1. Introduction
Gerstmann syndrome is a rare neurocognitive disorder defined by a specific set of four deficits: agraphia (or dysgraphia), acalculia, finger agnosia, and right–left disorientation [
1,
2]. Josef Gerstmann first described the syndrome in the early 20th century, and clinicians traditionally associate it with lesions in the dominant inferior parietal lobule, especially the left angular gyrus [
3,
4]. Cognitive impairments are believed to result from the disruption of multimodal association areas that are engaged in language, arithmetic processing, body schema, and spatial orientation [
5].
Gerstmann syndrome, while traditionally linked to focal lesions like ischaemic strokes, has also been documented in instances of brain tumours, traumatic brain injury, demyelinating diseases, and epileptic activity impacting the parietal cortex [
6,
7,
8,
9]. A developmental variant of the syndrome has been identified in children exhibiting learning disabilities and structural anomalies in the parietal lobe [
10].
In adults, Gerstmann syndrome typically arises from vascular events, especially infarctions affecting the parietal lobe. The parieto-occipital region, located at the convergence of the parietal and occipital lobes, plays a crucial role in the integration of sensory perception, visuospatial awareness, and symbolic processing [
11]. Damage to this region can result in complex neurocognitive syndromes that exceed typical stroke manifestations. Emerging evidence indicates that Gerstmann syndrome represents a disconnection syndrome, resulting from disruption of white matter tracts connecting the angular gyrus with adjacent parietal and frontal regions, rather than stemming from an isolated cortical lesion [
12,
13]. The syndrome is rare, with a reported prevalence of 0.04–0.3% among stroke patients, predominantly those with involvement of the dominant parietal lobe [
14,
15].
Recent studies indicate an increasing incidence of stroke among younger and middle-aged individuals, linked to modifiable risk factors including sedentary lifestyle, poor diet, hypertension, and psychological stress [
16]. The patient in this report exceeds the conventional “young stroke” threshold of 50 years; however, his case exemplifies the increasing incidence of lifestyle-related cerebrovascular disease across a wider age demographic.
This case report describes an atypical presentation of Gerstmann syndrome associated with bilateral parietal infarctions. This study illustrates how lesions impacting both hemispheres can mimic or amplify the classical tetrad initially described by Gerstmann, while also emphasising the evolving comprehension of the syndrome’s neuroanatomical foundation. A comprehensive evaluation of this case could assist clinicians in identifying subtle cognitive deficits after parietal strokes and encourage more extensive neuropsychological evaluations in stroke patients.
2. Case Report
A 52-year-old right-handed male engineer with no significant prior medical history presented to the Accident and Emergency (A&E) department after his general practitioner referred him due to new-onset palpitations. On presentation, his blood pressure was markedly elevated at 228/147 mmHg, and electrocardiogram confirmed fast atrial fibrillation (AF). Laboratory investigations revealed mild hypokalaemia (serum potassium 3.2 mmol/L); blood count, renal and liver function, and inflammatory markers were normal.
The cardiology team reviewed the patient and started him on bisoprolol, bendroflumethiazide, and apixaban, with plans for outpatient transthoracic echocardiogram and elective direct current cardioversion (DCCV). He was discharged within 24 h with safety-netting advice.
Four hours post-discharge, the patient’s family observed acute onset of confusion, agitation, repetitive speech (“yes/no”), and autonomic symptoms such as sweating and clamminess. He was urgently readmitted. On re-examination, his blood pressure was 260/140 mmHg with a heart rate of 130 bpm. Neurological examination showed disorientation, impaired command following, expressive and receptive dysphasia, and right-sided hemineglect, but no focal motor deficits. The initial NIH Stroke Scale (NIHSS) score was 8. Due to increasing agitation, lorazepam was administered and intravenous antihypertensive therapy initiated using labetalol and glyceryl trinitrate (GTN) infusion.
Non-contrast computed tomography (CT) of the head demonstrated an evolving acute infarct in the right posterior parieto-occipital region with poorly defined hypodensity, white matter oedema, and mild mass effect (
Figure 1). Apixaban was immediately withheld, and aspirin was commenced after coagulation screening. MRI brain was requested to rule out a space-occupying lesion.
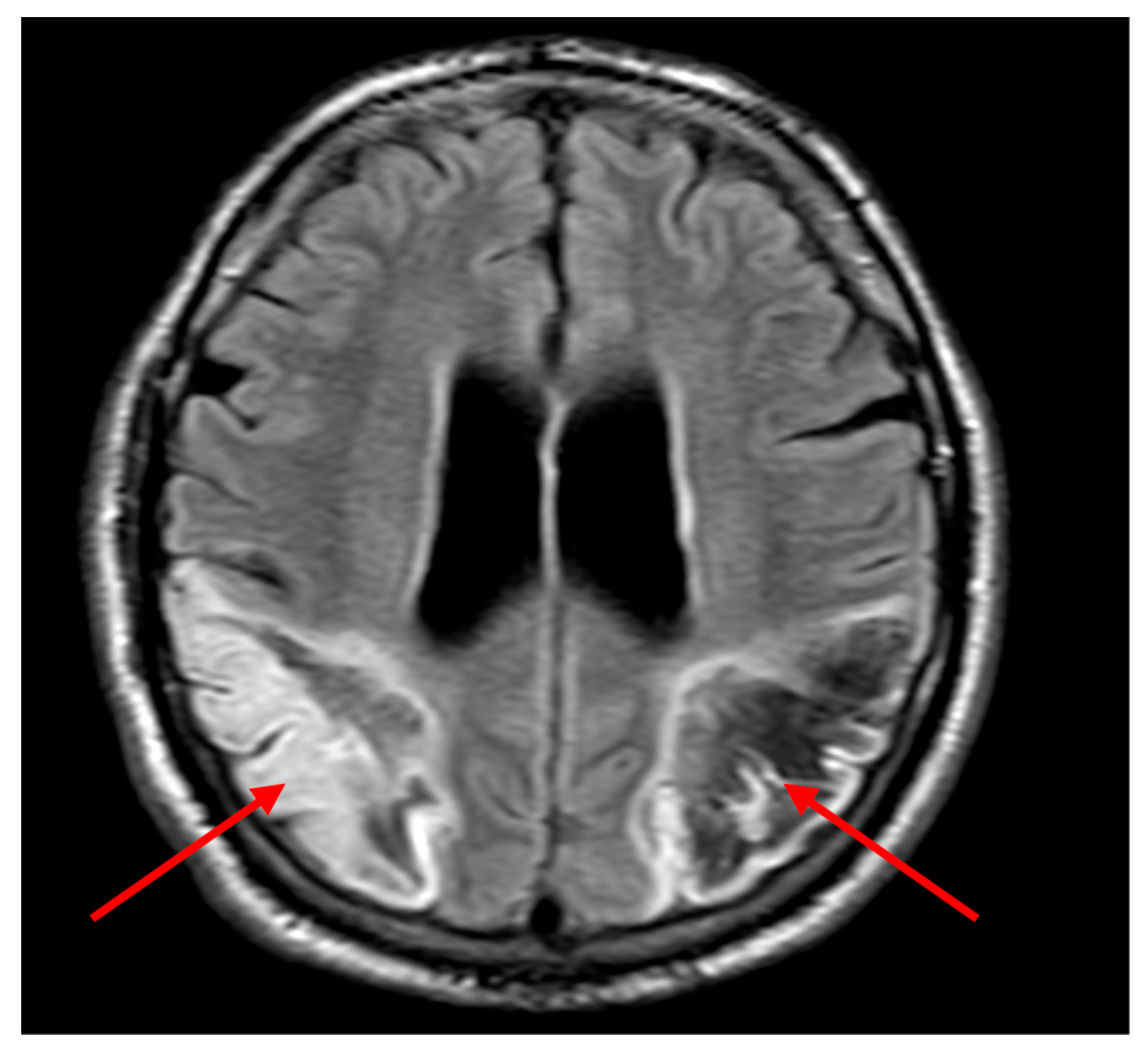
On day 2, further neurological signs evolved including cortical visual loss, persistent dysphasia, and neurocognitive impairment. By day 3, the patient demonstrated visual inattention, inability to follow two-step commands, and absent visual threat on the left. Diffusion-weighted MRI revealed a large acute infarct in the left parietal lobe and a subacute infarct in the right parietal region (
Figure 2a). Fast field echo sequences indicated haemorrhagic transformation of the left-sided infarct (
Figure 2b). Aspirin was discontinued, with plans for repeat imaging to guide anticoagulation restart.
The medical team transferred the patient to the stroke rehabilitation unit on day 5. Repeat CT showed stable findings, and apixaban was re-initiated. During his rehabilitation admission, clinicians observed persistent visual and cognitive deficits. He was unable to localise objects in his visual field, misidentified finger positions, and demonstrated right–left disorientation.
Two weeks into rehabilitation, a neurology review identified classical features of Gerstmann syndrome—acalculia, finger agnosia, right–left disorientation, and alexia without agraphia. The patient was unable to perform basic arithmetic (e.g., 2 + 2), could write but not read simple words, and showed preserved colour perception. His Mini-Addenbrooke’s Cognitive Examination (M-ACE) score was 13/30, consistent with moderate cognitive impairment. Orthoptic evaluation confirmed he has right homonymous hemianopia. These findings were consistent with dominant (left) parietal lobe involvement. To further characterise his cognitive profile, a structured bedside neuropsychological assessment was undertaken by the multidisciplinary team. For acalculia, the patient was asked to solve simple arithmetic problems (e.g., 2 + 2, 5 − 3), all of which he answered incorrectly despite preserved verbal comprehension. Finger agnosia was assessed using a finger identification task on both hands; the patient could correctly name only 3 out of 10 fingers. Right–left disorientation was demonstrated during a command-following task that required the patient to touch his left ear with his right hand, which he repeatedly failed. For writing, he was able to copy and write single words with support, indicating partial preservation of graphomotor skills. Reading tasks however revealed marked alexia, with the patient unable to read printed or handwritten words. These findings were consistent with classical Gerstmann syndrome, alongside alexia and visual neglect.
The patient completed a total of 56 days in the stroke rehabilitation unit, receiving multidisciplinary input from speech and language therapy and occupational therapy supported neuropsychology. While mild improvements in orientation and speech were observed, key features of Gerstmann syndrome persisted. He was discharged home with community-based rehabilitation and referred to a specialised neurocognitive rehabilitation facility. Outpatient cardiology follow-up led to the addition of digoxin for further rate control, with ongoing monitoring.
Follow-up contrast-enhanced MRI confirmed stable bilateral parietal infarcts with no evidence of new lesions (
Figure 3).
3. Discussion
This case illustrates the complex clinical and neuroanatomical interplay underpinning bilateral parieto-occipital infarcts and their association with Gerstmann syndrome (GS), a rare and multifaceted neuropsychological disorder traditionally linked to lesions in the dominant inferior parietal lobule. Bilateral involvement of parieto-occipital regions remains uncommon, typically resulting from embolic events, hypoxic–ischemic injury, or systemic causes [
17]. In this patient, a cardioembolic stroke secondary to atrial fibrillation was the presumed aetiology, highlighting the critical role of anticoagulation therapy in preventing recurrent embolic cerebrovascular events [
17]. Bilateral parietal lobe infarcts presenting with Gerstmann syndrome are exceptionally rare. Notably, a large clinical series of 1000 consecutive patients with acute cerebrovascular disease did not report any cases of bilateral parietal involvement manifesting as Gerstmann syndrome [
18]. This underscores the unique and educational value of the current case in expanding our understanding of atypical stroke presentations.
Notably, this patient—aged 52—reflects an increasing incidence of stroke among younger adults, a trend attributed to both traditional vascular risk factors and emerging lifestyle influences. Early recognition and preventive strategies, including lifestyle modification and public health initiatives, are essential to mitigate this growing burden [
17].
Clinically, the patient presented with an atypical and rapidly evolving neurological syndrome. Initial confusion and agitation, coupled with impaired command following, likely delayed recognition of the cerebrovascular insult. Neuroimaging subsequently revealed bilateral parieto-occipital infarctions, accounting for the constellation of cognitive, sensory, and visual disturbances observed. Imaging revealed infarcts predominantly involving the left angular gyrus (Brodmann area 39) and adjacent inferior parietal lobule, regions classically associated with Gerstmann syndrome. The right parieto-occipital lesion affected the non-dominant hemisphere’s homologous areas, contributing to spatial neglect and impaired visual awareness. On diffusion-weighted MRI (
Figure 2a), the infarcted regions aligned with cortical areas subserving symbolic representation and spatial mapping. Notably, the haemorrhagic transformation (
Figure 2b) likely limited early re-initiation of anticoagulation, underscoring the clinical utility of sequential imaging. These anatomical correlates correspond well to the observed deficits and support a network-based model of cognitive disruption.
The classical GS tetrad—finger agnosia, agraphia, acalculia, and right-left disorientation—was fully manifested alongside additional deficits including alexia without agraphia, homonymous hemianopia, and persistent visuospatial impairments. This combination underscores the extensive involvement of not only the dominant parietal cortex but also adjacent occipital regions, emphasising the integrated nature of multimodal cognitive processing within this cortical network [
16,
19]. Beyond cortical damage, the bilateral parietal infarcts likely disrupted key white matter association tracts critical for multimodal integration. The left-sided lesion may have interrupted the superior longitudinal fasciculus (SLF), which connects parietal areas to the frontal cortex and is implicated in arithmetic processing and spatial attention. Damage to the inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFOF) may have contributed to the patient’s alexia by impairing visual word form processing. The right hemisphere lesion, although subacute, may have compounded deficits through interhemispheric disconnection, particularly involving the splenium of the corpus callosum. These patterns support recent theories conceptualising Gerstmann syndrome as a disconnection syndrome rather than a focal cortical phenomenon [
12,
20].
Recent theoretical and imaging studies provide further support for this network-based perspective. Rusconi et al. (2009) proposed a “disconnection account” of GS, showing that lesions interrupting communication between distinct parietal-frontal-occipital subnetworks can produce the classical tetrad even without a single focal lesion [
21]. More recently, Ranzini et al. (2023) used diffusion MRI and tractography to demonstrate that disruption of long association bundles—particularly segments of the SLF and IFOF—correlates with the co-occurrence of acalculia, agraphia, finger agnosia, and right–left disorientation [
13]. These observations align with broader reviews emphasising GS as a distributed network disorder rather than a focal lesion syndrome [
12].
The initial discrepancy between impaired writing ability and the later diagnosis of alexia without agraphia may reflect evolving cognitive recovery or the complex interplay of overlapping deficits. Early in the course, expressive and receptive dysphasia likely contributed to writing difficulties, while subsequent improvement in motor coordination and language output revealed relatively preserved agraphia. Meanwhile, persistent visual word form deficits were consistent with alexia. This evolution suggests the presence of a mixed or partial variant, possibly related to bilateral cortical and subcortical involvement. Such heterogeneity is well-documented in the literature and reinforces the need for formal, repeated cognitive assessments over time to delineate true dissociations.
Anatomically, GS is conventionally attributed to lesions of the dominant angular gyrus, integral to numerical cognition and writing processes [
22,
23]. Yet, emerging evidence suggests that GS should be conceptualised as a disconnection syndrome resulting from interruption of association fibre tracts linking the parietal cortex with frontal and occipital areas [
12,
20]. Functional imaging and cortical stimulation studies have identified discrete cortical regions responsible for individual GS components, supporting limited anatomical overlap and implicating white matter pathways in the syndrome’s pathogenesis [
5,
20,
24]. This network-based view accounts for the diverse symptomatology and atypical presentations seen in bilateral or non-angular lesions, as observed in this case.
The early identification of GS facilitated a tailored, multidisciplinary rehabilitation approach encompassing cognitive, motor, and speech therapies, essential for addressing the complex symptom constellation. Neuroimaging played a pivotal role not only in confirming the diagnosis but also in guiding treatment decisions, particularly anticoagulation management in the context of haemorrhagic transformation.
Despite therapeutic efforts, the patient demonstrated persistent visuospatial deficits and residual features of GS at discharge, consistent with the syndrome’s often chronic and debilitating nature. This underscores the need for ongoing cognitive assessment and long-term rehabilitation in similar patients.
From a research perspective, this case highlights the importance of advancing our understanding of GS within the framework of large-scale brain networks rather than isolated cortical loci. Future studies employing functional MRI and diffusion tensor imaging hold promise in elucidating the precise neural mechanisms underlying GS, particularly in bilateral and atypical presentations. Furthermore, systematic investigation into the heterogeneity of alexia and agraphia manifestations within GS is warranted to refine diagnostic criteria and therapeutic approaches. This case report pertains to a single patient, which limits the generalizability of the findings. Continued documentation and analysis of similar cases will be necessary to deepen our understanding of the spectrum and neuroanatomical correlates of Gerstmann syndrome following bilateral parietal infarcts.
In summary, Gerstmann syndrome remains a challenging diagnostic entity characterised by diverse clinical and neuroanatomical profiles. Integrating comprehensive neuropsychological assessment with advanced neuroimaging enhances diagnostic accuracy and informs rehabilitation, ultimately improving patient outcomes in this rare but impactful syndrome.