building Organs from Scratch. Res. Output J. Biol. Appl. Sci. 2025, 5, 15–18. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, D.; Li, G.; Liu, J.; Chen, X.; Su, J. Engineering bone/cartilage organoids: Strategy, progress, and application. Bone Res. 2024, 12, 66. [Google Scholar] [CrossRef]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in tissue and organ 3D bioprinting: Current techniques, applications, and future perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Shyam, R.; Reddy, L.V.K.; Palaniappan, A. Fabrication and characterization techniques of in vitro 3D tissue models. Int. J. Mol. Sci. 2023, 24, 1912. [Google Scholar] [CrossRef]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Santos, J.D.; Atayde, L.; Alves, N.; Maurício, A.C. Three-Dimensional Printing/Bioprinting and Cellular Therapies for Regenerative Medicine: Current advances. J. Funct. Biomater. 2025, 16, 28. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Liu, L.; Nasar, N.K.A.; Gu, X.; Davis, T.P.; Zheng, X.; Wang, L.; Qiao, R. Fabrication of Organic/Inorganic nanocomposites: From traditional synthesis to additive manufacturing. Adv. Mater. 2025, 37, 2505504. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Innovative Micro- and Nano-Architectures in Biomedical engineering for therapeutic and diagnostic applications. Micromachines 2025, 16, 419. [Google Scholar] [CrossRef]
- Chand, R.; Kamei, K.-I.; Vijayavenkataraman, S. Advances in microfluidic bioprinting for multi-material multi-cellular tissue constructs. Cell Eng. Connect. 2025, 1, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, S.; Kim, J.I. 3D cotton-type anisotropic biomimetic scaffold with low fiber motion electrospun via a sharply inclined array collector for induced osteogenesis. Sci. Rep. 2024, 14, 7365. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.K.; Molnár, K. Current trends and future prospects of integrating electrospinning with 3D printing techniques for mimicking bone extracellular matrix scaffolds. J. Polym. Sci. 2025, 63, 1481–1504. [Google Scholar] [CrossRef]
- Hashemi, M.; Finklea, F.B.; Hammons, H.; Tian, Y.; Young, N.; Kim, E.; Halloin, C.; Triebert, W.; Zweigerdt, R.; Mitra, A.K.; et al. Hydrogel microsphere stem cell encapsulation enhances cardiomyocyte differentiation and functionality in scalable suspension system. Bioact. Mater. 2024, 43, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, K.; Wang, S. Practical guide to the design of granular hydrogels for customizing complex cellular microenvironments. Adv. Healthc. Mater. 2025, e01947, 1–23. [Google Scholar] [CrossRef]
- Far, B.F.; Safaei, M.; Nahavandi, R.; Gholami, A.; Naimi-Jamal, M.R.; Tamang, S.; Ahn, J.E.; Farani, M.R.; Huh, Y.S. Hydrogel Encapsulation Techniques and its clinical Applications in drug delivery and regenerative Medicine: A systematic review. ACS Omega 2024, 9, 29139–29158. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and spheroid tumor models: Techniques and applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, B.; Johandes, E.; Gramm, D.; Hanjaya-Putra, D. Bottom-up Biomaterial strategies for creating tailored stem cells in regenerative medicine. Front. Bioeng. Biotechnol. 2025, 13, 1581292. [Google Scholar] [CrossRef]
- Carvalho, V.; Bañobre-López, M.; Minas, G.; Teixeira, S.F.C.F.; Lima, R.; Rodrigues, R.O. The integration of spheroids and organoids into organ-on-a-chip platforms for tumour research: A review. Bioprinting 2022, 27, e00224. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, D.; Wang, F.; Chen, X.; Li, M.; Su, J. Organoids for tissue repair and regeneration. Mater. Today Bio 2025, 33, 102013. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The current status and biomedical applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef]
- Wang, H.; Ning, X.; Zhao, F.; Zhao, H.; Li, D. Human organoids-on-chips for biomedical research and applications. Theranostics 2024, 14, 788–818. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, K.; Zhang, C.; Zhao, Y.; Guo, Y.; He, J.; Chang, S.; Fang, X.; Liu, K.; Zhu, P.; et al. Bioprinted Organoids: An innovative engine in biomedicine. Adv. Sci. 2025, 12, e07317. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Ye, L.; Zhang, Y.; Hu, S.; Lei, W. Advances in humanoid organoid-based research on inter-organ communications during cardiac organogenesis and cardiovascular diseases. J. Transl. Med. 2025, 23, 380. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liu, Y.; Liu, Y. Organ-on-a-Chip applications in microfluidic platforms. Micromachines 2025, 16, 201. [Google Scholar] [CrossRef]
- Cao, U.M.N.; Zhang, Y.; Chen, J.; Sayson, D.; Pillai, S.; Tran, S.D. Microfluidic Organ-on-A-Chip: A guide to biomaterial choice and fabrication. Int. J. Mol. Sci. 2023, 24, 3232. [Google Scholar] [CrossRef]
- Ahmed, T. Organ-on-a-chip microengineering for bio-mimicking disease models and revolutionizing drug discovery. Biosens. Bioelectron. X 2022, 11, 100194. [Google Scholar] [CrossRef]
- Zheng, F.; Xiao, Y.; Liu, H.; Fan, Y.; Dao, M. Patient-Specific organoid and Organ-on-a-Chip: 3D Cell-Culture meets 3D printing and numerical simulation. Adv. Biol. 2021, 5, e2000024. [Google Scholar] [CrossRef]
- Aziz, A.; Geng, C.; Fu, M.; Yu, X.; Qin, K.; Liu, B. The role of microfluidics for organ on chip simulations. Bioengineering 2017, 4, 39. [Google Scholar] [CrossRef]
- Gong, J.; Li, M.; Kang, J.; Yin, Z.; Cha, Z.; Yang, J.; Xu, H. Microfluidic techniques for Next-Generation Organoid Systems. Adv. Mater. Interfaces 2022, 9, 2200846. [Google Scholar] [CrossRef]
- Vyas, H.K.N.; Xia, B.; Mai-Prochnow, A. Clinically relevant in vitro biofilm models: A need to mimic and recapitulate the host environment. Biofilm 2022, 4, 100069. [Google Scholar] [CrossRef]
- Lazarus, E.; Meyer, A.S.; Ikuma, K.; Rivero, I.V. Three dimensional printed biofilms: Fabrication, design and future biomedical and environmental applications. Microb. Biotechnol. 2023, 17, e14360. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Ning, E.; Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Vendrell, M.; Graham, D.; Wark, A.W.; Faulds, K.; Shu, W. 3D bioprinting of mature bacterial biofilms for antimicrobial resistance drug testing. Biofabrication 2019, 11, 045018. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.-K.; Son, K.-H.; Lee, J.-W. PCL/Sodium-Alginate Based 3D-Printed Dual Drug Delivery System with Antibacterial Activity for Osteomyelitis Therapy. Gels 2022, 8, 163. [Google Scholar] [CrossRef]
- Mills, D.K.; Jammalamadaka, U.; Tappa, K.; Weisman, J. Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly(methyl methacrylate) beads. Bioact. Mater. 2018, 3, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D printing of bacteria into functional complex materials. Sci. Adv. 2017, 3, eaao6804. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, W.; Roh, T.; Estes, M.K.; Kaplan, D.L. In vitro enteroid-derived three-dimensional tissue model of human small intestinal epithelium with innate immune responses. PLoS ONE 2017, 12, e0187880. [Google Scholar] [CrossRef] [PubMed]
- Adelfio, M.; Martin-Moldes, Z.; Erndt-Marino, J.; Tozzi, L.; Duncan, M.J.; Hasturk, H.; Kaplan, D.L.; Ghezzi, C.E. Three-Dimensional humanized model of the periodontal gingival pocket to study oral microbiome. Adv. Sci. 2023, 10, 2205473. [Google Scholar] [CrossRef]
- Biagini, F.; Calvigioni, M.; Lapomarda, A.; Vecchione, A.; Magliaro, C.; De Maria, C.; Montemurro, F.; Celandroni, F.; Mazzantini, D.; Mattioli-Belmonte, M.; et al. A novel 3D in vitro model of the human gut microbiota. Sci. Rep. 2020, 10, 21499. [Google Scholar] [CrossRef]
- Brown, J.L.; Townsend, E.; Short, R.D.; Williams, C.; Woodall, C.; Nile, C.J.; Ramage, G. Assessing the inflammatory response to in vitro polymicrobial wound biofilms in a skin epidermis model. Npj Biofilms Microbiomes 2022, 8, 19. [Google Scholar] [CrossRef]
- Beamer, M.A.; Zamora, C.; Nestor-Kalinoski, A.L.; Fernando, V.; Sharma, V.; Furuta, S. Novel 3D Flipwell system that models gut mucosal microenvironment for studying interactions between gut microbiota, epithelia and immunity. Sci. Rep. 2023, 13, 870. [Google Scholar] [CrossRef]
- Edwards, V.L.; McComb, E.; Gleghorn, J.P.; Forney, L.; Bavoil, P.M.; Ravel, J. Three-dimensional models of the cervicovaginal epithelia to study host–microbiome interactions and sexually transmitted infections. Pathog. Dis. 2022, 80, ftac026. [Google Scholar] [CrossRef]
- Wiegand, C.; Fink, S.; Mogrovejo, D.C.; Ruhlandt, M.; Wiencke, V.; Eberlein, T.; Brill, F.H.H.; Tittelbach, J. A standardized wound infection model for antimicrobial testing of wound dressings in vitro. Int. Wound J. 2024, 21, e14811. [Google Scholar] [CrossRef]
- Connell, J.L.; Ritschdorff, E.T.; Shear, J.B. Three-Dimensional printing of photoresponsive biomaterials for control of bacterial microenvironments. Anal. Chem. 2016, 88, 12264–12271. [Google Scholar] [CrossRef]
- Kurow, O.; Nuwayhid, R.; Stock, P.; Steinert, M.; Langer, S.; Krämer, S.; Metelmann, I.B. Organotypic 3D Co-Culture of Human Pleura as a Novel In Vitro Model of Staphylococcus aureus Infection and Biofilm Development. Bioengineering 2023, 10, 537. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).