Abstract
Dry eye disease (DED) is a multifactorial ocular surface disorder that significantly affects vision and quality of life. While artificial tears are the standard first-line therapy, their effectiveness is limited by the complex pathophysiology of DED. This study evaluated DayDrop® Triple Action, a novel formulation combining hyaluronic acid (HA), ectoine, and carboxymethylcellulose (CMC), designed to enhance tear film stability and ocular surface protection. Physicochemical and rheological properties were assessed, including viscosity, pseudoplasticity, and viscoelastic behaviour under dynamic conditions, along with ectoine release over 24 h. An in vitro allergic conjunctivitis model using conjunctival fibroblasts exposed to a pro-allergic cytokine cocktail was employed to examine immunomodulatory effects. DayDrop® Triple Action demonstrated high viscosity with pronounced pseudoplasticity and stable viscoelasticity, supporting improved mucoadhesion. The formulation provided sustained ectoine release and exhibited a positive immunomodulatory effect, likely linked to ectoine’s preferential hydration mechanism, which stabilizes membranes and reduces inflammatory signalling. These findings suggest that DayDrop® Triple Action integrates viscoelastic optimization, osmoprotection, and targeted anti-inflammatory action, offering a promising non-pharmacological strategy for managing DED and allergic ocular surface disorders.
1. Introduction
Dry eye disease (DED), also referred to as dry eye syndrome (DES), keratoconjunctivitis sicca, or dysfunctional tear syndrome, is a multifactorial, progressive, and complex ophthalmic disorder affecting approximately 10–20% of the global adult population with an increasing prevalence [1,2]. Specifically, DED arises when the eye fails to produce a sufficient volume of high-quality tears, resulting in disturbances in tear production, absorption, and drainage [3]. Alternatively, DED may also occur when tear production is adequate, but the tear film is deficient in providing effective lubrication, protection against infections, and support for ocular surface healing [4,5]. In this regard, tear film instability is primarily classified into mild, moderate, severe, evaporative, and aqueous-deficient DED subtypes [6]. Notably, DED symptoms incidence and severity have worsened following the COVID-19 pandemic primarily due to environmental influences, lifestyle modifications, and the heightened use of screen-based digital devices leading to a significant decline in patients’ quality of life [7].
HA-based eye drops or artificial tears are among the most widely used therapeutic interventions for managing DED [8,9]. Hyaluronic Acid (HA) is a naturally occurring glycosaminoglycan present in the vitreous humour, cornea, and tear film [10], composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine linked by alternating β-(1→3) and β-(1→4) glycosidic bonds [11]. Its distinctive hydrophilic chemical structure underlies its advanced functionality and versatility [12], supporting its broad use in biomedical fields such as esthetics, traumatology, drug delivery, and particularly ophthalmology [13,14,15,16,17]. In ocular applications, HA provides numerous therapeutic benefits, including water retention, hydration, lubrication, moisturization, viscoelasticity, tear film stabilization, reduction in corneal surface shear force and tear elimination, as well as cytoprotective, anti-inflammatory, and antioxidant effects [18]. Moreover, by forming a protective film on the ocular surface, HA-based artificial tears not only alleviate DED symptoms but are also considered safe for stabilizing intraocular pressure (IOP), which is often compromised in patients with DED and those at risk of glaucoma [19].
Nowadays, single-ingredient HA artificial tears are distinguished from other ophthalmic agents, including polyvinyl alcohol, carboxymethylcellulose (CMC), hydroxypropyl methylcellulose, osmoprotectants, lipids and serums, among others [20]. Nevertheless, no differences in DED clinical efficacy have been reported among single-ingredient HA commercial eye drops, being practically the same in all of them [21]. Moreover, they exhibit a major drawback of reduced effectiveness over time due to rapid clearance from ocular surface [22]. Hence, HA-only formulations are being used today as standard baseline in DED treatment studies.
To enhance the effectiveness and extend the duration of HA-based eye drops for DED, significant research has focused on combining HA with complementary ophthalmic bioactive compounds [23]. Among these, osmoprotectants and CMC have shown synergistic effects on the chemical and therapeutic properties of HA. Ectoine, a naturally occurring osmoprotectant, has gained prominence in ophthalmic formulations due to its strong water-attracting and -retaining capacity, which protects against desiccation and supports corneal hydration and epithelial stability [24,25,26,27].
CMC is a widely used viscoelastic polymer in ophthalmic applications, valued for its mucoadhesive, lubricating, moisturizing, and water-retention properties [28,29]. As a core component in many artificial tear formulations, CMC enhances tear film stability and alleviates ocular surface discomfort [30]. Its bioadhesive capacity allows it to form a protective layer on the corneal epithelium, prolonging retention time on the ocular surface, reducing tear evaporation, and improving hydration—ultimately contributing to long-lasting symptomatic relief in DED patients [31,32].
Similarly, ectoine also contributes to maintaining tear film integrity, increasing the fluidity of cellular membranes, reducing oxidative stress, and protecting corneal epithelial cells from environmental stressors, ultimately offering anti-inflammatory and anti-allergic benefits [33]. These properties make HA–ectoine combinations particularly attractive for enhancing ocular hydration, soothing irritation, and improving long-term DED management. Commercial products such as Hylo-Dual® and Yeloin® exemplify the successful integration of HA and ectoine in therapeutic eye drops.
In recent years, multi-ingredient eye drops combining HA, CMC, and osmoprotectants have gained attention for their good tolerability and synergistic benefits in relieving DED symptoms [34,35]. Optive Fusion® is one example, incorporating HA, CMC, and glycerol/erythritol-based osmoprotectants; however, it contains preservatives that may damage the corneal epithelium and reduce tolerability [36,37]. To our knowledge, this is the first study to evaluate a triple-combination formulation integrating HA, CMC, and ectoine. While HA and ectoine are known for hydration and osmoprotection, and CMC for mucoadhesion, their combined effects have not been previously assessed. This formulation targets two key limitations of current therapies: rapid clearance and insufficient protection against inflammatory and allergic triggers. By coupling HA water retention with CMC adherence-enhancing properties, it aims to prolong ocular residence and improve tear film stability. Moreover, although ectoine anti-inflammatory and anti-allergic benefits are documented for the single molecule [38] and binary mixtures with other polymers [39], its performance within an HA–CMC matrix has not been reported. Here, we provide the first evidence that this triple-action system reduces pro-inflammatory cytokines (IL-6 and IL-8) in an in vitro allergic conjunctivitis model, supporting its potential as a novel non-pharmacological strategy for managing DED with inflammatory and allergic components.
Considering these factors, here we evaluate three leading HA–ectoine eye drop formulations—DayDrop® Triple Action, Hylo-Dual®, and Yeloin®—to determine their relative efficacy and ocular residence time in treating DED. Although Hylo-Dual® and Yeloin® were selected for comparison with DayDrop® Triple Action based on their market prominence, published data on their efficacy based on physico-chemical analysis are missing. We therefore hypothesized that these three products would differ significantly in therapeutic performance and duration of action due to their distinct physicochemical and rheological properties. DayDrop® Triple Action was further assessed for its sustained-release profile of ectoine, and its potential relief of DED associated inflammatory and allergy related symptoms. Overall, the next-generation DayDrop® Triple Action eye drops—combining hyaluronic acid, carboxymethylcellulose, and ectoine—demonstrate promising potential for improving DED management in the ophthalmic field.
2. Materials and Methods
2.1. Materials and Chemicals
DayDrop® Triple Action efficacy, as a function of its physicochemical properties, was evaluated and compared against two different commercial eye drops with formulations based on HA and ectoine (Hylo-Dual® and Yeloin®). While DayDrop® Triple Action is manufactured by i+Med S. Coop., Hylo-Dual® and Yeloin® are commercialized by Brill Pharma and Bausch + Lomb, respectively. All eyedrops were kept at 23 °C prior to use. The most important specifications have been summarized in Table 1. Acetonitrile (≥99.9%, Chem-Lab, Zedelgem, Belgium), water (LabKem, Casablanca, Morocco), and ethanol (≥99.8%, Sigma-Aldrich, St. Louis, MO, USA) were utilized for chromatographic assays. Simulated or artificial tear fluids (STF/ATF) were prepared using sodium chloride (NaCl, Fischer Scientific, Pittsburgh, PA, USA), calcium chloride (CaCl2, LabKem), sodium hydrogen carbonate (NaHCO3, LabKem) and potassium chloride (KCl, LabKem). Simulated or artificial tear fluids (STF/ATF, pH 7.4, 290–310 mOsmol·kg−1 osmotic pressure) was prepared according to the electrolyte composition of tears: 0.67% NaCl, 0.008% CaCl2, 0.20% NaHCO3 and 0.14% KCl in distilled water. QuixSep Micro Dilyzers (Seguin, TX, USA) equipped with 3500 Da molecular weight cut-off (MWCO) dialysis membranes (Medicell, Hwasun-eup, Jeollanam-do, South Korea) were employed for ectoine (142.16 g·mol−1) delivery studies from HA plus CMC polymeric matrix. Such membrane ensures unrestricted diffusion of ectoine while effectively retaining the high-molecular-weight polymeric network, which is in megadalton (MDa) range, allowing a precise evaluation of ectoine release.

Table 1.
Available specifications in the respective Instructions For Use (IFU) document of each commercial eye drop.
2.2. Physicochemical Characterization
2.2.1. HA
HA concentration (%) of commercial eye drops was quantitatively determined using a high-performance liquid chromatography (HPLC) coupled to a diode array detector (DAD) equipped with a PolySep-GFC-P precolumn (35 × 7.8 mm) and a PolySep-GFC-P linear chromatographic column (300 × 7.8 mm). Phosphate buffer 0.05 M (isocratic) was the mobile phase, flow rate was 0.8 mLmin−1, 20 µL was injection volume, 210 nm was detection wavelength, retention time was set at 9.00 min. HA concentration was calculated following the methods previously described in the literature [40]. The HA used in the tested formulations was produced by Bacillus subtilis fermentation and classified as high molecular weight (>1 MDa), consistent with the range typically associated with enhanced ocular surface protection and hydration [35].
2.2.2. CMC
The CMC used in the tested formulations exhibits a viscosity of approximately 2500 Pa·s, a property that ensures adequate thickening, mucoadhesion, and prolonged ocular surface residence [32].
2.2.3. pH
A SensION pH metre (HACH, pH31 model) equipped with XS sensor pH electrode (pH 0–14, 0–100 °C, KCl 3 M reference solution, 4.00 ± 0.01, 7.00 ± 0.01 and 9.21 ± 0.01 at 20 °C calibration standards) was used to measure the pH of commercial eye drops.
2.2.4. Osmolality
An Osmomat 030-D cryoscopic osmometer (Gonotec, 0–3000 mOsmol/kg measuring range, 1 mOsmol/kg resolution) was employed to determinate the osmolality of eye drops. Each measurement was performed by adding 50 µL.
2.2.5. Refractive Index
Refractive index of each commercial eye drop was measured by Optika 2WAJ refractometer. Before and after each measurement, surfaces in contact with polymeric solutions were cleaned with cotton cloth soaked with water and ethanol (1:1) mixture.
2.3. Rheological Characterization
The rheological characterization of commercial eye drops was conducted using an ARES-G2 rheometer (TA Instruments, New Castle, DE, USA). The viscoelastic properties of each hydrogel were evaluated at low (1 s−1) and high (5000 s−1) shear rates to simulate eye movement and blinking, respectively [41]. These measurements were performed at 25 °C using a 40 mm parallel plate geometry (steel plate), with data acquisition every 60 s. The first normal stress difference (N1) was determined using a standard DIN concentric cylinder geometry (Peltier Aluminum, 28 mm bob diameter, 30 mm cup diameter), following a previously described method [42]. Viscosity was examined varying shear rate from 1 to 1000 s−1.
2.4. Sustained Release of Ectoine
Ectoine release from different DayDrop® Triple Action formulations were determined in simulated or artificial tear fluids (STF/ATF) using QuixSep Micro Dilyzers. Briefly, 1 mL of DayDrop® Triple Action was placed in the upper compartment (donor chamber) with a hydrated dialysis membrane (3500 Da) in the middle. Then, 2 mL of simulated tear fluid (STF/ATF) was added to the lower compartment (receiver chamber). After that, prepared device is placed in an orbital shaker at 37 °C for 24 h measuring released ectoine to the receiver chamber at different times.
Cumulative release profile was obtained after quantification at 210 nm and retention time of 2.60–2.90 min by high-performance liquid chromatography coupled to a diode array detector (HPLC-DAD, Agilent 1260 Infinity II, Agilent Technologies, Waldbronn, Germany). Chromatographic column was a Jupiter® C18 300 Å (4.6 × 150 mm, 5 μm particle size) (Torrance, CA, USA) coupled to a security guard column and filters. Flow was adjusted to 0.8 mL·min−1, injection volume to 20 µL and column temperature to 30 °C. Water (eluent A) and acetonitrile (eluent B) were mobile phases. Gradient analyses of 0–5 min, 0% B; 5–10 min 0–100% B; 10–11 min, 100% B; 11–12 min, 100–0% B; 12–15 min, 0% B were used for analytes chromatographic separation.
Regarding ectoine concentration measurement, standard curve was performed using HPLC-grade ectoine standard (99.9%, Sigma-Aldrich, 142.16 g·mol−1). The standard curve was generated using ectoine reference solutions at 1, 2, 5, 10, 20, and 50 ppm, prepared from a 1000 ppm stock solution. These standards were analysed under the same HPLC-DAD conditions as the release samples to confirm linearity and accuracy across the expected concentration range. Ectoine quantification was realised using the following calibration curve: Signal (AU) = 52.32 C (mg·L−1) − 11.70, R2 = 0.999.
2.5. In Vitro Allergic Immunomodulation Assay
Human conjunctival fibroblasts (Innoprot, P10876 (Innovative Technologies in Biological Systems, S.L., Bizkaia, Spain)) were cultured using fibroblasts medium (Innoprot, P60108), following the manufacturer’s protocol. Cells were maintained in a humidified incubator at 37 °C with 5% CO2.
Prior to the assay, a cytotoxicity test, 1 × 105 conjunctival fibroblasts/well were seeded into 96-well plates pre-coated with poly-L-lysine and incubated at 37 °C with 5% CO2 for 24 h. Subsequently, the media was aspirated from all wells, and 200 µL of each product concentration suspended in culture media was added (ranging from 50% to 0.39% v/v). After the incubation period, cells were washed with pre-warmed PBS and stained with MTT solution. The plates were then incubated at 37 °C for an additional 4 h to allow the formation of the formazan crystals. Subsequently, the medium was removed, and 100 µL of DMSO per well was added to solubilize the coloured precipitate. Absorbance was measured at 570 nm using a microplate reader. Cell viability was expressed as a percentage relative to the negative control (C−) based on the absorbance values. To correct for background signal, the absorbance of blank wells—containing the test substance at the same concentrations but without cells—was subtracted from all measurements.
The percentage (%) of cell viability was calculated using the following formula:
where
- Abs (t) is the absorbance at 570 nm of the cell culture after exposure to a concentration of the test item after subtracting the absorbance of its corresponding blank.
- Abs (c) is the absorbance at 570 nm of the negative (untreated) control after subtracting the absorbance of its corresponding blank.
Three product dilutions were chosen based on cell viability: 25% > 70%, 12.5% > 85%, 1.5% > 95%.
To evaluate the anti-allergic effects of the three ophthalmic solutions on human conjunctival fibroblasts, an in vitro assay was performed in two phases: prevention and protection. Human conjunctival fibroblasts were seeded and cultured until confluent in 96-well plates, four replicates per experimental groups were included. Cells were treated with the selected concentrations of the ophthalmic solutions (25%, 12.5% and 1.5%) for 5 h, to assess their preventive and protective effect against an allergic/inflammatory stimulus. After this 5 h pre-treatment, the medium was replaced with fresh medium containing both the ophthalmic solutions and a cocktail of pro-inflammatory cytokines and mediators, IL-4 (10 ng/mL), IL-13 (10 ng/mL), TNF-α (10 ng/mL), and histamine (10 µM), to induce an inflammatory/allergenic response. After an additional 24 h of incubation, the cell culture supernatants were collected and stored properly for analysis. The levels of the pro-inflammatory cytokines IL-6 and IL-8 in the supernatants were quantified by ELISA, as markers of the inflammatory response following manufacturer instructions.
2.6. Statistical Analyses
Quantitative variables of assays are represented as means ± standard deviations. Statistical analysis was performed using multiple comparisons were performed employing One-way ANOVA (analysis of variance) with 0.05 significance level Tukey test and unpaired two-tailed t-tests (* p < 0.05) using OriginPro software 8.5 version. Graphical representation was performed using SigmaPlot software 15.0 version.
3. Results
3.1. Physicochemical Characterization
The chemical composition of eye drops is a critical factor influencing their clinical efficacy. While HA concentration has previously been considered a primary determinant of effectiveness in HA-only formulations, with higher concentrations presumed to yield better performance, it is now well established that HA concentration alone does not fully predict therapeutic outcomes [41]. In this regard the most important specifications of products analysed in this study have been summarized in Table 1.
Additional formulation parameters—such as the molecular weight of HA, rheological properties, as well as osmolarity, pH, and the presence of electrolytes or buffering agents—play essential roles in overall performance maintaining epithelial integrity and patient comfort.
Here, we analysed the composition of all three eye-drops as provided by the manufacturer. In terms of composition, all three products contain ectoine at 2%; however, the HA concentration and additional formulation vary (Table 1). The three products vary significantly in their HA concentration, with Hylo-Dual® having the lowest, 0.05%, DayDrop® Triple Action 0.1% and Yeloin® the highest, 0.24%. To verify the HA-content of all eye-drops, we measured the HA concentration in eye-drop samples by HPLC. As shown in Figure 1A, Hylo-Dual® contains significantly less HA (0.053 ± 0.001%) compared to DayDrop® Triple Action (0.103 ± 0.004%; p < 0.05), while Yeloin® shows the highest HA concentration (0.291 ± 0.021%; p < 0.05), 0.05% above the HA concentration provided by the manufacturer.
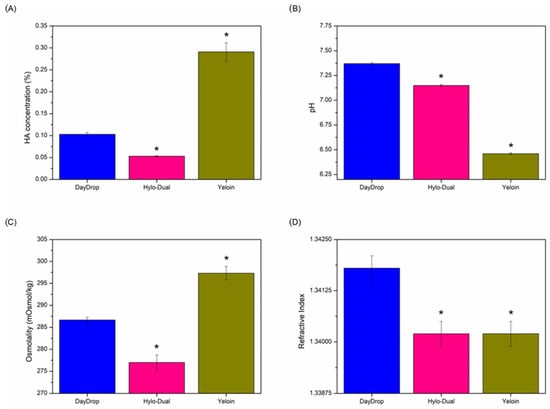
Figure 1.
(A) HA concentration, (B) pH, (C) osmolality and (D) refractive index physicochemical results of commercial eye drops. Statistical analysis was performed comparing DayDrop® Triple Action with Hylo-Dual® and Yeloin® using unpaired two-tailed t-tests (* p < 0.05). Replicates, n = 3.
While pH and osmolality are often considered to have a minor role in the clinical efficacy of eye drops, deviations in pH—6.5 to 8—can influence the viscosity of HA [41,43]. Some artificial tear formulations mimic the osmolarity of natural tears (~304 mOsm/L or ~304 mOsm/kg) [44], while others are slightly hypotonic to help counteract the hyperosmolarity associated with DED. Elevated tear osmolarity is known to damage the ocular surface, triggering inflammation and destabilizing the tear film [45]. We found that both DayDrop® Triple Action (pH 7.37 ± 0.01; 286.67 ± 0.58 mOsmol/kg) and Hylo-Dual® (pH 7.15 ± 0.01; 277.00 ± 1.73 mOsmol/kg) have osmolarities slightly hypotonic (pH Figure 1B, osmolality Figure 1C). Yeloin®, while the closest to the osmolality range of natural tears (297.33 ± 1.53 mOsmol/kg), is significantly more acidic (pH 6.46 ± 0.01) than DayDrop® Triple Action and Hylo-Dual®, thus on the lower end of the optimal pH range for HA.
Refractive index measurements for the tested formulations fell within the established physiological range of the human cornea (1.335–1.4391) as reported in prior studies [46]. Specifically, values of 1.3418 ± 0.0003 were obtained for DayDrop® Triple Action, while Hylo-Dual® and Yeloin®, each exhibited a value of 1.3402 ± 0.0003. These measurements are consistent with known variations in the corneal refractive index, which ranges from approximately 1.335 in earlier estimates to over 1.430 in more recent, in vivo assessments.
In summary, while all three formulations contain 2% ectoine, their differing HA concentrations, pH, and osmolality profiles highlight the importance of comprehensive physicochemical characterization. These variations may influence both the bioavailability and therapeutic performance of each eye drop, underscoring that HA content alone is not a sufficient predictor of clinical efficacy. Subsequent rheological measurements will further elucidate how these compositional differences impact the functional properties of these formulations.
3.2. Rheological Characterization
Natural tears are non-Newtonian fluids with shear-dependent properties. Accordingly, optimal ophthalmic formulations should also display pseudoplastic, shear-thinning behaviour. At low shear rates, such as when the eyes are open, increased viscosity supports tear film stability by reducing evaporation and delaying tear film breakup. Conversely, at high shear rates, as occur during blinking, a lower viscosity minimizes mechanical stress on the epithelial surface, thereby helping to prevent discomfort, inflammation, and transient blurred vision [47]. The physicochemical properties of HA artificial tears determine their viscosity and resistance to shear forces, which directly impact their clinical effectiveness [48]. Rheological modelling is used to study how viscosity changes under different shear rates, simulating how the drops behave on the eye’s surface during blinking and eye movements [49].
All tested artificial tears exhibited non-Newtonian shear-thinning behaviour, which supports tear-film stability and protects the ocular surface under static conditions, while reducing friction and mechanical stress during blinking up to 5000 s−1 (Figure 2) [50]. However, DayDrop® Triple Action and Hylo-Dual® demonstrated more pronounced shear-thinning properties compared to Yeloin®, indicating a rheological profile more favourable for HA ophthalmic formulations.
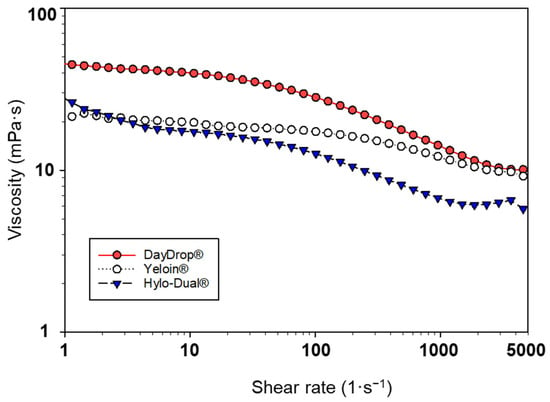
Figure 2.
Viscosity (mPa·s) versus shear rate (1/s) of DayDrop® Triple Action, Hylo-Dual® and Yeloin® commercial eye drops.
Thus, non-Newtonian rheological behaviour of artificial tears becomes more pronounced as the pseudoplastic index (n) decreases. A lower pseudoplastic index indicates enhanced shear-thinning properties. Among the evaluated formulations, DayDrop® Triple Action (n = 0.75) and Hylo-Dual® (n = 0.77) exhibited the most pronounced pseudoplastic behaviour, demonstrating greater shear-dependent viscosity reduction compared to Yeloin® (n = 0.89). Thus, enhanced pseudoplasticity of DayDrop® Triple Action and Hylo-Dual® may contribute to an improved biomechanical adaptability under dynamic physiological conditions.
Among the tested artificial tears, viscosity values varied significantly across the evaluated shear rate range, all within the range of healthy human tears [48,51]. DayDrop® Triple Action exhibited the highest viscosity at all measured shear rates: 39.31 mPa·s at 10 s−1 (representing open eye), 12.20 mPa·s at 1000 s−1 (transition phase of the blink cycle), and 10.09 mPa·s at 5000 s−1 (upstroke or downstroke of blinking). In contrast, Hylo-Dual® and Yeloin® showed lower viscosities than DayDrop® Triple Action, measuring Hylo-Dual 17.51 mPa·s, 6.8 mPa·s, and 5.78 mPa·s (as measured by others [52]), and Yeloin® 20.20 mPa·s, 11.41 mPa·s, and 9.27 mPa·s at the respective shear rates. Higher viscosity at low shear rates, as seen with DayDrop® Triple Action, can enhance tear film stability and reduce evaporation during interblink periods, while sufficient viscosity at high shear rates helps maintain lubrication and minimize mechanical stress during blinking. The lower viscosity of Hylo-Dual® and Yeloin® may therefore limit both retention time and lubricating efficacy on the ocular surface.
To assess the structural integrity and mechanical resilience of the formulations under dynamic conditions, the viscoelastic response of the eye drops was evaluated by measuring the first normal stress difference (N1, mPa) as a function of shear rate (Figure 3). N1 reflects the elastic component of a fluid’s response to shear; in viscoelastic materials, it arises from the alignment and stretching of polymer chains under flow, generating normal stresses perpendicular to the direction of shear [47]. A stable or high N1 indicates a robust elastic network capable of resisting deformation, which is critical for maintaining tear film structure during blinking and ensuring rapid recovery between blinks [52].
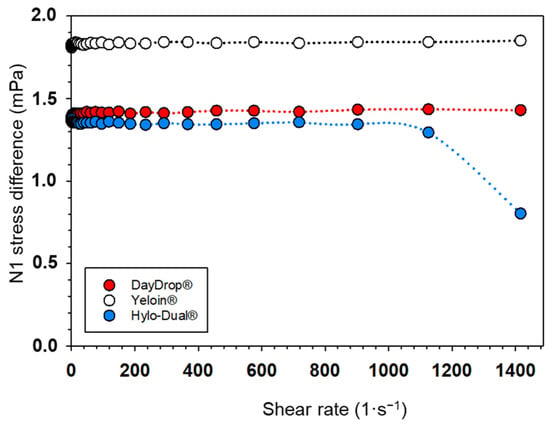
Figure 3.
The first normal stress difference (N1) represented against shear rate (1/s) of DayDrop® Triple Action, Hylo-Dual® and Yeloin® commercial eye drops.
Two formulations, DayDrop® Triple Action and Yeloin®, exhibited stable N1 values across the entire tested shear rate range (1–1400 s−1), maintaining approximately 1.8 mPa and 1.4 mPa, respectively. This consistency suggests a robust internal polymeric network that can withstand mechanical stress. In contrast, Hylo-Dual® showed a marked decline in N1 beyond ~900 s−1, dropping from ~1.3 mPa to ~0.8 mPa, indicating a breakdown of its viscoelastic structure under high shear. Such a collapse may compromise performance during rapid eyelid motion. Overall, DayDrop® Triple Action and Yeloin® demonstrate optimal viscoelastic response due to their higher and more consistent N1 values, although this must be balanced with viscosity and pseudoplastic behaviour.
Taken together, these results underscore the importance of a well-balanced rheological profile in the design of ophthalmic formulations. While all tested eye drops exhibited non-Newtonian, shear-thinning behaviour beneficial for ocular surface protection, DayDrop® Triple Action consistently demonstrated a consistent performance across all evaluated parameters. It combined the highest viscosity values at both low and high shear rates—supporting tear film stability during interblink periods and reducing mechanical stress during blinking—with the most pronounced pseudoplastic behaviour (n = 0.75), indicating excellent shear adaptability. Additionally, DayDrop® Triple Action maintained stable N1 values, reflecting a robust viscoelastic network capable of withstanding dynamic mechanical forces. In contrast, Hylo-Dual® showed a decline in viscoelastic integrity at high shear rates, and Yeloin®, despite stable N1 values, exhibited weaker pseudoplasticity and lower viscosity, potentially limiting its effectiveness under physiological conditions. Overall, DayDrop® Triple Action emerges as a rheologically balanced formulation, offering optimal viscosity, pseudoplasticity, and viscoelasticity to enhance tear film stability, prolong ocular surface retention, and improve comfort and protection in the management of DED.
The practical significance of DayDrop® Triple Action rheological properties lies not only in enhancing lubrication and comfort, but also in promoting stronger mucoadhesion to the ocular mucin layer. This increased mucoadhesion prolongs ocular surface residence time despite tear film washout [53], thereby increasing contact time and improving ectoine bioavailability. As a result, the formulation enables more sustained therapeutic action. These optimizations support reduced dosing frequency and greater therapeutic efficacy, ultimately contributing to improved adherence and clinical outcomes in the treatment of DED.
3.3. Sustained Release of Ectoine
HA-based eye drops with ectoine offer a nonpharmacological approach to treating DED by combining the hydrating and film-stabilizing effects of HA with the osmoprotective properties of ectoine. Sustained ectoine release helps reduce tear evaporation and maintain ocular surface stability, contributing to prolonged therapeutic benefits and improved patient comfort [54]. Given these potential benefits, it is important to determine whether the DayDrop® Triple Action formulation can effectively support the sustained release of ectoine under physiologically relevant conditions. To investigate this, we quantified the ectoine release profile from DayDrop® Triple Action using HPLC-DAD in simulated tear fluid (STF), mimicking its gradual diffusion into the tear film. A dialysis membrane was employed as a physical barrier to simulate ocular surface diffusion. Since DayDrop® Triple Action is a liquid formulation containing both HA and CMC, we also investigated whether the presence of CMC influences ectoine retention and delivery, and whether it enhances or impairs the overall efficacy of the formulation.
Hence, we measured ectoine delivery profiles from DayDrop® Triple Action over a 24 h period (Figure 4A). Our measurements indicate that ectoine release to the media reaches 50% (49.22 ± 5.31%) in just 4 h, and more than 90% is released after 12 h (90.92 ± 7.04%). Such a gradual increase in released ectoine suggests a controlled release mechanism, which supports extended hydration and protective effects against tear film instability, oxidative stress, and inflammatory or reactions to effectively and durably manage DED.
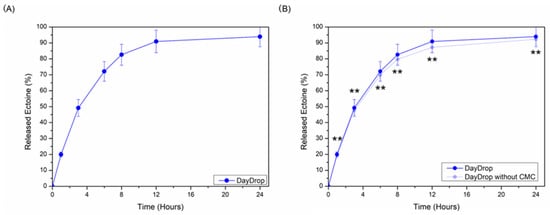
Figure 4.
(A) Cumulative release profiles of ectoine (%) from DayDrop® Triple Action. (B) CMC effect on ectoine delivery properties from DayDrop® Triple Action formulation. Replicates, n = 5 samples each condition. * Significant differences (p < 0.05), ** non-significant differences (p > 0.05).
We compared ectoine release from DayDrop® Triple Action formulations with and without the viscosity-enhancing polymer CMC (Figure 4B). No significant differences were observed at any time point (p > 0.05), indicating that the presence of CMC does not hinder ectoine release. On the contrary, the addition of CMC to the HA–ectoine formulation (DayDrop® Triple Action) slightly improved ectoine release, albeit not significantly (p > 0.05). This modest increase suggests that CMC may support a more sustained release profile over 24 h.
The well-established mucoadhesive and viscosity-enhancing properties of CMC may contribute to prolonged ectoine retention on the ocular surface, potentially enhancing its therapeutic effect [51]. Unlike formulations containing only HA and ectoine (e.g., Hylo-Dual® and Yeloin®), the inclusion of CMC in DayDrop® Triple Action may improve ectoine bioavailability and ensure a more consistent and sustained therapeutic action.
This extended bioavailability is particularly advantageous for DED management, as it promotes continuous ocular protection, reduces tear evaporation, and minimizes the need for frequent reapplication. Overall, the HA–ectoine–CMC triple-action system in DayDrop® Triple Action represents an advanced ophthalmic solution that enhances hydration, protection, and therapeutic longevity, reinforcing its potential as an effective nonpharmacological therapy for DED.
3.4. Protection Against Allergic Inflammation
Ectoine has emerged as a promising non-pharmacological compound with demonstrated anti-inflammatory properties and clinical efficacy alleviating allergic conditions [55]. Recent in vitro studies have further highlighted its protective capacity, showing that ectoine can shield cells from osmotic stress and modulate inflammatory signalling pathways [38]. These findings support its role as a cytoprotective agent, particularly in mucosal and epithelial tissues. In vitro evidence indicates that ectoine exerts its cytoprotective effects through a mechanism known as preferential exclusion, which promotes the structuring of water molecules around macromolecules such as proteins and lipid membranes. This structured hydration shell stabilizes the native conformations of these macromolecules by reducing their direct interaction with destabilizing solutes or environmental stressors. As a result, ectoine helps preserve the functional integrity of cellular components under osmotic, oxidative, or inflammatory stress, thereby attenuating downstream signalling cascades associated with cellular damage and inflammation [24,56,57,58].
To investigate the potential of DayDrop® Triple Action in a relevant allergic model, we employed conjunctival fibroblasts, which are key effector cells in ocular surface inflammation. Cells were exposed to a pro-allergic signalling cocktail consisting of IL-4, IL-13, TNF-α, and histamine, simulating the inflammatory environment of allergic conjunctivitis.
As shown in Figure 5, DayDrop® Triple Action significantly reduced the secretion of pro-inflammatory cytokines IL-6 and IL-8 in conjunctival fibroblasts exposed to the cytokine cocktail. All tested concentrations (1.5%, 12.5%, and 25%) led to a reduction in IL-6 levels compared to the positive control (C+), with the 12.5% and 25% concentrations showing the most pronounced effects. The response was dose-dependent, indicating a robust anti-inflammatory effect across a range of concentrations.
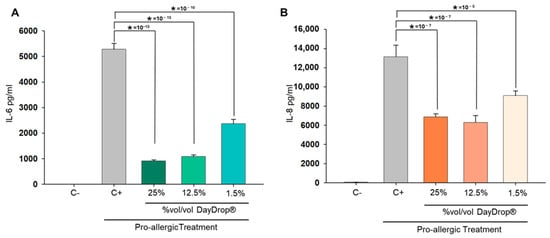
Figure 5.
Protective effects of DayDrop® Triple Action on cytokine release following a pro-allergic challenge. Cells were pre-incubated for 5 h with DayDrop® Triple Action at 25%, 12.5%, and 1.5% (%v/v), followed by a 24 h treatment with a pro-allergenic cocktail (IL-4, IL-13, TNF-α, histamine) in the continued presence of the eye drop. Supernatants were collected and analysed for IL-6 (A) and IL-8 (B) levels by ELISA. DayDrop® Triple Action significantly reduced cytokine release compared to the positive control (C+), indicating a protective effect. Data represents mean pg/mL protein plus SD (n = 6). Statistical analysis was performed using unpaired two-tailed t-tests (* p < 0.05).
A similar pattern was observed for IL-8. While all concentrations of DayDrop® Triple Action reduced IL-8 secretion relative to the positive control, the 12.5% and 25% concentrations were particularly effective, with the 25% concentration achieving the greatest suppression. This is notable given central role of IL-8 in neutrophil recruitment and amplification of allergic inflammation [59].
Taken together, these findings highlight the dual efficacy of DayDrop® Triple Action in reducing both IL-6 and IL-8 levels in an in vitro model of allergic conjunctivitis, suggesting that the presence of ectoine in DayDrop® Triple Action contributes significantly to the modulation of allergic cellular response, likely through stabilization of cellular membranes and interference with inflammatory signalling pathways.
3.5. Biological Safety
Despite the widespread utilization of HA alone, as well as its combinations separately with osmoprotectants or CMC, in the formulation, production, and commercialization of ophthalmic medical devices, new generation eye drops currently lack scientific literature supporting their biological safety profile. In this context, biological safety of medical devices like DayDrop® Triple Action must be strictly regulated under ISO 10993 [60] to protect humans from potential biological risks. Considering its classification (Class IIb: surface medical device in contact with mucosal membrane for more than 30 days), an in-depth physicochemical characterization and biocompatibility tests must be performed to validate biological safety.
A comprehensive physicochemical characterization was performed to assess the biological safety of DayDrop® Triple Action, including potential residual processing aids, in accordance with ISO 15798 [61] and the European Pharmacopoeia. DayDrop® Triple Action met all safety criteria: it is a transparent liquid free of suspended particles, with a viscosity of 5–50 cP, pH between 6.8 and 7.8, and osmolality of 200–400 mOsmol/kg. Microbiological evaluations confirmed the absence of hazardous contaminants. Bioburden testing, compliant with ISO 11737-1 [62], verified that microbial counts (aerobic, anaerobic, fungal/yeast) remained below 50 CFU/g. Filtration sterilization was validated per EP 2.6.1, ensuring a sterility assurance level (SAL) of at least 10−6.
Biocompatibility testing was conducted under ISO 10993 standards to support CE marking, including evaluations for genotoxicity, cytotoxicity, implantation, irritation, sensitization, and systemic toxicity across various exposure durations. Additionally, DayDrop® Triple Action was tested for compatibility with soft daily contact lenses (Bausch & Lomb®, BC 8.6, 14.2 mm, PWR −0.25 D, batch W92001217) following ISO 11981 [63]. It also demonstrated physical compatibility with hydrophilic silicone hydrogel and rigid gas permeable lenses, meeting the optical and physical criteria outlined in ISO 18369-2 [64].
Preservatives and phosphates in ophthalmic formulations are known to contribute to ocular toxicity, potentially worsening DED symptoms. Preservative-free and phosphate-free eye drops, such as DayDrop® Triple Action, are increasingly favoured for their safety and tolerability. Common preservatives like benzalkonium chloride (BAK) can induce oxidative stress, disrupt epithelial membranes, and trigger inflammation, especially with chronic use—leading to tear film instability and exacerbation of DED. Similarly, phosphate buffers, though used to stabilize pH, carry a risk of corneal calcification in patients with compromised corneal epithelium, impairing vision and delaying healing [65].
The absence of these excipients in DayDrop® Triple Action enhances its suitability for long-term use in chronic DED and post-surgical care. By avoiding preservatives and phosphates, such formulations help maintain ocular surface integrity, reduce irritation, and improve patient compliance. This is particularly important in pediatric populations, where physiological differences in the ocular surface and tear film require careful consideration. While HA- and ectoine-based drops are generally well tolerated, their use in children should be guided by healthcare professionals. Preservative-free artificial tears remain the safest option, offering effective relief with minimal risk of adverse effects in younger patients.
4. Discussion
In this study, we evaluated the efficacy and ocular retention time of three commercially available eye drops for the treatment of DED: DayDrop® Triple Action, Hylo-Dual®, and Yeloin®. DayDrop® Triple Action is a formulation that combines HA, CMC, and ectoine. It is designed to mimic the properties of natural tears and support ocular surface health under both mechanical and inflammatory stress. This is achieved through a barrier effect and hydration capacity, which result from the optimized physicochemical properties and the synergistic action of its three components.
When compared to other formulations containing HA and ectoine, we observed only minor differences in pH, osmolarity, and refractive index. However, DayDrop® Triple Action demonstrated optimization, with a pH that closely mimics natural tears. Its hypoosmotic ionic strength is particularly well-suited to the physiological requirements of eye drops intended for DED management, contributing to improved hydration.
The most significant differences among the tested formulations were found in their viscosity and shear-thinning behaviour. DayDrop® Triple Action, which includes CMC in addition to HA and ectoine, exhibited higher viscosity and more pronounced pseudoplastic behaviour compared to the other formulations (Figure 2). While Hylo-Dual® showed better pseudoplasticity than Yeloin®, it had lower overall viscosity. These findings suggest that the inclusion of CMC in DayDrop® Triple Action measurably increases viscosity, which may enhance mucoadhesion, reduce mechanical stress, and improve ocular retention time.
Furthermore, both DayDrop® Triple Action and Yeloin® demonstrated greater resistance to viscoelastic deformation than Hylo-Dual®, as indicated by measurements of the first normal stress difference (N1). This suggests that DayDrop® Triple Action and Yeloin® may provide longer-lasting support to the tear film and reduce epithelial friction. Overall, our rheological analysis indicates that DayDrop® Triple Action possesses optimal mechanical properties for minimizing water loss and extending the duration of action, likely facilitating more effective delivery of ectoine compared to formulations lacking CMC.
We evaluated the release profile of ectoine in DayDrop® Triple Action and observed a gradual release, with more than 90% delivered within 12 h. This sustained delivery is essential for maintaining osmoprotective and anti-inflammatory effects, particularly in relation to the formulation’s rheological characteristics. Ectoine plays a pivotal role in stabilizing the tear film and enhancing hydration through preferential exclusion, a mechanism that strengthens the hydration shell surrounding biomolecules [57,60]. This process improves membrane fluidity under conditions of limited water availability, decreases the generation of reactive oxygen species, and mitigates the activation of stress signalling pathways (Figure 6B). Additionally, ectoine contributes to lipid layer stability and reduces tear evaporation.
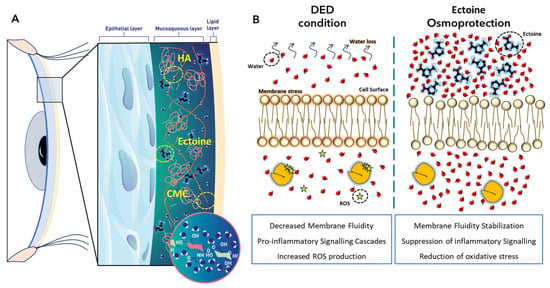
Figure 6.
Model illustrating the physicochemical and biological actions of DayDrop® Triple Action on the ocular surface. (A) Schematic cross-section of the human eye showing the tear film architecture, including the epithelial, mucin, and lipid layers. The distribution of HA, CMC, and ectoine is depicted. Molecular inset illustrates the structural features of HA. (B) Ectoine-mediated protection through preferential exclusion promoting hydration of lipid bilayers and ROS-production reduction.
To evaluate the anti-inflammatory and anti-allergic potential of DayDrop® Triple Action, we conducted in vitro assays under pro-allergic stimulation. The results showed that DayDrop® Triple Action significantly reduced the secretion of pro-inflammatory cytokines, particularly IL-6 and IL-8 (Figure 5). This cytokine suppression has important implications for allergic eye disease. IL-6 is a key mediator of chronic inflammation and immune cell activation, and its reduction may help restore epithelial integrity and reduce ocular discomfort. IL-8, a potent neutrophil chemoattractant, plays a central role in amplifying allergic responses and sustaining inflammation. Lowering IL-8 levels can limit neutrophil infiltration, reduce tissue damage, and break the cycle of inflammation, thereby improving tear film stability and reducing the severity of allergic flare-ups. Together, the suppression of IL-6 and IL-8 suggests that DayDrop® Triple Action may offer meaningful anti-allergic benefits, particularly in patients with mixed-type DED and seasonal or perennial allergies.
Based on our results, we propose that DayDrop® Triple Action exerts a triple therapeutic action on the ocular surface (Figure 6): (1) Ectoine provides anti-inflammatory and membrane-stabilizing effects, protecting the eye from environmental stressors such as allergens and dry air; (2) HA delivers deep hydration and supports epithelial regeneration; and (3) CMC increases viscosity, prolongs ocular residence time, and enhances the sustained release of active ingredients. Together, these components offer long-lasting hydration, protection, and symptom relief for patients with DED.
5. Conclusions
DayDrop® Triple Action, combining HA, CMC, and ectoine, shows favourable physicochemical and rheological properties compared to other HA–ectoine formulations, with enhanced viscosity, pseudoplasticity, and viscoelasticity that support tear film stability and ocular surface retention. Its gradual ectoine release enables sustained anti-inflammatory and osmoprotective effects, while in vitro data suggest additional anti-allergic potential. This triple-action formulation provides a well-balanced, long-lasting relief and ocular surface support.
These findings support DayDrop® Triple Action as a promising non-pharmacological option for managing dry eye disease, particularly in patients with inflammatory or allergic components.
Author Contributions
Conceptualization, J.A.d.O., A.M., A.P., J.M.A. and R.P.G.; methodology, J.A.d.O., A.M., A.P., N.M.d.C. and O.G.; software, J.A.d.O., A.M. and S.B.C.; validation, J.A.d.O., A.M. and J.M.A.; formal analysis, J.A.d.O., A.M. and J.T.; investigation, J.A.d.O., A.M., A.P., N.M.d.C. and O.G.; resources, O.G., J.T. and L.G.; data curation, J.A.d.O., A.M., O.G., N.M.d.C. and S.B.C.; writing—original draft preparation, J.A.d.O. and A.M.; writing—review and editing, J.A.d.O., A.M. and J.M.A.; visualization, J.A.d.O. and A.M.; supervision, R.P.G., M.U.L.d.H. and L.G.; project administration, S.B.C. and R.P.G.; funding acquisition, M.U.L.d.H., S.B.C. and R.P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by i+Med S. Coop.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and legal reasons.
Acknowledgments
Authors acknowledge technical and human support provided by i+Med S. Coop.
Conflicts of Interest
The authors declare that they are employees of i+Med S. Coop., the company that commercializes DayDrop® Triple Action eye drops. In any case, this fact has not influenced the scientific rigour of the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of Variance |
| CFU | Colony Forming Units |
| CMC | Carboxymethylcellulose |
| DAD | Diode Array Detector |
| DED | Dry Eye Disease |
| EP | European Pharmacopoeia |
| HA | Hyaluronic Acid |
| HPLC | High-Performance Liquid Chromatography |
| IFN | Interferon |
| IFU | Instructions For Use |
| IL | Interleukin |
| ISO | International Organization for Standardization |
| MDR | Medical Device Regulation |
| OA | Ocular allergy |
| SAL | Sterility Assurance Level |
| SD | Standard deviation |
| STF/ATF | Simulated or Artificial Tear Fluids |
| TNF | Tumour Necrosis Factor |
References
- Mohamed, H.B.; Abd El-Hamid, B.N.; Fathalla, D.; Fouad, E.A. Current Trends in Pharmaceutical Treatment of Dry Eye Disease: A Review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef]
- Benítez-del-Castillo, J.M.; Burgos-Blasco, B. Prevalence of Dry Eye Disease in Spain: A Population-Based Survey (PrevEOS). Ocul. Surf. 2025, 36, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Roucaute, E.; Huertas-Bello, M.; Sabater, A.L. Novel Treatments for Dry Eye Syndrome. Curr. Opin. Pharmacol. 2024, 75, 102431. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Wu, M.; Sun, C.; Shi, Q.; Luo, Y.; Wang, Z.; Wang, J.; Qin, Y.; Cui, W.; Yan, C.; Dai, H.; et al. Dry Eye Disease Caused by Viral Infection: Past, Present and Future. Virulence 2024, 15, 2289779. [Google Scholar] [CrossRef]
- Phadatare, S.P.; Momin, M.; Nighojkar, P.; Askarkar, S.; Singh, K.K. A Comprehensive Review on Dry Eye Disease: Diagnosis, Medical Management, Recent Developments, and Future Challenges. Adv. Pharm. 2015, 2015, 704946. [Google Scholar] [CrossRef]
- Barabino, S. A Narrative Review of Current Understanding and Classification of Dry Eye Disease with New Insights on the Impact of Dry Eye during the COVID-19 Pandemic. Ophthalmol. Ther. 2021, 10, 495–507. [Google Scholar] [CrossRef]
- Ouyang, X.W.; Fang, S.; Yi, Y.M.; Zou, S.P.; Hu, Q.Y.; Huang, Z.X.; Li, Q.X.; Luo, J.Y. Different Concentrations of Hyaluronic Acid Eye Drops for Dry Eye Syndrome: A Systematic Review and Meta-Analysis. Int. J. Ophthalmol. 2024, 17, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Andrade del Olmo, J.; Sáez Martínez, V.; Martínez de Cestafe, N.; Alonso, J.M.; Olavarrieta, C.; Ucelay López de Heredia, M.; Benito Cid, S.; Pérez González, R. Effectiveness Evaluation of Hyaluronic Acid-Based Commercial Eye Drops to Treat Ophthalmic Dry Eye Disease. Carbohydr. Polym. Technol. Appl. 2024, 8, 100577. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic Acid in the Treatment of Dry Eye Disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Landi, M.R.; Massa, A.; D’Amora, U.; Guarino, V. Hyaluronic Acid in Biomedical Fields: New Trends from Chemistry to Biomaterial Applications. Int. J. Mol. Sci. 2022, 23, 14372. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Liu, P.Y.; Lin, M.H.; Lu, C.J.; Chou, H.Y.; Nian, C.Y.; Jiang, Y.T.; Hsu, Y.H.H. Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Sáez Martínez, V.; Pérez González, R.; María Alonso, J. Sustained Drug Release from Biopolymer-Based Hydrogels and Hydrogel Coatings. In Hydrogels—From Tradition to Innovative Platforms with Multiple Applications; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Ye, H.; Zhang, R.; Zhang, C.; Xia, Y.; Jin, L. Advances in Hyaluronic Acid: Bioactivity, Complexed Biomaterials and Biological Application: A Review. Asian J. Surg. 2025, 48, 49–61. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Alonso, J.M.; Sáez Martínez, V.; Ruiz-Rubio, L.; Pérez González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Biocompatible Hyaluronic Acid-Divinyl Sulfone Injectable Hydrogels for Sustained Drug Release with Enhanced Antibacterial Properties against Staphylococcus Aureus. Mater. Sci. Eng. C 2021, 125, 112102. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, J.A.; Pérez-álvarez, L.; Martínez, V.S.; Cid, S.B.; González, R.P.; Vilas-Vilela, J.L.; Alonso, J.M. Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications. Gels 2022, 8, 223. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Moschowits, E.; Tønseth, K.A.; Vehof, J.; Utheim, T.P. A Comparison between Hyaluronic Acid and Other Single Ingredient Eye Drops for Dry Eye, a Review. Acta Ophthalmol. 2024, 102, 25–37. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic Acid in Ocular Drug Delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Lee, W.-Y.; Kim, Y.; Hong, Y. A Meta-Analysis of the Efficacy of Hyaluronic Acid Eye Drops for the Treatment of Dry Eye Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 2383. [Google Scholar] [CrossRef]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of Hyaluronan-Based Eye Drop Formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef]
- Sánchez-González, J.-M.; De-Hita-Cantalejo, C.; González-Rodríguez, M.L.; Fernández-Trueba-Fagúndez, A.; Ballesteros-Sánchez, A.; Martinez-Perez, C.; Caro-Díaz, R.; Guzman, C.M.; González-Oyarce, M.F.; Sánchez-González, M.C. Efficacy Assessment of Liposome Crosslinked Hyaluronic Acid and Standard Hyaluronic Acid Eye Drops for Dry Eye Disease Management: A Comparative Study Employing the Ocular Surface Analyzer and Subjective Questionnaires. Front. Med. 2024, 11, 1264695. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Chen, X.; Lin, N.; Li, J.M.; Liu, H.; Abu-Romman, A.; Yaman, E.; Bian, F.; de Paiva, C.S.; Pflugfelder, S.C.; Li, D.Q. Ectoine, from a Natural Bacteria Protectant to a New Treatment of Dry Eye Disease. Pharmaceutics 2024, 16, 236. [Google Scholar] [CrossRef]
- Ng, H.S.; Wan, P.K.; Kondo, A.; Chang, J.S.; Lan, J.C.W. Production and Recovery of Ectoine: A Review of Current State and Future Prospects. Processes 2023, 11, 339. [Google Scholar] [CrossRef]
- Wang, K.; Cui, B.; Wang, Y.; Luo, W. Microbial Production of Ectoine: A Review. ACS Synth. Biol. 2025, 14, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiao, D.; Yuan, T.; Feng, Y.; Zhang, P.; Wang, X.; Zhang, L. Biotechnological Production of Ectoine: Current Status and Prospects. Folia Microbiol. 2024, 69, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Groß, D.; Childs, M.; Piaton, J.M. Comparative Study of 0.1% Hyaluronic Acid versus 0.5% Carboxymethylcellulose in Patients with Dry Eye Associated with Moderate Keratitis or Keratoconjunctivitis. Clin. Ophthalmol. 2018, 12, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Arif, F.A.C.; Hilmi, M.R.; Rejab, N.S.M.; Wolffsohn, J.S. Immediate Effects of Artificial Tears with and without Preservatives Containing Hyaluronic Acid and Carboxymethyl Cellulose. Med. Hypothesis Discov. Innov. Optom. 2023, 4, 102–111. [Google Scholar] [CrossRef]
- Salim, S.; Kamath, S.J.; Jeganathan, S.; Pai, S.G.; Mendonca, T.M.; Kamath, A.R. Comparing the Efficacy of Sodium Hyaluronate Eye Drops and Carboxymethylcellulose Eye Drops in Treating Mild to Moderate Dry Eye Disease. Indian. J. Ophthalmol. 2023, 71, 1593–1597. [Google Scholar] [CrossRef]
- Aragona, P.; Benítez-Del-castillo, J.M.; Coroneo, M.T.; Mukherji, S.; Tan, J.; Vandewalle, E.; Vingrys, A.; Liu, H.; Carlisle-Wilcox, C.; Vehige, J.; et al. Safety and Efficacy of a Preservative-Free Artificial Tear Containing Carboxymethylcellulose and Hyaluronic Acid for Dry Eye Disease: A Randomized, Controlled, Multicenter 3-Month Study. Clin. Ophthalmol. 2020, 14, 2951–2963. [Google Scholar] [CrossRef]
- Paugh, J.R.; Chatelier, R.C.; Huff, J.W. Ocular Residence Time of Carboxymethylcellulose Solutions. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2: Basic Science and Clinical Relevance; Sullivan, D.A., Dartt, D.A., Meneray, M.A., Eds.; Springer: Boston, MA, USA, 1998; pp. 761–767. ISBN 978-1-4615-5359-5. [Google Scholar]
- Allegri, P.; Marrazzo, G.; Ciurlo, C.; Mastromarino, A.; Autuori, S.; Murialdo, U. Retrospective Study to Evaluate the Efficacy on Vernal Kerato-Conjunctivitis (VKC) of 2% Ectoine versus 0.05% Ketotifen Eye-Drops. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2492. [Google Scholar]
- Labetoulle, M.; Chiambaretta, F.; Shirlaw, A.; Leaback, R.; Baudouin, C. Osmoprotectants, Carboxymethylcellulose and Hyaluronic Acid Multi-Ingredient Eye Drop: A Randomised Controlled Trial in Moderate to Severe Dry Eye. Eye 2017, 31, 1409–1416. [Google Scholar] [CrossRef]
- Mateo Orobia, A.J.; Saa, J.; Lorenzo, A.O.; Herreras, J.M. Combination of Hyaluronic Acid, Carmellose, and Osmoprotectants for the Treatment of Dry Eye Disease. Clin. Ophthalmol. 2018, 12, 453–461. [Google Scholar] [CrossRef]
- Schrage, N.; Frentz, M.; Spoeler, F. The Ex Vivo Eye Irritation Test (EVEIT) in Evaluation of Artificial Tears: Purite®-Preserved versus Unpreserved Eye Drops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1333–1340. [Google Scholar] [CrossRef]
- Pathak, Y.; Sutariya, V.; Hirani, A.A. (Eds.) Nano-Biomaterials for Ophthalmic Drug Delivery; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Li, J.M.; Lin, N.; Zhang, Y.; Chen, X.; Liu, Z.; Lu, R.; Bian, F.; Liu, H.; Pflugfelder, S.C.; Li, D.Q. Ectoine Protects Corneal Epithelial Survival and Barrier from Hyperosmotic Stress by Promoting Anti-Inflammatory Cytokine IL-37. Ocul. Surf. 2024, 32, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Bilstein, A.; Werkhäuser, N.; Rybachuk, A.; Mösges, R. The Effectiveness of the Bacteria Derived Extremolyte Ectoine for the Treatment of Allergic Rhinitis. Biomed Res. Int. 2021, 2021, 5562623. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Sáez Martínez, V.; Martínez de Cestafe, N.; Alonso, J.M.; Goenaga Ibeas, C.; Ucelay López de Heredia, M.; Benito Cid, S.; Pérez González, R. Resistance to Enzymatic Degradation and Efficacy Evaluation of Crosslinked Hyaluronic Acid Based Commercial Viscosupplements for Knee Osteoarthritis Treatment. Carbohydr. Polym. Technol. Appl. 2023, 6, 100392. [Google Scholar] [CrossRef]
- Aragona, P.; Simmons, P.A.; Wang, H.; Wang, T. Physicochemical Properties of Hyaluronic Acid–Based Lubricant Eye Drops. Transl. Vis. Sci. Technol. 2019, 8, 2. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Balazs, E.A.; Cowman, M.K.; Briller, S.O.; Cleland, R.L. On the Limiting Viscosity Number of Hyaluronate in Potassium Phosphate Buffers between PH 6.5 and 8. Biopolymers 1983, 22, 589–591. [Google Scholar] [CrossRef]
- Gilbard, J.P. Human Tear Film Electrolyte Concentrations in Health and Dry-Eye Disease. Int. Ophthalmol. Clin. 1994, 34, 27–36. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of Hyperosmolarity in the Pathogenesis and Management of Dry Eye Disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Patel, S.; Tutchenko, L. The Refractive Index of the Human Cornea: A Review. Contact Lens Anterior Eye 2019, 42, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Arshinoff, S.A.; Hofmann, I.; Nae, H. Role of Rheology in Tears and Artificial Tears. J. Cataract Refract. Surg. 2021, 47, 655–661. [Google Scholar] [CrossRef]
- Müller-Lierheim, W.G.K. Why Chain Length of Hyaluronan in Eye Drops Matters. Diagnostics 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Simmons, P.A.; Vehige, J.G. Investigating the Potential Benefits of a New Artificial Tear Formulation Combining Two Polymers. Clin. Ophthalmol. 2017, 11, 1637–1642. [Google Scholar] [CrossRef]
- Tiffany, J.M. The Viscosity of Human Tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M. Viscoelastic Properties of Human Tears and Polymer Solutions. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes. Advances in Experimental Medicine and Biology; Sullivan, D.A., Ed.; Springer: Boston, MA, USA, 1994; Volume 350. [Google Scholar]
- Arshinoff, S.; Hofmann, I.; Nae, H. Rheological Behavior of Commercial Artificial Tear Solutions. J. Cataract Refract. Surg. 2021, 47, 649–654. [Google Scholar] [CrossRef]
- Ludwig, A. The Use of Mucoadhesive Polymers in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Gupta, P.K.; Toyos, R.; Sheppard, J.D.; Toyos, M.; Mah, F.S.; Bird, B.; Theriot, P.E.; Higgins, D. Tolerability of Current Treatments for Dry Eye Disease: A Review of Approved and Investigational Therapies. Clin. Ophthalmol. 2024, 18, 2283–2302. [Google Scholar] [CrossRef]
- Bilstein, A.; Heinrich, A.; Rybachuk, A.; Mösges, R. Ectoine in the Treatment of Irritations and Inflammations of the Eye Surface. Biomed. Res. Int. 2021, 2021, 8885032. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, M.; Brinkkötter, M.; Harishchandra, R.K.; Galla, H.J. Biophysical Investigations of the Structure and Function of the Tear Fluid Lipid Layers and the Effect of Ectoine. Part B: Artificial Lipid Films. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, M.; Backers, H.; Harishchandra, R.K.; Galla, H.J. Biophysical Investigations of the Structure and Function of the Tear Fluid Lipid Layer and the Effect of Ectoine. Part A: Natural Meibomian Lipid Films. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2708–2715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harishchandra, R.K.; Sachan, A.K.; Kerth, A.; Lentzen, G.; Neuhaus, T.; Galla, H.J. Compatible Solutes: Ectoine and Hydroxyectoine Improve Functional Nanostructures in Artificial Lung Surfactants. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2830–2840. [Google Scholar] [CrossRef][Green Version]
- Shute, J. Interleukin-8 Is a Potent Eosinophil Chemo-Attractant. Clin. Exp. Allergy 1994, 24, 203–206. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 15798; Ophthalmic Implants—Ophthalmic Viscosurgical Devices. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 11737-1; Sterilization of Health Care Products—Microbiological Methods—Part 1: Determination of a Population of Microorganisms on Products. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 11981; Ophthalmic Optics—Contact Lenses and Contact Lens Care Products—Determination of Physical Com-patibility of Contact Lens Care Products with Contact Lenses. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 18369-2; Ophthalmic Optics—Contact Lenses—Part 2: Tolerances. International Organization for Standardization: Geneva, Switzerland, 2017.
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in Eyedrops: The Good, the Bad and the Ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).