The Invisible Threat That Leaves You Breathless—A Literature Review on Pneumothorax in the Emergency Department
Abstract
:1. Introduction
2. Pathogenesis
2.1. Primary Spontaneous Pneumothorax
2.1.1. Structural Lung Changes
2.1.2. Environmental Factors
2.1.3. Genetic Predisposition
2.2. Secondary Spontaneous Pneumothorax
2.3. Traumatic Pneumothorax
2.3.1. Penetrating Chest Trauma
2.3.2. Blunt Chest Trauma
- Sudden Lung Compression: High-impact forces, such as those from motor vehicle collisions or falls, may compress the lung tissue, leading to alveolar rupture and subsequent air leakage into the pleural space [19].
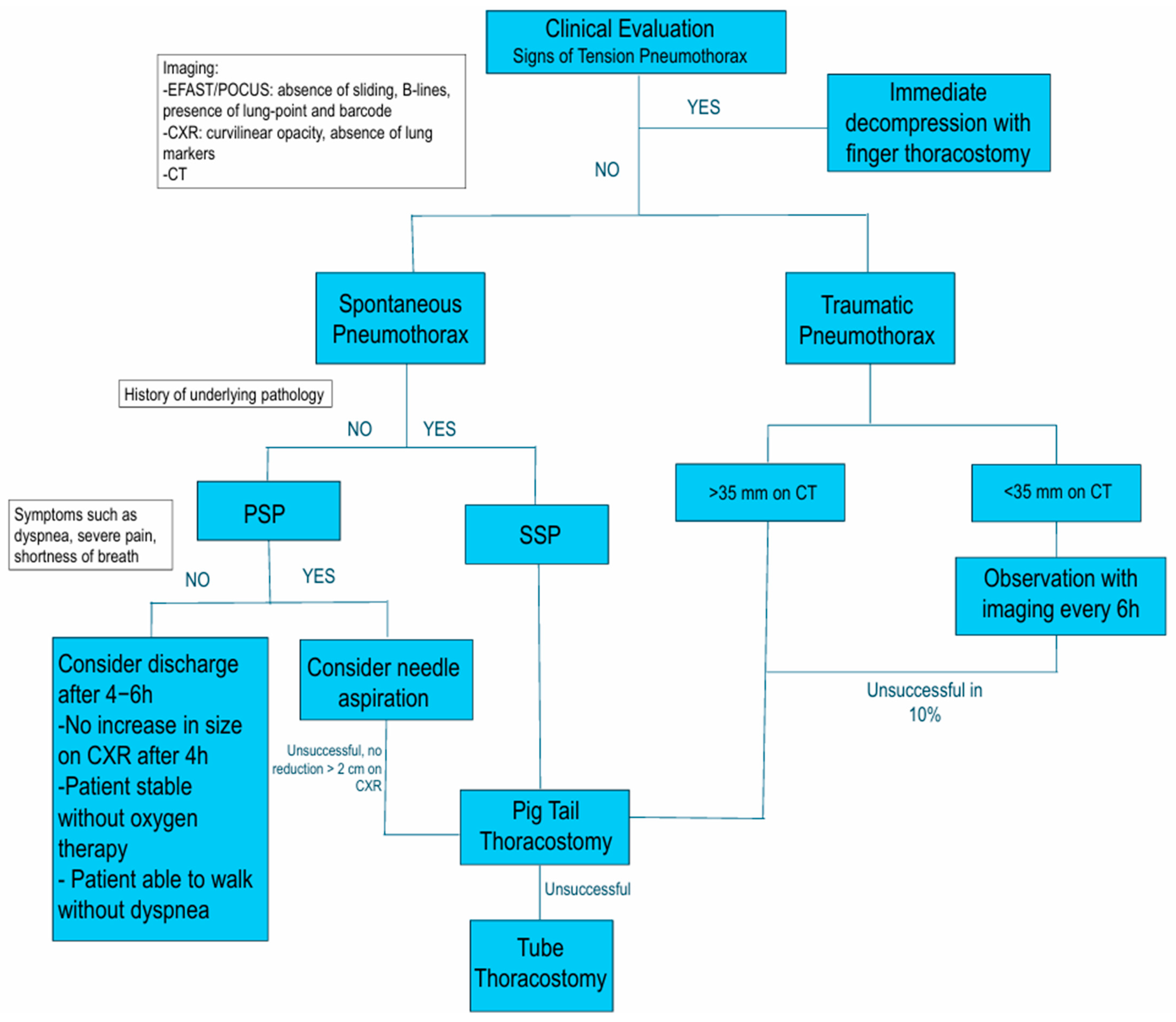
3. Diagnosis
3.1. Clinical Presentation
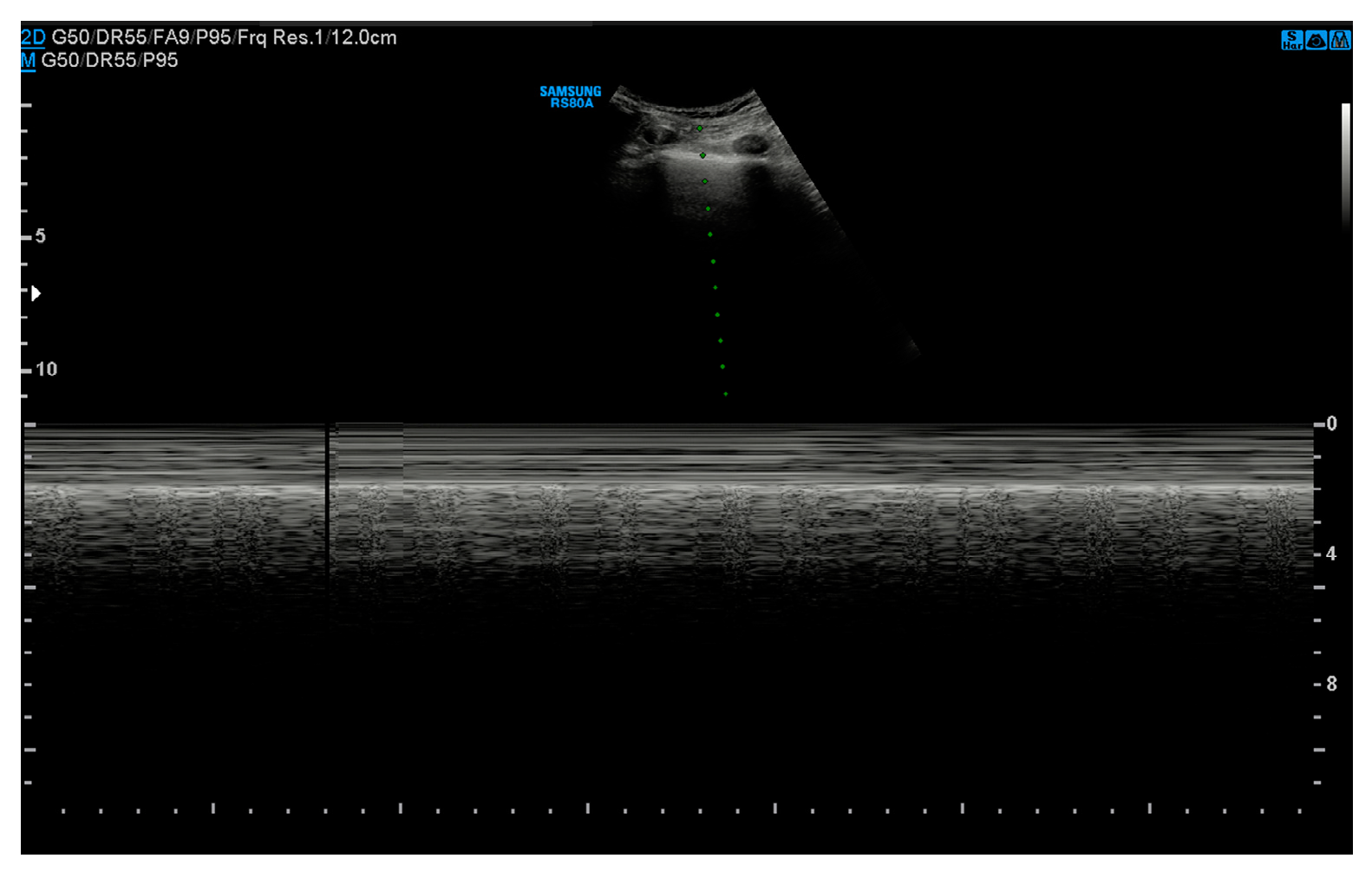
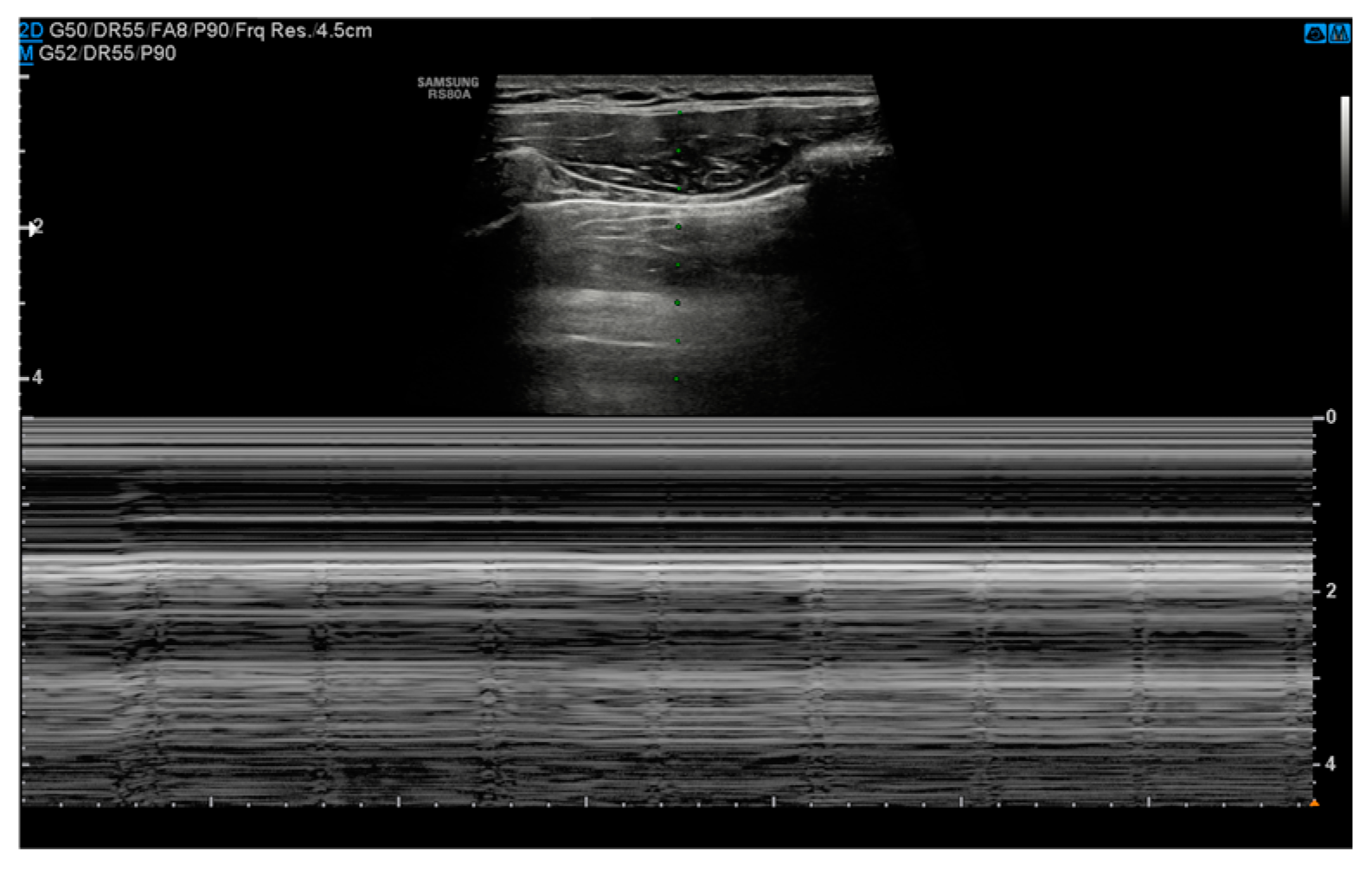
3.2. Ultrasound
- Absence of pleural sliding: Normally, the visceral pleura glides against the parietal pleura with respiration. In pneumothorax, this movement is absent;
- Absence of B-lines: Comet-tail artifacts (B-lines) are reverberation anomalies that disappear with the presence of air in the pleural space;
- Lung point sign: The point where normal lung sliding meets the absent sliding of the pneumothorax; this is highly specific for pneumothoraces;
- Barcode or stratosphere sign: A static, uniform appearance on M-mode ultrasound indicating loss of lung movement (Figure 3).
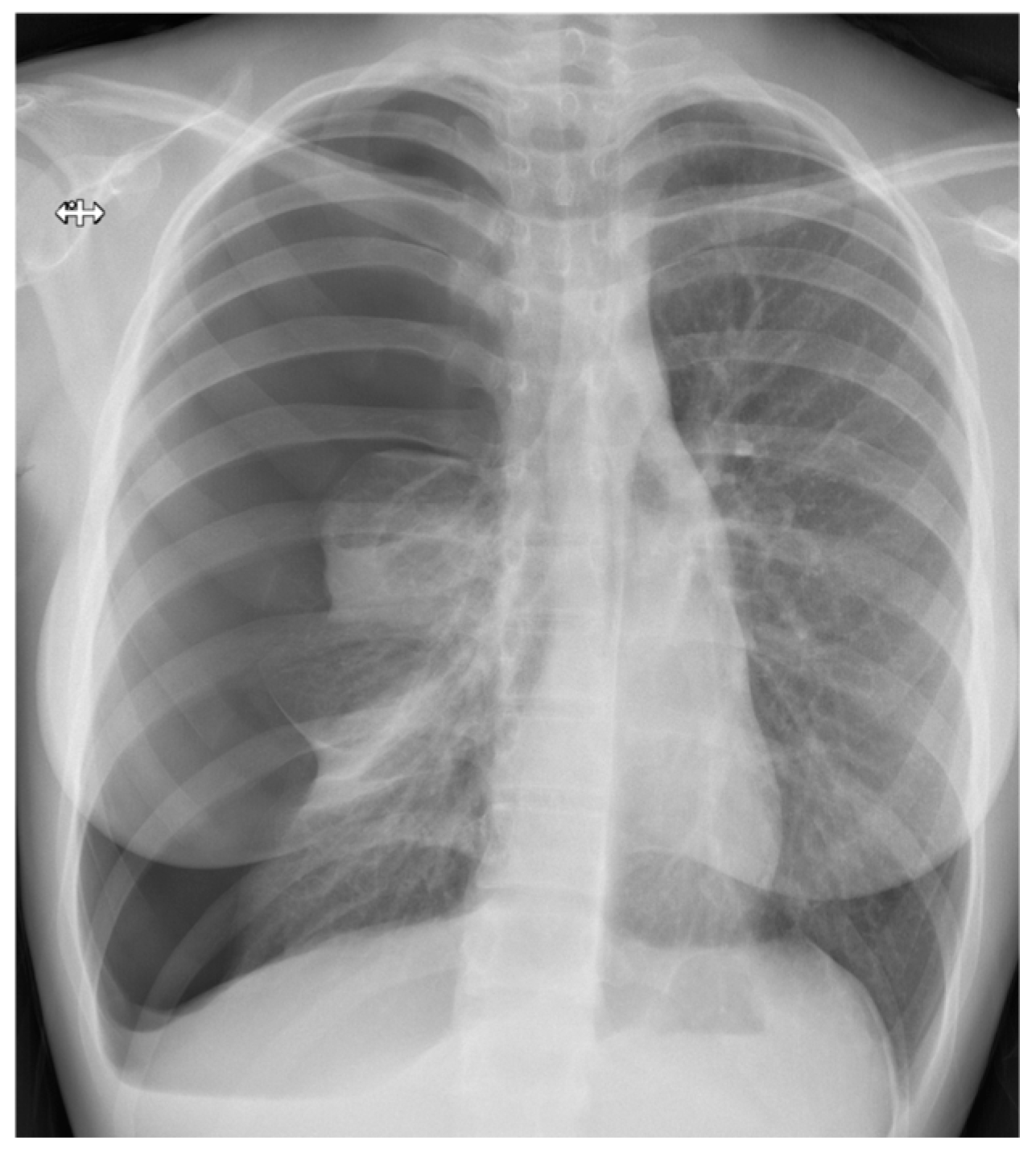
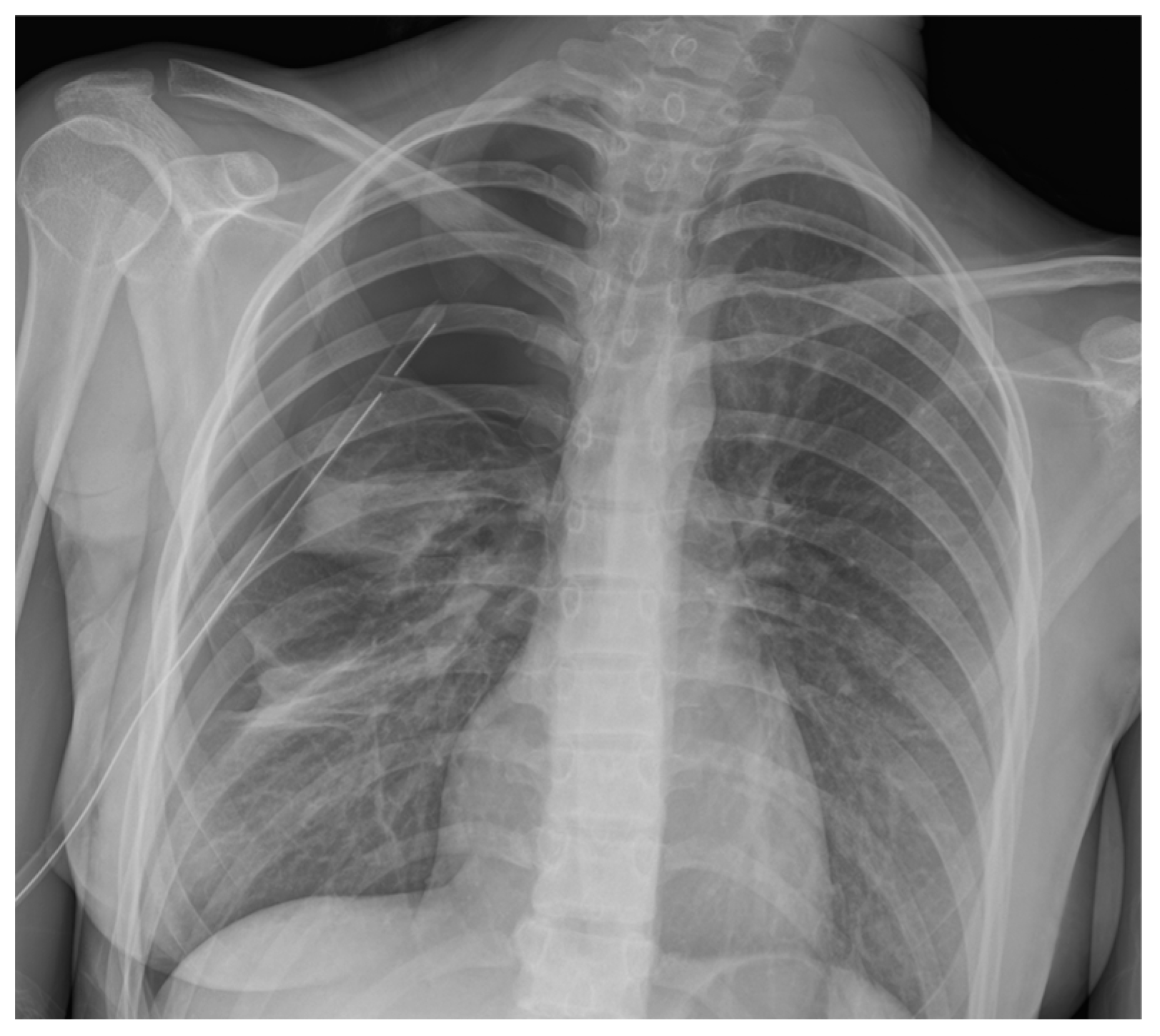
3.3. Chest Radiography
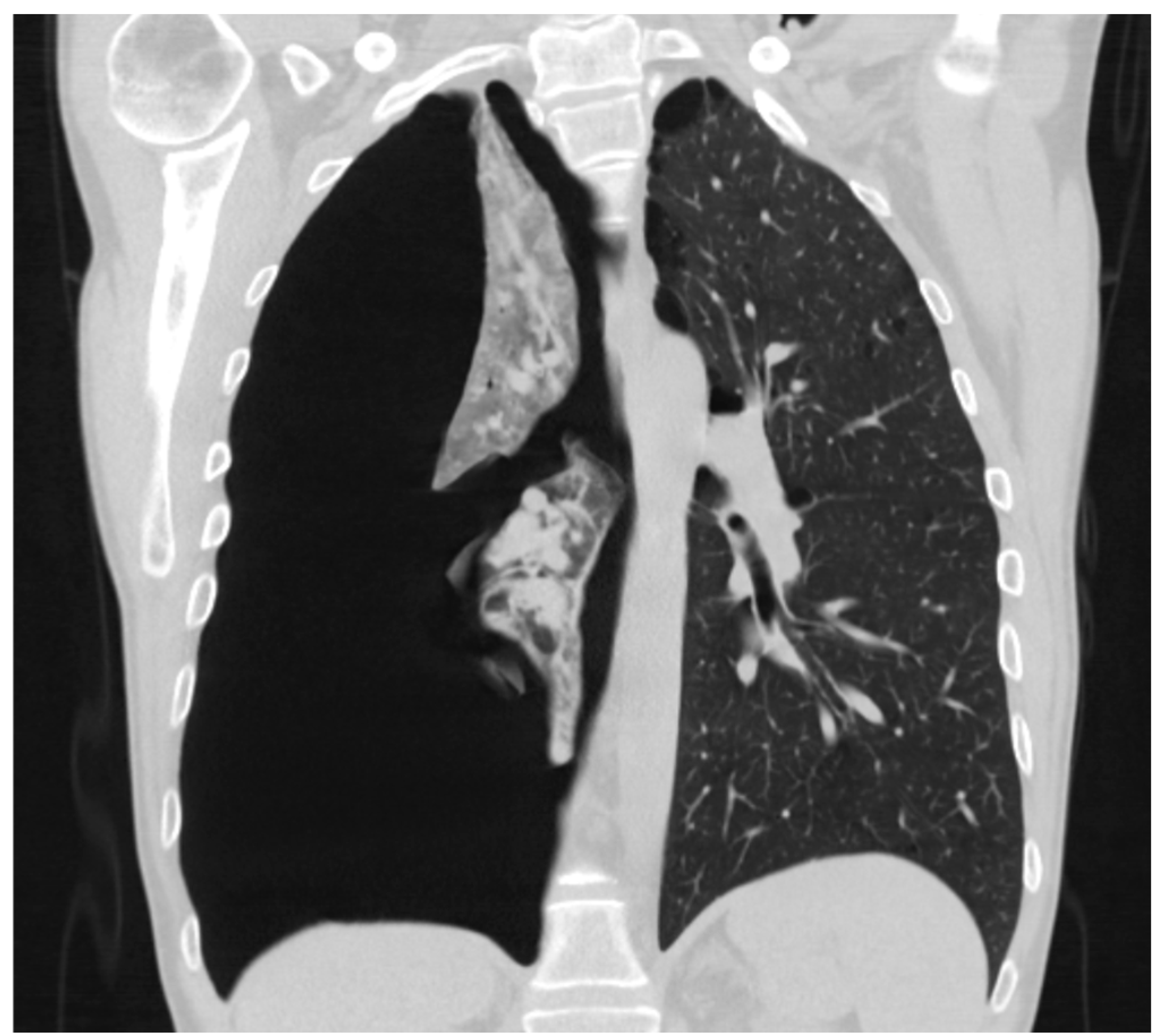
3.4. Computed Tomography Scan
3.5. Size of Pneumothorax
4. Treatment
4.1. Needle Decompression and Aspiration
4.2. Chest Drainage
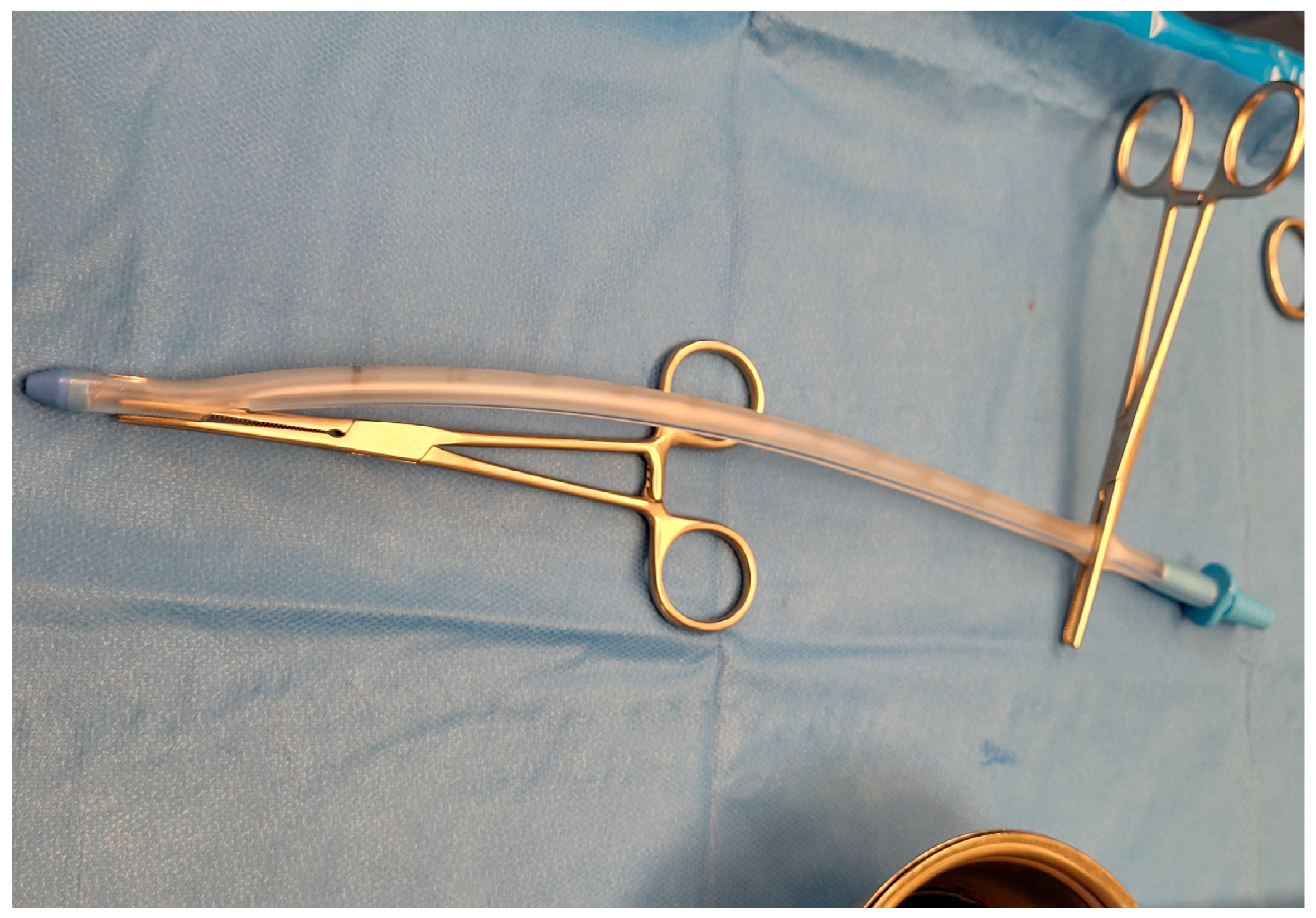
4.2.1. Insertion and Removal Techniques
- 1.
- Assemble the supplies and prepare the underwater seal collection device;
- 2.
- Place the patient in a Fowler position, with the upper body tilted around 30–45°, and, when feasible, the ipsilateral patient’s arm should be extended over the head and flexed at the elbow;
- 3.
- Prepare the sterile field and locate the 4th–5th intercostal space between the anterior and midaxillary lines for the insertion site. If there is no time to locate precisely the correct intercostal space or if the patient’s habitus prevents that, remember to place the tube inside the “triangle of safety”, delimited by the following:
- a.
- Inferiorly: the mammary fold in women or the inter-nipple line in men;
- b.
- Superiorly: the base of the axilla;
- c.
- Anteriorly: the lateral–posterior border of the pectoralis major muscle;
- d.
- Posteriorly: lateral–anterior border of the latissimus dorsi muscle.
- 4.
- Administer local anesthesia to the site, including the skin, subcutaneous tissue, rib periosteum, and parietal pleura. When deep, identify the pleural space aspirating air in the syringe;
- 5.
- Incise the skin 2–3 cm parallel to the ribs and blunt-dissect the subcutaneous tissue just above the superior margin of the inferior rib to avoid the neurovascular intercostal bundle;
- 6.
- Pierce the parietal pleura with the tip of the clamp, advance the clamp over the rib, and spread it to enlarge the pleural opening. Insert a sterile gloved finger into the breach and perform a 360° sweep to clear all the possible adhesions and confirm the correct location;
- 7.
- Secure the distal end of the tube with a clamp, and clamp the proximal end to use the instrument as a guide to advance the drain into the pleural space. Advance the tube aiming for the supraclavicular fossa, removing the proximal clamp once the tube is inside the pleural cavity (Figure 7). Signs such as fogging of the chest tube and the presence of drainage material may indicate the correct position;
- 8.
- Remove the distal clamp and connect the tube to the seal apparatus. Secure the tube with a non-absorbable suture using the shoelace technique. Apply dressing.
- 1.
- Assemble the supplies and the drainage system;
- 2.
- Identify the site of insertion based on diagnostic imaging and the location of the pneumothorax: 4–5th intercostal space between the anterior and midaxillary lines or 2nd–3rd intercostal space in the mid-clavicular line. If possible, the patient should be positioned with a 45–50° torso elevation, with the ipsilateral arm flexed and extended over the head. Mark the insertion site with a pen;
- 3.
- Administer local anesthesia as previously described;
- 4.
- Insert the introducer needle attached to a 5 mL syringe, aspirating while moving through the tissue on the superior margin of the inferior rib. Aspiration of air confirms the appropriate location for catheter insertion and ensures accurate access to the targeted space;
- 5.
- Insert the guidewire through the needle, advance it into the chest cavity, and then remove the introducer needle;
- 6.
- Make a small incision in the skin to enlarge the breach. To create an adequate pathway for the catheter insertion, a dilator may be used over the guidewire to widen the path;
- 7.
- Introduce the pigtail catheter into the pleural space by advancing it over the guidewire, and ensure all the holes are within the space;
- 8.
- Withdraw the trocar and the guidewire. As the catheter is released, its coiled or “pigtail” shape forms, securing it within the pleural cavity and reducing the risk of dislodgement;
- 9.
- Connect the catheter to the drainage system, secure the drain with sutures, and apply a dressing.
4.2.2. Complications of Chest Drainage
4.2.3. Role of Negative Pressure in Chest Drainage
4.3. Surgical Management
- 1.
- During forced expiration;
- 2.
- Expiration only;
- 3.
- Inspiration only;
- 4.
- Continuous bubbling.
4.4. Conservative Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSP | Primary Spontaneous Pneumothorax |
| SSP | Secondary Spontaneous Pneumothorax |
| COPD | Chronic Obstructive Pulmonary Disease |
| ELC | Emphysema-like Change |
| MMP | Matrix Metalloproteinase |
| US | Ultrasound |
| E-FAST | Extended Focused Assessment with Sonography for Trauma |
| POCUS | Point-of-care Ultrasound |
| CXR | Chest X-ray |
| ATLS | Advanced Trauma Life Support |
| PA | Posteroanterior |
| LL | Laterolateral |
| AP | Anteroposterior |
| CT | Computed Tomography Scan |
| REPE | Re-expansion Pulmonary Edema |
| TAE | Transarterial Embolization |
| VATS | Video-assisted Thoracic Surgery |
| PAL | Persistent Air Leak |
| LOS | Length of Stay |
References
- Mendogni, P.; Vannucci, J.; Ghisalberti, M.; Anile, M.; Aramini, B.; Congedo, M.T.; Nosotti, M.; Bertolaccini, L. Epidemiology and management of primary spontaneous pneumothorax: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sahn, S.A.; Heffner, J.E. Spontaneous Pneumothorax. N. Engl. J. Med. 2000, 342, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Epidemiology of pneumothorax in England. Thorax 2000, 55, 666–671. [Google Scholar] [CrossRef]
- Noppen, M.; De Keukeleire, T. Pneumothora. Respiration 2008, 76, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, G.; Gheda, S.; Altomare, M.; Cioffi, S.P.B.; Ferrazzi, D.; Cazzaniga, M.; Bonacchini, L.; Cimbanassi, S.; Aseni, P. Successful Needle Aspiration of a Traumatic Pneumothorax: A Case Report and Literature Review. Medicina 2024, 60, 548. [Google Scholar] [CrossRef]
- Fattori, S.; Reitano, E.; Chiara, O.; Cimbanassi, S. Predictive Factors of Ventilatory Support in Chest Trauma. Life 2021, 11, 1154. [Google Scholar] [CrossRef]
- Baumann, M.H.; Strange, C.; Heffner, J.E.; Light, R.; Kirby, T.J.; Klein, J.; Luketich, J.D.; Panacek, E.A.; Sahn, S.A.; ACCP Pneumothorax Consensus Group. Management of Spontaneous Pneumothorax: An American College of Chest Physicians Delphi consensus statement. Chest 2021, 119, 590–602. [Google Scholar] [CrossRef]
- MacDuff, A.; Arnold, A.; Harvey, J.; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65 (Suppl. S2), ii18–ii31. [Google Scholar] [CrossRef]
- Ohata, M.; Suzuki, H. Pathogenesis of Spontaneous Pneumothorax. Chest 1980, 77, 771–776. [Google Scholar] [CrossRef]
- Noppen, M.; Dekeukeleire, T.; Hanon, S.; Stratakos, G.; Amjadi, K.; Madsen, P.; Meysman, M.; D’Haese, J.; Vincken, W. Fluorescein-enhanced Autofluorescence Thoracoscopy in Patients with Primary Spontaneous Pneumothorax and Normal Subjects. Am. J. Respir. Crit. Care Med. 2006, 174, 26–30. [Google Scholar] [CrossRef]
- Huan, N.-C.; Sidhu, C.; Thomas, R. Pneumothorax. Clin. Chest Med. 2021, 42, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Seyama, K.; McCormack, F.X. Pulmonary manifestations of Birt-Hogg-Dubé syndrome. Fam. Cancer 2013, 12, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.B.; Nolasco, M.; Peterlin, B.; Garcia, C.K. Nonsense Mutations in Folliculin Presenting as Isolated Familial Spontaneous Pneumothorax in Adults. Am. J. Respir. Crit. Care Med. 2005, 172, 39–44. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, C.; Rodriguez, R.M.; Light, R.W. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005, 10, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Freixinet, J.L.; Caminero, J.A.; Marchena, J.; Rodríguez, P.M.; Casimiro, J.A.; Hussein, M. Spontaneous pneumothorax and tuberculosis: Long-term follow-up. Eur. Respir. J. 2011, 38, 126–131. [Google Scholar] [CrossRef]
- Iqbal, B.; Hallifax, R. Pneumothorax. Medicine 2023, 51, 888–892. [Google Scholar] [CrossRef]
- Tran, J.; Haussner, W.; Shah, K. Traumatic Pneumothorax: A Review of Current Diagnostic Practices And Evolving Management. J. Emerg. Med. 2021, 61, 517–528. [Google Scholar] [CrossRef]
- Liman, S. Chest injury due to blunt trauma. Eur. J. Cardiothorac. Surg. 2003, 23, 374–378. [Google Scholar] [CrossRef]
- Moore, E.E.; Feliciano, D.V.; Mattox, K.L. Trauma, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Sharma, A.; Jindal, P. Principles of diagnosis and management of traumatic pneumothorax. J. Emerg. Trauma Shock 2008, 1, 34. [Google Scholar] [CrossRef]
- Barton, E.D.; Rhee, P.; Hutton, K.C.; Rosen, P. The pathophysiology of tension pneumothorax in ventilated swine. J. Emerg. Med. 1997, 15, 147–153. [Google Scholar] [CrossRef]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef]
- Gottlieb, M.; Long, B. Managing Spontaneous Pneumothorax. Ann. Emerg. Med. 2023, 81, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Joo, D.A.; McRae, A.D.; Takwoingi, Y.; Premji, Z.A.; Lang, E.; Wakai, A. Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. Cochrane Database Syst. Rev. 2020, 2020, CD013031. [Google Scholar] [CrossRef]
- ATLS—Advanced Trauma Life Support, 10th ed.; American College of Surgeons: Chicago, IL, USA, 2018.
- Aswin, K.; Balamurugan, S.; Govindarajalou, R.; Saya, G.K.; Elamurugan, T.P.; Rajendran, G.; Murugan, B.; Elamurugan, T. Comparing Sensitivity and Specificity of Ultrasonography With Chest Radiography in Detecting Pneumothorax and Hemothorax in Chest Trauma Patients: A Cross-Sectional Diagnostic Study. Cureus 2023, 15, e44456. [Google Scholar] [CrossRef]
- Salama, K.M.; Elshaboury, I.M.; Huissen, W.M.; Eldomiaty, H.A.; Elghoboshy, K.I. Role of bedside sonography in the assessment of patients with chest trauma in the emergency department of Suez Canal University Hospital. Int. Surg. J. 2017, 4, 465. [Google Scholar] [CrossRef]
- Silva, D.; Schuch; Dalcin, P.D.T.R. Posteroanterior chest X-ray for the diagnosis of pneumothorax: Methods, usage, and resolution. Rep. Med. Imaging 2010, 3, 29–34. [Google Scholar] [CrossRef]
- Polireddy, K.; Hoff, C.; Kinger, N.P.; Tran, A.; Maddu, K. Blunt thoracic trauma: Role of chest radiography and comparison with CT—Findings and literature review. Emerg. Radiol. 2022, 29, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Almajid, F.M.; Aljehani, Y.M.; Alabkary, S.; Alsaif, H.S. The accuracy of computed tomography in detecting surgically resectable blebs or bullae in primary spontaneous pneumothorax. Radiol. Med. 2019, 124, 833–837. [Google Scholar] [CrossRef]
- Tschopp, J.-M.; Bintcliffe, O.; Astoul, P.; Canalis, E.; Driesen, P.; Janssen, J.; Krasnik, M.; Maskell, N.; Van Schil, P.; Tonia, T.; et al. ERS task force statement: Diagnosis and treatment of primary spontaneous pneumothorax. Eur. Respir. J. 2015, 46, 321–335. [Google Scholar] [CrossRef]
- Tsou, K.-C.; Huang, P.-M.; Hsu, H.-H.; Chen, K.-C.; Kuo, S.-W.; Lee, J.-M.; Chang, Y.-C.; Chen, J.-S.; Lai, H.-S. Role of computed tomographic scanning prior to thoracoscopic surgery for primary spontaneous pneumothorax. J. Formos. Med. Assoc. 2014, 113, 606–611. [Google Scholar] [CrossRef]
- Cropano, C.; Mesar, T.; Turay, D.; King, D.; Yeh, D.; Fagenholz, P.; Velmahos, G.; de Moya, M.A. Pneumothoraces on Computed Tomography Scan: Observation using the 35 Millimeter Rule is Safe. Panam. J. Trauma Crit. Care Emerg. Surg. 2015, 4, 48–53. [Google Scholar] [CrossRef]
- Eddine, S.B.Z.; Boyle, K.A.; Dodgion, C.M.; Davis, C.S.; Webb, T.P.; Juern, J.S.; Milia, D.J.; Carver, T.W.; Beckman, M.A.; Codner, P.A.; et al. Observing pneumothoraces: The 35-millimeter rule is safe for both blunt and penetrating chest trauma. J. Trauma Acute Care Surg. 2019, 86, 557–564. [Google Scholar] [CrossRef]
- De Moya, M.; Brasel, K.J.; Brown, C.V.; Hartwell, J.L.; Inaba, K.; Ley, E.J.; Moore, E.E.; Peck, K.A.; Rizzo, A.G.; Rosen, N.G.; et al. Evaluation and management of traumatic pneumothorax: A Western Trauma Association critical decisions algorithm. J. Trauma Acute Care Surg. 2022, 92, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Hallifax, R.; Ricciardi, S.; Fitzgerald, D.; Keijzers, M.; Lauk, O.; Petersen, J.; Bertolaccini, L.; Bodtger, U.; Clive, A.; et al. Joint ERS/EACTS/ESTS clinical practice guidelines on adults with spontaneous pneumothorax. Eur. Respir. J. 2024, 63, 2300797. [Google Scholar] [CrossRef]
- Inaba, K.; Lustenberger, T.; Recinos, G.; Georgiou, C.; Velmahos, G.C.; Brown, C.; Salim, A.; Demetriades, D.; Rhee, P. Does size matter? A prospective analysis of 28–32 versus 36–40 French chest tube size in trauma. J. Trauma Acute Care Surg 2012, 72, 422–427. [Google Scholar] [CrossRef]
- Tanizaki, S.; Maeda, S.; Sera, M.; Nagai, H.; Hayashi, M.; Azuma, H.; Kano, K.-I.; Watanabe, H.; Ishida, H. Small tube thoracostomy (20–22 Fr) in emergent management of chest trauma. Injury 2017, 48, 1884–1887. [Google Scholar] [CrossRef]
- Kulvatunyou, N.; Erickson, L.; Vijayasekaran, A.; Gries, L.; Joseph, B.; Friese, R.F.; O’Keeffe, T.; Tang, A.L.; Wynne, J.L.; Rhee, P. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. J. Br. Surg. 2014, 101, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sorino, C.; Feller-Kopman, D.; Mei, F.; Mondoni, M.; Agati, S.; Marchetti, G.; Rahman, N.M. Chest Tubes and Pleural Drainage: History and Current Status in Pleural Disease Management. J. Clin. Med. 2024, 13, 6331. [Google Scholar] [CrossRef]
- Ukeh, I.; Fang, A.; Patel, S.; Opoku, K.; Nezami, N. Percutaneous Chest Tube for Pleural Effusion and Pneumothorax. Semin. Interv. Radiol. 2022, 39, 234–247. [Google Scholar] [CrossRef]
- Lewis, M.R.; Georgoff, P. Minimally invasive management of thoracic trauma: Current evidence and guidelines. Trauma Surg. Acute Care Open 2024, 9 (Suppl. S2), e001372. [Google Scholar] [CrossRef]
- Becker, J.C.; Zakaluzny, S.A.; Keller, B.A.; Galante, J.M.; Utter, G.H. Clamping trials prior to thoracostomy tube removal and the need for subsequent invasive pleural drainage. Am. J. Surg. 2020, 220, 476–481. [Google Scholar] [CrossRef]
- Asciak, R.; Bedawi, E.O.; Bhatnagar, R.; Clive, A.O.; Hassan, M.; Lloyd, H.; Reddy, R.; Roberts, H.; Rahman, N.M. British Thoracic Society Clinical Statement on pleural procedures. Thorax 2023, 78 (Suppl. S3), s43–s68. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, A.; Gupta, S.; Priyadarshini, P.; Kumar, A.; Alam, J.; Bagaria, D.; Choudhary, N.; Sagar, S.; Gupta, A.; Mishra, B.; et al. Role of low-pressure negative pleural suction in patients with thoracic trauma—a randomized controlled trial. Eur. J. Trauma Emerg. Surg. 2024, 50, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Choudhary, I.S.; Dutt, A.; Banerjee, N.; Chauhan, A.S.; Rodha, M.S.; Sharma, N.; Puranik, A.K.; Chauhan, N.K.; Gupta, M.K.; et al. Efficacy of slow negative pleural suction in thoracic trauma patients undergoing tube thoracostomy—A randomised clinical trial. Injury 2025, 56, 111928. [Google Scholar] [CrossRef] [PubMed]
- Dugan, K.C.; Laxmanan, B.; Murgu, S.; Hogarth, D.K. Management of Persistent Air Leaks. Chest 2017, 152, 417–423. [Google Scholar] [CrossRef]
- Cerfolio, R.J. Advances in thoracostomy tube management. Surg. Clin. N. Am. 2002, 82, 833–848. [Google Scholar] [CrossRef]
- Banks, K.C.; Mooney, C.M.; Mazzolini, K.; Browder, T.D.; Victorino, G.P. Comparison of outcomes between observation and tube thoracostomy for small traumatic pneumothoraces. Am. J. Emerg. Med. 2023, 66, 36–39. [Google Scholar] [CrossRef]
- Sum, S.-K.; Peng, Y.-C.; Yin, S.-Y.; Huang, P.-F.; Wang, Y.-C.; Chen, T.-P.; Tung, H.-H.; Yeh, C.-H. Using an incentive spirometer reduces pulmonary complications in patients with traumatic rib fractures: A randomized controlled trial. Trials 2019, 20, 797. [Google Scholar] [CrossRef]
- Namwaing, P.; Ngamjarus, C.; Sakaew, W.; Sawunyavisuth, B.; Sawanyawisuth, K.; Khamsai, S.; Srichaphan, T. Chest physical therapy and outcomes in primary spontaneous pneumothorax: A systematic review. J. Med. Assoc. Thail. 2021, 104, S165–S168. [Google Scholar] [CrossRef]
- Park, C.B.; Moon, M.H.; Jeon, H.W.; Cho, D.G.; Song, S.W.; Won, Y.D.; Kim, Y.H.; Kim, Y.-D.; Jeong, S.C.; Kim, K.S.; et al. Does oxygen therapy increase the resolution rate of primary spontaneous pneumothorax? J. Thorac. Dis. 2017, 9, 5239–5243. [Google Scholar] [CrossRef]
| Ultrasound Signs | |
|---|---|
| Normal Lung | Pneumothorax |
| Lung sliding | Absence of lung sliding |
| Lung pulse | Stratosphere sign on M-mode |
| B-lines | Lung point |
| Seashore sign on M-mode | Visible air in pleural cavity |
| Type of Pneumothorax | Clinical Features | Diagnostic Criteria | Therapeutic Indications | Possible Complications |
|---|---|---|---|---|
| Primary Spontaneous | Young, healthy patients in absence of clinically apparent lung disease | POCUS, Chest X-ray, CT in complicated PSP | Observation in minimally symptomatic; oxygen therapy, aspiration, and small-bore chest tube if symptomatic | Recurrence, infection, PAL |
| Secondary Spontaneous | Patients over 55 y.o. with underlying lung disease (e.g., COPD) | POCUS, Chest X-ray, CT often required | Small-bore chest tube; if unsuccessful, consider a larger caliber | PAL, recurrence, infection |
| Traumatic | Penetrating or blunt chest trauma | E-FAST, Chest X-ray, CT | Observation if <35 mm on CT, chest tube if >35 mm. Treatment of the concomitant injuries | Tension pneumothorax, infection, pain |
| Tension | Hemodynamic instability, acute dyspnea, tracheal deviation | Clinical diagnosis | Emergency decompression with finger thoracostomy | Cardiac arrest, death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattori, S.; Bellio, G.; Cimino, M.M.; Kurihara, H. The Invisible Threat That Leaves You Breathless—A Literature Review on Pneumothorax in the Emergency Department. Emerg. Care Med. 2025, 2, 24. https://doi.org/10.3390/ecm2020024
Fattori S, Bellio G, Cimino MM, Kurihara H. The Invisible Threat That Leaves You Breathless—A Literature Review on Pneumothorax in the Emergency Department. Emergency Care and Medicine. 2025; 2(2):24. https://doi.org/10.3390/ecm2020024
Chicago/Turabian StyleFattori, Silvia, Gabriele Bellio, Matteo Maria Cimino, and Hayato Kurihara. 2025. "The Invisible Threat That Leaves You Breathless—A Literature Review on Pneumothorax in the Emergency Department" Emergency Care and Medicine 2, no. 2: 24. https://doi.org/10.3390/ecm2020024
APA StyleFattori, S., Bellio, G., Cimino, M. M., & Kurihara, H. (2025). The Invisible Threat That Leaves You Breathless—A Literature Review on Pneumothorax in the Emergency Department. Emergency Care and Medicine, 2(2), 24. https://doi.org/10.3390/ecm2020024






