Abstract
Dried blood spots (DBSs) are a practical tool for diagnosing infectious diseases, especially in remote or resource-limited settings. This study assessed the efficacy of DBS-based serological assays for syphilis screening. EDTA blood samples from 171 syphilis-seropositive and 122 seronegative individuals were used to prepare DBSs by spotting whole blood onto filter paper. After drying, 12 mm disks were punched, incubated overnight in buffered solution, and centrifuged. Syphilis serological screening was conducted using the Liaison® Treponema Screen assay, Macro-Vue™ Reagin Plasma Rapid (RPR) card test, and Dual Path Platform (DPP) Syphilis Screen and Confirm test. The Liaison® assay demonstrated 100% sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with an optimized cut-off. The nontreponemal RPR test showed very low sensitivity (2.9%) on DBS but perfect specificity (100%). The DPP test for treponemal antibodies achieved high sensitivity (92.1%) and specificity (98.2%) with microreader adjustment. Visual reading of the DPP test had variable accuracy, with sensitivity reaching 100% but lower specificity (42.1%). Nontreponemal antibody detection by DPP showed moderate sensitivity and specificity. Although nontreponemal testing requires refinement, DBS testing combined with point-of-care tests like DPP holds promise for expanding syphilis screening accessibility and decentralization globally, particularly in resource-constrained environments.
1. Introduction
Syphilis remains a major global public health concern, with an estimated 6 million new cases occurring annually among individuals aged 15 to 49 years according to the World Health Organization (WHO) [1]. A disproportionate burden of infections continues to be reported among men, especially among gay, bisexual, and other men who have sex with men (GBMSM), who constitute the most affected population worldwide. This epidemiological pattern has been consistently observed in Europe, where the European Centre for Disease Prevention and Control (ECDC) has documented a steady increase in reported syphilis cases over the past decade. In 2019 alone, 33,709 cases were recorded within the European Union/European Economic Area (EU/EEA), corresponding to a notification rate of 7.9 cases per 100,000 population [2]. Notably, the incidence was nine times higher in men than in women, with the highest rates being observed among those aged 25–34 years. Approximately 74% of cases occurred in MSM, a trend that has been linked to multiple behavioral and biomedical factors, including high rates of condomless sex, serosorting practices among HIV-positive MSM, increased numbers of sexual partners among HIV-negative MSM, and the widespread uptake of HIV pre-exposure prophylaxis (PrEP). While PrEP is highly effective in preventing HIV transmission, its availability may have inadvertently contributed to risk behaviors associated with syphilis transmission [2].
The persistence and resurgence of syphilis in both high-income and resource-limited settings highlight the central importance of accurate and accessible diagnostic tools. Traditionally, syphilis screening has relied on serological testing of serum or plasma samples. The classical diagnostic algorithm involves initial nontreponemal testing using assays such as the Rapid Plasma Reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) test, followed by confirmatory treponemal-specific assays such as the Treponema pallidum Hemagglutination Assay (TPHA) or the Fluorescent Treponemal Antibody Absorption (FTA-Abs) test [3]. In recent years, however, many high-volume laboratories have adopted the so-called reverse sequence algorithm, in which automated treponemal assays—typically chemiluminescence immunoassays (CLIA)—are employed as the initial screening step, with reactive results being subsequently confirmed using nontreponemal tests. This reverse approach has been shown to enhance sensitivity, particularly for latent or previously treated infections, while improving laboratory efficiency and reducing overall testing costs.
Despite their proven utility, both traditional and reverse algorithms require access to laboratory infrastructure, specialized equipment, and trained personnel, limiting their applicability in decentralized or resource-constrained environments. To address these limitations, several point-of-care (POC) tests have been developed to enable syphilis screening outside of formal laboratory settings [3,4,5]. Most of these rapid assays detect only treponemal antibodies and therefore cannot differentiate between an active infection and prior exposure. This lack of distinction is clinically relevant, as serological scarring from previous syphilis episodes may persist indefinitely, complicating diagnosis and case management. However, syphilis POC tests improves screening coverage in vulnerable populations such as pregnant women, for whom timely diagnosis is critical to preventing congenital syphilis, or hard to reach populations such as indigenous communities, rural residents, migrants, and groups experiencing social or structural barriers to healthcare. Syphilis POC tests can provide rapid results that enable same-visit diagnosis and treatment; and so, POC tests can reduce loss to follow-up and substantially improve equity in syphilis control programs.
To address this diagnostic gap, newer dual POC platforms have been developed, such as the Dual Path Platform (DPP) Syphilis Screen and Confirm Assay. This test detects both treponemal and nontreponemal antibodies from plasma, serum, or whole-blood (including finger pick specimens) samples, providing a more comprehensive diagnostic tool that facilitates timely screening, diagnosis, and treatment in non-laboratory settings [6].
Simultaneously, research efforts have also focused on alternative sampling strategies to broaden the scope of syphilis surveillance. Dried blood spot (DBS) samples have shown promising performance as an alternative to serum or plasma in serological assays for Treponema pallidum (TP) diagnosis. DBS collection is minimally invasive, requires only a small blood volume, and can be performed with limited training. Moreover, DBS samples offer excellent stability, can be transported and stored at room temperature, and are easily shipped using standard postal services, making them especially valuable for hard-to-reach populations and resource-limited environments [7]. Several studies have validated the feasibility of using DBS samples for treponemal antibody detection, and recent investigations have assessed their compatibility with both reference laboratory assays and dual POC platforms [7,8].
Regarding the detection of nontreponemal antibodies with the RPR test, the presence of red or white blood cells may interfere with assay performance. When whole blood dries on the filter paper card, cellular fractions rupture, and their contents are released into DBS eluates, which will vary in their sensitivity to potential interference [9].
The global resurgence of syphilis, particularly among gay, bisexual, and other men who have sex with men (GBMSM), underscores the urgent need for diagnostic strategies that are not only highly sensitive and specific but also scalable, cost-effective, and adaptable to diverse healthcare infrastructures. The integration of innovative POC technologies with simplified sampling strategies such as DBS offers a promising solution to enhance diagnostic coverage, particularly in underserved populations, and represents a critical step toward achieving more equitable and effective global syphilis control.
The aim of this study was to evaluate the diagnostic performance of two routinely used laboratory-based serological assays and a dual point-of-care (POC) test for syphilis screening using dried blood spot (DBS) samples, with matched serum samples serving as the reference standard.
2. Materials and Methods
2.1. Study Design and Population
This retrospective study included 293 individuals, of whom 171 were classified as syphilis-positive and 122 as syphilis-negative based on serum results obtained using the routine serological assays employed in our laboratory for syphilis diagnosis (Liaison® Treponema Screen assay (Diasorin, Saluggia, Italy) and Macro-VueTM Reagin Plasma Rapid (RPR) card flocculation test (Beckton Dickinson, Franklin Lakes, NJ, USA). Remaining EDTA blood samples from each individual were collected and used to prepare dried blood spots. This study was approved by the local Research Ethics Committee (PI22-051) as a secondary objective of the TESTATE PrEP project, a randomized controlled trial to assess the feasibility and impact of online HIV/STI screening addressed to men who have sex with men and transgender women who are users of pre-exposure prophylaxis (PrEP) in Spain (number project: PI21/01589).
2.2. DBS Sample Collection
DBS samples were prepared by spotting 4 drops (50 μL) of whole-blood samples onto Whatman filter paper card (Whatman no.903, G&E Healthcare, Little Chalfont, Buckinghamshire, UK). The cards were air-dried overnight at room temperature and subsequently stored in a single, gas-impermeable zipper bag containing desiccant and a humidity indicator. This bag was stored at room temperature (20–25 °C) for up to 14 days according to the instructions reported by Grüner et al. [10]. After drying overnight, 12 mm diameter disks were punched from each DBS card and placed into individual Falcon tubes containing 500 μL of phosphate-buffered saline (PBS, pH 7.2). The tubes were incubated overnight at room temperature with gentle agitation. The resulting eluates were transferred into an Eppendorf microcentrifuge tubes and centrifuged for 2 min at 10,500× g. The supernatants were then subjected to syphilis serological testing to detect both treponemal and nontreponemal antibodies (Figure 1).
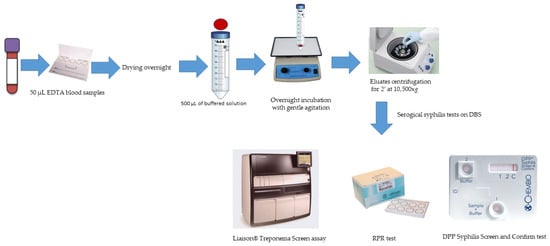
Figure 1.
Workflow for serological syphilis screening on DBS samples.
2.3. Serological Syphilis Screening on DBS
To assess the performance of conventional serological assays on DBS samples, we initially selected 69 syphilis-seropositive and 65 seronegative individuals. DBS eluates from these participants were tested using the Liaison® Treponema Screen assay (Diasorin, Saluggia, Italy) and Macro-VueTM Reagin Plasma Rapid (RPR) card flocculation test (Beckton Dickinson, Franklin Lakes, NJ, USA) for treponemal and nontreponemal antibodies, respectively. These tests were performed according to the manufacturer’s instructions.
Subsequently, a separate cohort comprising 102 syphilis-seropositive and 57 syphilis-seronegative individuals was selected to evaluate the performance of the POC test, Dual Path Platform (DPP) Syphilis Screen and Confirm test (Chembio Diagnostic Systems Inc., Medford, NY, USA). The DPP test is an immunochromatographic device containing a plastic cassette with two nitrocellulose membrane strips perpendicular to each other in a T formation, which allows for independent delivery of the test sample and detecting the conjugate reagent. For each test, 10 µL of DBS eluate was added to the first well, along with two drops of buffer solution. The diluted sample migrates toward the second strip, which has two test lines (a recombinant Treponema pallidum antigen and a nontreponemal antigen), alongside a control line. After 5 min, five drops of buffer were added to the second well to hydrate colloidal gold particles conjugated to protein A and anti-human IgM antibody. The conjugate migrates along the second strip to the test area, where the presence of total antibodies (IgM and IgG) against treponemal and nontreponemal antigens results in the formation of visible colored lines. Results were interpreted after 15 min either visually or using a microreader, which quantifies band intensity. All procedures were conducted following the manufacturer’s instructions.
2.4. Data Analyses
Serum-based results obtained from the Liaison® Treponema Screen Assay and the Rapid Plasma Reagin (RPR) test served as the reference standards for evaluating the performance of DBS-based assays. Receiver Operating Characteristic (ROC) curve analysis was conducted to determine the optimal cut-off of the Liaison® Treponema Screen and the DPP Syphilis Screen and Confirm assays to detect treponemal antibodies on DBS samples. The diagnostic performance of the DPP Syphilis Screen and Confirm test was assessed for both treponemal and nontreponemal antibody detection, using serum-based results from the Liaison® Treponema Screen Assay and the RPR test, respectively, as a reference. Concordance between the visual interpretation and microreader results was evaluated using Cohen’s kappa statistic. Additionally, we also assessed the diagnostic performance of the RPR test on DBS samples to detect nontreponemal antibodies by RPR titer. All analyses were performed using R version 4.3.2.
3. Results
3.1. Performance of Routine Syphilis Serological Assays in DBS Samples
All DBS samples from the 65 seronegative patients tested negative for both treponemal and nontreponemal antibodies. Among the 69 syphilis-seropositive patients, treponemal antibodies were detected initially on 67 DBS samples according to the Liaison® Treponema Screen assay cut-off (S/CO: 1.10). The sensitivity, specificity, PPV, and NPV were 95.6% (95% CI: 87.9–98.5), 100% (95% CI: 94.4–100), 100% (95% CI: 100–100), and 95.8% (95% CI: 91.6–100), respectively. ROC analysis revealed that the optimal cut-off was 0.395, yielding 100% sensitivity and specificity (95% CI: 100–100 for both) (Figure 2). For nontreponemal antibodies, only two DBS samples tested positive using the RPR assay. The sensitivity, specificity, PPV, and NPV of the RPR test were 2.9% (95% CI: 0.7–1.0), 100% (95% CI: 94.4–100), 100% (95% CI: 100–100), and 49.2% (95% CI: 40.4–58.1), respectively (Figure 3).
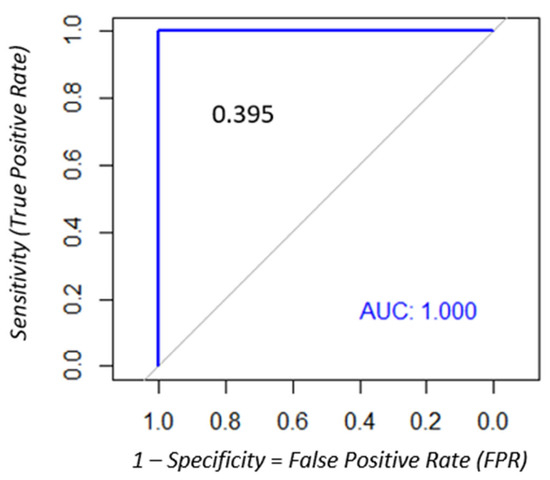
Figure 2.
ROC analysis to determine the optimal cut-off of the Liaison® Treponema Screen assay to identify a positive result on DBS samples.
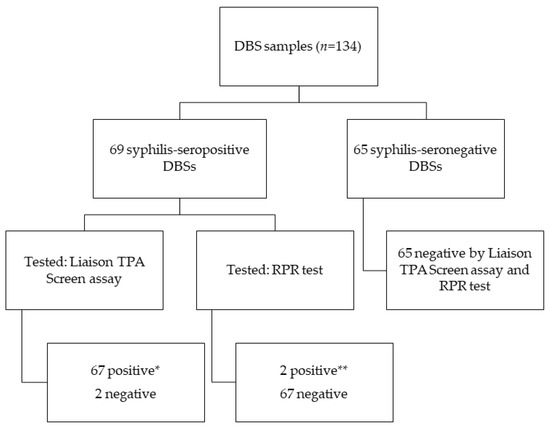
Figure 3.
Results obtained by the syphilis reference serological assays on DBS samples. * Results obtained according to the Liaison Treponema Screen assay cut-off (1.10) ** RPR titers of 1/32 and 1/16 from serum samples with RPR titers of 1/1024 and 1/4096, respectively.
3.2. Performance of the DPP Syphilis Screen and Confirm Test in DBS Samples
The diagnostic performance of the DPP Syphilis Screen and Confirm test using DBS samples was evaluated for both treponemal and nontreponemal antibody detection. Data are displayed in Figure 4. Sensitivity and specificity of the DPP to detect treponemal antibodies were 100% (95% CI: 96.3–100) and 17.5% (95% CI: 9.8–29), respectively, according to the microreader cut-off value of 9.0 that was recommended by the manufacturer. The ROC curve analysis identified that an optimal cut-off of this test to detect treponemal antibodies on DBS samples was 32.5, which improved performance, with sensitivity and specificity of 92.1% (95% CI: 89.3–99.9) and 98.2 (95% CI: 85.8–96.9), respectively (Figure 5, Table 1). Visual interpretation of the test yielded lower sensitivity, specificity, PPV, and NPV (Table 2). The discrepancy between visual and microreader readings was statistically significant (p < 0.05). Cohen’s kappa coefficient for treponemal antibody detection was 0.435 (95% CI: 0.41–0.60), indicating moderate agreement.
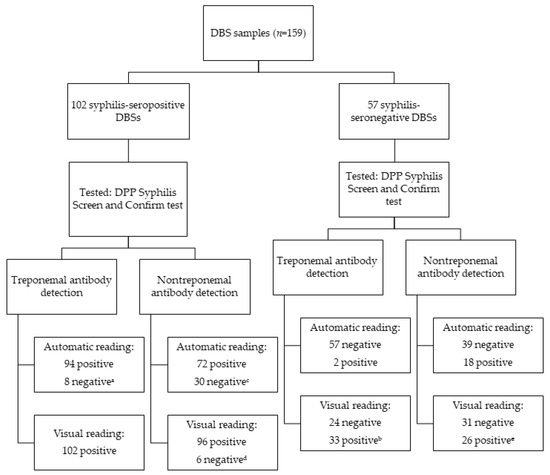
Figure 4.
Results of the DPP Syphilis Screen and Confirm test on DBS samples. a Eight DBS samples with results below 32.5; b thirty-three DBS samples showed a weak treponemal band, which were considered positive; c thirty DBS samples had values below 8.5, of which fourteen had serum RPR titers of 1:1; d six negative DBS samples, with serum RPR titers ranging from 1:2 to 1:8; e twenty-six samples showed a weak nontreponemal band, which were considered positive.
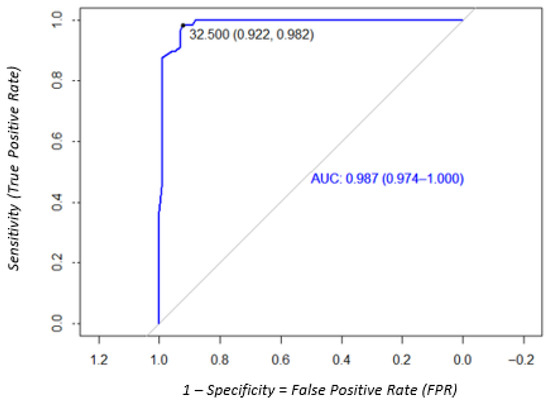
Figure 5.
ROC analysis to determine the optimal cut-off of the DPP Syphilis Screen and Confirm test to detect treponemal antibodies on DBS samples.

Table 1.
Performance of the DPP Syphilis Screen and Confirm test on DBS samples with visual and automatic reading (according to the cut-off obtained with ROC analysis).

Table 2.
Distribution of microreader nontreponemal mean values by RPR titer.
For the nontreponemal antibody detection, the sensitivity, specificity, PPV, and NPV were 70.5% (95% CI: 61.1–78.5), 68.4% (95% CI: 55.5–78.9), 69% (95% CI: 60.5–77.6), and 69.9% (95% CI: 62.4–77.4) using automatic readings. The ROC curve analysis showed that the optimal cut-off was 8.5, with the same values of sensitivity, specificity, PPV, and NPV (Figure 6, Table 1). For visual reading, the sensitivity, specificity, PPV, and NPV data are shown in Table 1. Agreement between visual and microreader readings for nontreponemal antibodies was also moderate, with a Cohen’s kappa of 0.432 (95% CI: 0.41–0.60).
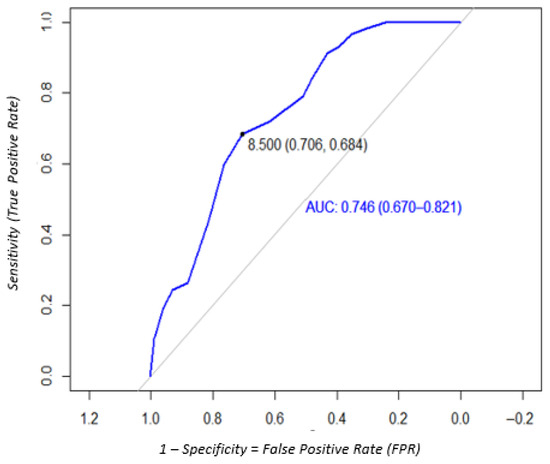
Figure 6.
ROC analysis to determine the optimal cut-off of the DPP Syphilis Screen and Confirm test to detect nontreponemal antibodies on DBS samples.
The sensitivity of nontreponemal antibody detection varied by RPR titer. The nontreponemal microreader median values increase when RPR titers are higher (Table 2). We found a sensitivity of 58.4% (95% CI: 50–70) and 92% (95% CI: 80–100) for RPR titers of ≤1/4 and ≥1/8, respectively. These findings suggest improved detection performance at higher RPR titers.
4. Discussion
In this study, we evaluated the performance of syphilis screening using DBS samples. Dried blood samples offer several operational advantages, including the requirement of only a small volume of blood, ease of collection with minimal training, simplified transport logistics, and stable storage conditions. Previous studies have demonstrated the utility of DBS samples for detection of HIV, HCV, HVB, and Treponema pallidum antibodies, especially in resource-limited settings and difficult-to-access populations for surveillance contexts [11,12,13]. Our findings contribute to this core of evidence by validating DBS-based serological assays for syphilis screening. Specifically, we observed high concordance between DBSs and serum for treponemal antibody detection using the chemiluminescence immunoassay (CLIA). After adjusting the assay to an optimized cut-off value, the sensitivity and specificity of the CLIA on DBS samples were comparable to those obtained with serum, supporting its use in research and potentially in clinical practice. These results align with studies that have reported high diagnostic accuracy of treponemal antibodies using DBS samples [8], reinforcing the feasibility of integrating DBS-based testing into syphilis screening programs aimed at improving access and equity.
Despite the high specificity (100%) of the RPR test on DBS samples, its sensitivity was markedly low (2.9%), rendering the data suboptimal for diagnosing active syphilis and monitoring treatment response. While the small sample volume of blood is advantageous, the final concentration of antibodies in DBS eluate was more diluted than with serum, which may contribute to reduced sensitivity—particularly in samples with low antibody titers. To our knowledge, there are no published studies evaluating the performance of the RPR test on DBS samples, limiting our ability to compare our findings with the existing literature. Given the critical role of nontreponemal testing in identifying active infection and guiding treatment decisions, the lack of robust data on DBS-based RPR testing highlights an important gap in syphilis diagnostics. Further research is needed to optimize DBS protocols for nontreponemal antibody detection. This includes evaluating elution procedures, adjusting sample volumes, and systematically assessing assay performance characteristics such as intra- and inter-assay variability, analytical accuracy, sample stability, and concordance with matched serum or plasma samples [9].
The DPP Syphilis Screen and Confirm test demonstrated excellent sensitivity for treponemal antibody detection using both visual and automated readings, showing strong agreement with the reference laboratory-based treponemal assay. These findings are consistent with previous studies that evaluated the DPP test in several samples such as serum, plasma, and whole-blood (including finger prick) samples [14,15].
However, the specificity varied widely between reading methods. Visual interpretation yielded a lower specificity (42.1%) compared to automated microreader analysis (98.2%). This discrepancy contrasts with the specificities reported by Vargas et al., who observed values of 85.9% and 83.2% using serum samples. The reduced specificity in visual readings may be attributed to the subjective interpretation of weak test lines, which can be challenging to classify consistently as positive or negative. In contrast, the microreader provides objective, quantitative measurements, minimizing reader variability and enhancing diagnostic accuracy—particularly in settings with limited training or experience. These results underscore the importance of standardized, automated interpretation methods to improve reliability and reduce false positives in syphilis screening programs using DBS samples.
Regarding nontreponemal antibody detection, we observed that the sensitivity increased among individuals with high levels of RPR titers in their serum. More false negative results occurred in DBS samples from patients with serum RPR titers ≤ 1:2, suggesting that the DPP Syphilis Screen and Confirm test may be more effective in identifying active syphilis cases, which typically present with higher titers. However, early or late latent syphilis cases often exhibit low antibody levels and may not be reliably detected using DBS-based nontreponemal testing. Therefore, individuals with clinical suspicion of syphilis but negative DPP results should be retested after 1–2 weeks or referred for conventional serological screening. These data are consistent with findings reported by Causer et al. using serum samples [3].
This study has several limitations. The retrospective design and reliance on residual EDTA blood samples may introduce selection bias. Additionally, the sample size for nontreponemal testing on DBS was relatively small, limiting the generalizability of our results. The study population consisted exclusively of HIV-positive individuals and those receiving PrEP, representing a high-risk group for syphilis. Future studies should evaluate the performance of DBS-based syphilis serological assays in lower-risk populations, such as pregnant women or the general population.
In 2007, The World Health Organization (WHO) launched the Initiative for the Global Elimination of Mother to Child Transmission (MTCT) of both HIV and Syphilis, recognizing the value of the syphilis POC testing for universal screening of pregnant women [16]. The combination of DBS samples with a dual POC test like the DPP Syphilis Screen and Confirm could significantly enhance access to syphilis screening and treatment in remote areas with limited diagnostic infrastructure, contributing to improved maternal and neonatal health outcomes.
5. Conclusions
DBS samples represent a reliable alternative to serum for syphilis screening, applicable to both laboratory-based assays and POC tests. While further improvement is needed for nontreponemal testing, DBS testing has significant potential to expand access to syphilis diagnostics, particularly in resource-constrained settings. The combination of DBS samples with POC tests like the DPP Syphilis Screen and Confirm could play a crucial role in decentralizing and scaling up syphilis screening efforts on a global scale.
Author Contributions
C.A., V.G.S., G.F.-R., P.J.C.I., J.C.B. and H.M.R. designed the study. V.G.S., G.F.-R., P.P.A. and H.M.R. carried out sample collection and processing. V.G.S., G.F.-R. and P.P.A. conducted and performed laboratory assays. Y.D.R., M.M.F. and M.S.M. analyzed the data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors contributed to and reviewed the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved on 27 May 2022 by the Germans Trias i Pujol Research Ethics Committee (PI22-051) as a secondary objective of the TESTATE PrEP project, a randomized controlled trial to assess the feasibility and impact of online HIV/STI screening addressed to men who have sex with men and transgender women users of pre-exposure prophylaxis (PrEP) in Spain; described elsewhere [17]. The protocol has been registered at www.clinicaltrials.gov; identifier number: NCT05752643. Protocol version 1, 2 March 2023.
Informed Consent Statement
The analyses were carried out exclusively on remnant samples that were originally obtained for routine clinical purposes. Therefore, in accordance with the Ethics Committee’s approval, informed consent was waived for this sub-study.
Data Availability Statement
All data relevant to the study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| POC | Point-of-care test |
| DPP | Dual Path Platform |
References
- World Health Organization. Report on Global Sexually Transmitted Infection Surveillance; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- European Centre for Disease Prevention and Control. Syphilis—Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Causer, L.M.; Kaldor, J.M.; Fairley, C.K.; Donovan, B.; Karapanagiotidis, T.; Leslie, D.E.; Robertson, P.W.; McNulty, A.M.; Anderson, D.; Wand, H.; et al. A laboratory-based evaluation of four rapid point-of-care tests for syphilis. PLoS ONE 2014, 9, e91504. [Google Scholar] [CrossRef] [PubMed]
- Constantine, N.T.; Sill, A.M.; Paulus, E.G.S.; Saleh, A. Assessment of Two Rapid Assays for Diagnostic Capability to Accurately Identify Infection by Treponema pallidum. J. Appl. Lab. Med. 2017, 1, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Mabey, D.C. The introduction of syphilis point of care tests in resource limited settings. Expert Rev. Mol. Diagn. 2017, 17, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.R.; Esfandiari, J.; Kumar, S.; Ashton, M.; Kikkert, S.E.; Park, M.M.; Ballard, R.C. Novel point-of-care test for simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. J. Clin. Microbiol. 2010, 48, 4615–4619. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ren, Y.; He, L.; He, X.; Xing, W.; Jiang, Y. An efficient method for simultaneously screening for HIV, syphilis, and HCV based on one dried blood spot sample. Antivir. Res. 2020, 181, 104775. [Google Scholar] [CrossRef] [PubMed]
- van Loo, I.H.M.; Dukers-Muijrers, N.H.T.M.; Heuts, R.; van der Sande, M.A.B.; Hoebe, C.J.P.A. Screening for HIV, hepatitis B and syphilis on dried blood spots: A promising method to better reach hidden high-risk populations with self-collected sampling. PLoS ONE 2017, 12, e0186722. [Google Scholar] [CrossRef] [PubMed]
- McDade, T.W. Development and validation of assay protocols for use with dried blood spot samples. Am. J. Hum. Biol. 2014, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Grüner, N.; Stambouli, O.; Ross, R.S. Dried blood spots—Preparing and processing for use in immunoassays and in molecular techniques. J. Vis. Exp. 2015, 13, 52619. [Google Scholar] [CrossRef]
- Not, A.; Saludes, V.; Gálvez, M.; Miralpeix, A.; Bordoy, A.E.; González, N.; González-Gómez, S.; Muntané, L.; Reyes-Urueña, J.; Majó, X.; et al. Usefulness of dried blood spot samples for monitoring hepatitis C treatment outcome and reinfection among people who inject drugs in a test-and-treat program. J. Med. Virol. 2023, 95, e28544. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Raimondo, A.; Pénaranda, G.; Camus, C.; Ouzan, D.; Ravet, S.; Bourlière, M.; Khiri, H.; Dukan, P.; Olive, D.; et al. Dried blood spot sampling for hepatitis B virus serology and molecular testing. PLoS ONE 2013, 8, e61077. [Google Scholar] [CrossRef] [PubMed]
- Vojnov, L.; Carmona, S.; Zeh, C.; Markby, J.; Boeras, D.; Prescott, M.R.; Mayne, A.L.H.; Sawadogo, S.; Adje-Toure, C.; Zhang, G.; et al. The performance of using dried blood spot specimens for HIV-1 viral load testing: A systematic review and meta-analysis. PLoS Med. 2022, 19, e1004076. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.K.; Qquellon, J.; Vasquez, F.; Konda, K.A.; Calvo, G.; Reyes-Diaz, M.; Caceres, C.; Klausner, J.D. Laboratory Evaluation of the DPP Syphilis Screen & Confirm Assay. Microbiol. Spectr. 2022, 10, e0264221. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-P.; Chen, X.-S.; Wei, W.-H.; Gong, K.-L.; Cao, W.-L.; Yong, G.; Feng, L.; Huang, S.-J.; Wang, D.-M.; Han, Y.; et al. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: A multisite evaluation study in China. Clin. Infect. Dis. 2013, 56, 659–665. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. The Global Elimination of Congenital Syphilis: Rationale and Strategy for Action. 2007. Available online: https://www.who.int/publications/i/item/the-global-elimination-of-congenital-syphilis-rationale-and-strategy-for-action (accessed on 2 October 2025).
- Agustí, C.; Riveros, H.M.; García-Pérez, J.; Descalzo, V.; Fernandez, G.; Ramírez-Marinero, A.; Gonzalez, M.V.; Díaz, Y.; Montoro-Fernandez, M.; Gea, P.R.-D.; et al. Feasibility and impact of online HIV/STI screening addressed to men who have sex with men and transgender women users of pre-exposure prophylaxis (PrEP) in Spain (TESTATE PrEP): A study protocol for a non-blinded randomised controlled trial. BMJ Open 2023, 16, e073459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).