Establishing and Validating a Predictive Model for the Risk of In-Hospital Mortality Post-Resuscitation in Patients with Sudden Death, as Well as Conducting Clinical Analysis Research: A Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Inclusion/Exclusion Criteria
- (1)
- Inclusion criteria: This study includes the medical records of patients who experienced out-of-hospital cardiac arrest as diagnosed by pre-hospital emergency services. Eligible participants are those who were successfully resuscitated and subsequently admitted to the hospital for further treatment, as well as individuals with a documented history of underlying medical conditions, such as hypertension, diabetes, and coronary artery disease.
- (2)
- Exclusion criteria: patients with identifiable causes such as trauma, pregnancy, drowning, and similar conditions, as well as those with an unclear medical history, significant deficiencies in research data, and discrepancies or conflicts between the patient and the medical team, will be excluded.
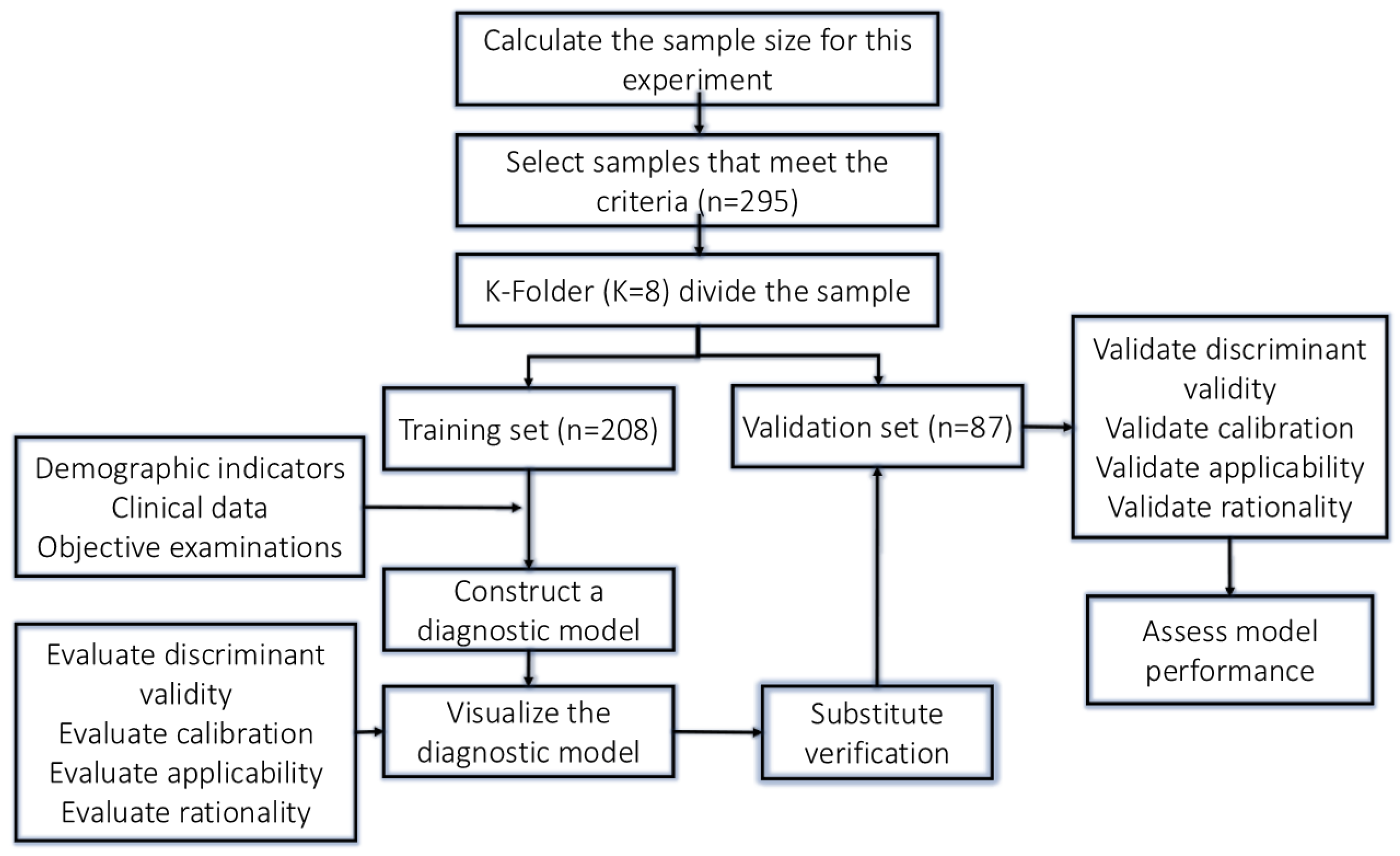
2.2. Sample Size Calculation
2.3. Research Sample Grouping
2.4. Data Collection
2.5. Selection of Independent Variables
2.6. Statistical Analysis
3. Results
3.1. Comparison of Patient Characteristics and Baseline Data
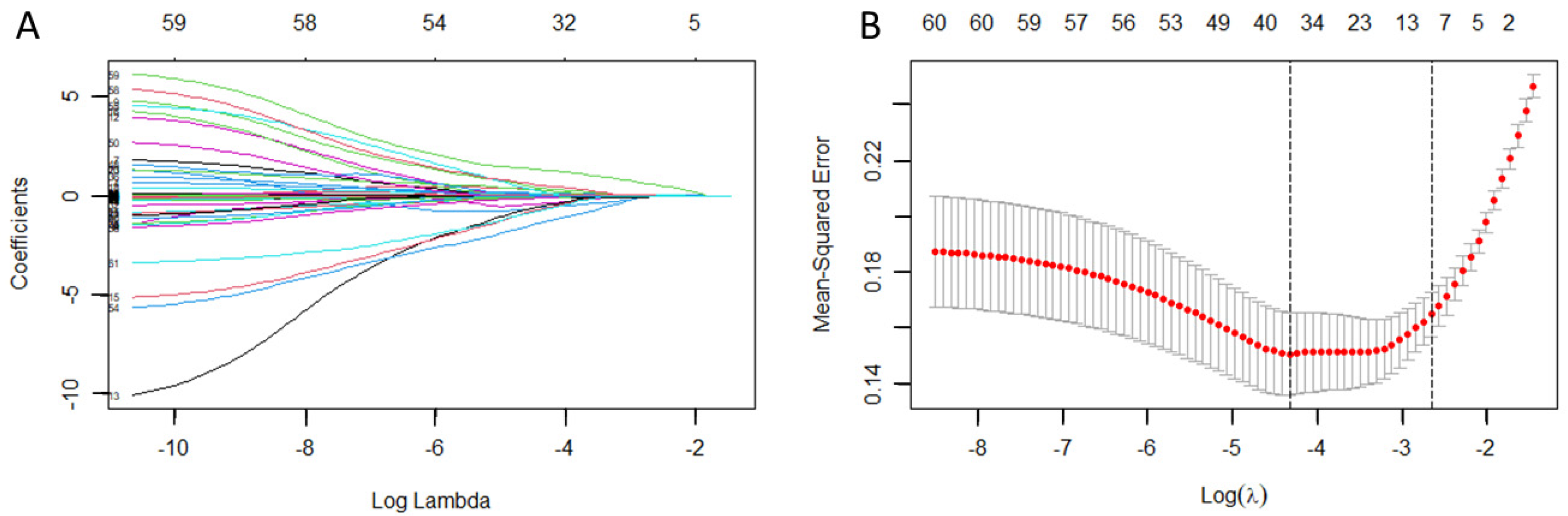
3.2. Identification of Risk Prediction Factors
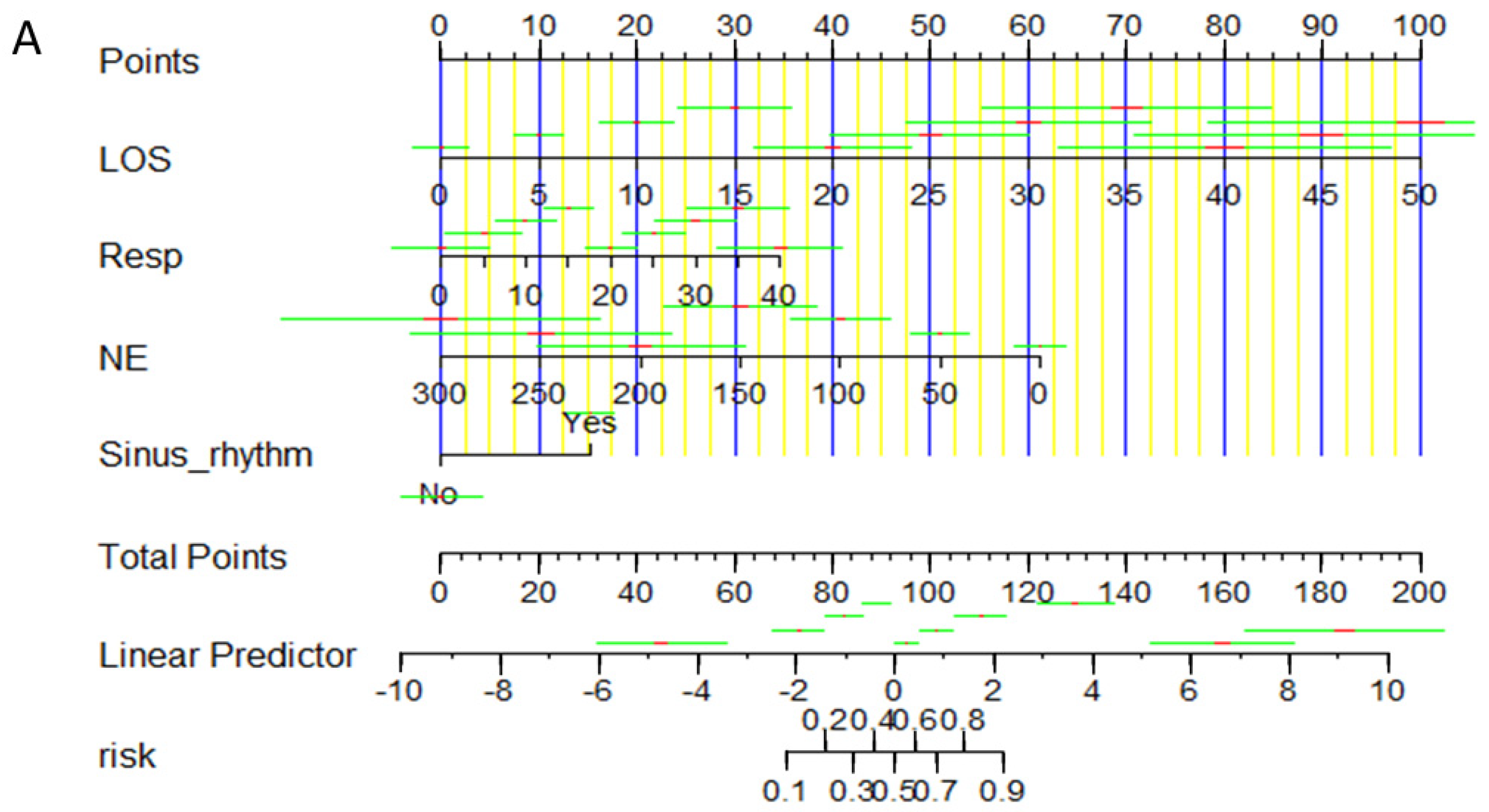
3.3. Construction of the Training Set Nomogram Prediction Model
3.4. Evaluation of the Prediction Model
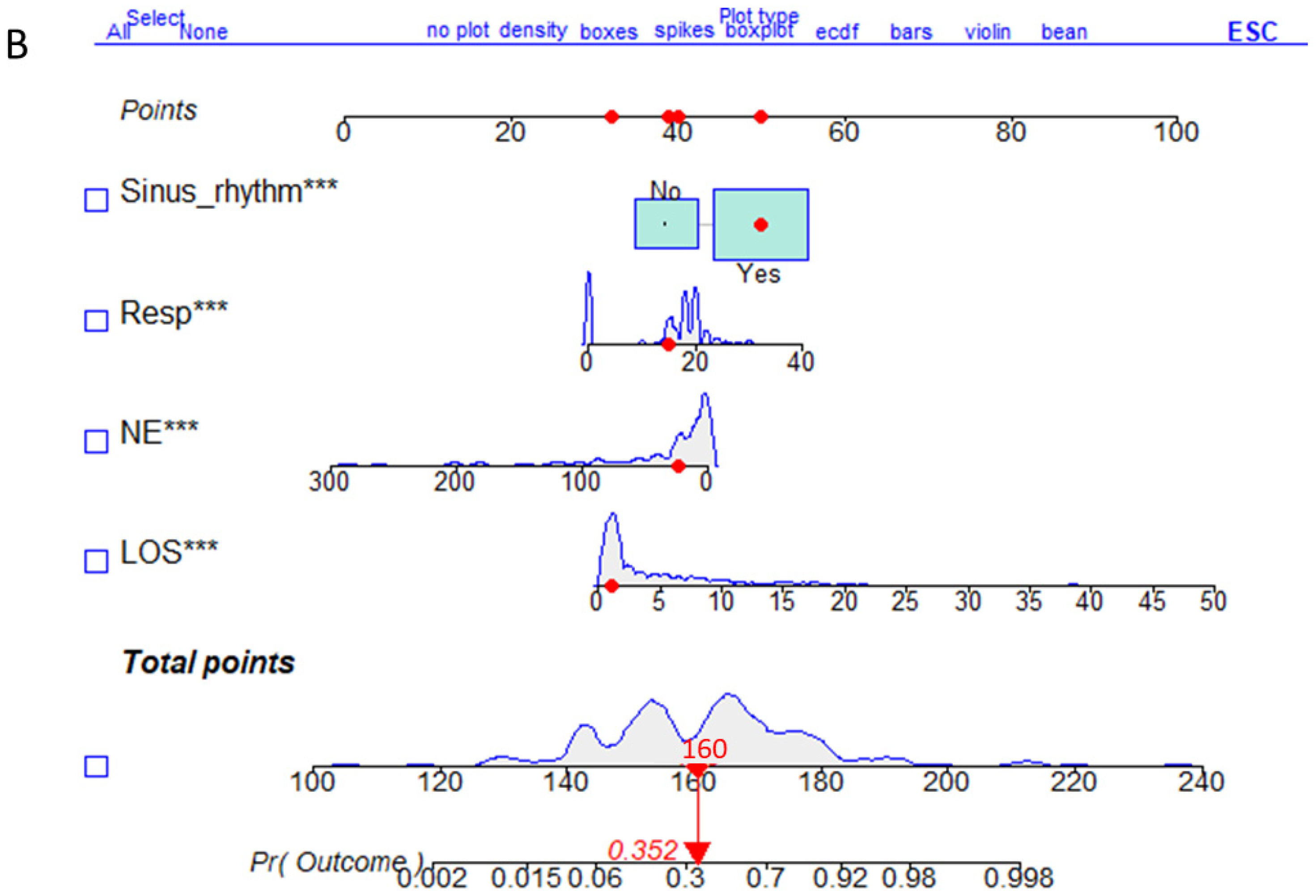
3.5. Validation Set Nomogram for the Risk Prediction Model
3.6. Verification of the Risk Prediction Model
4. Discussion
4.1. Clinical Significance of the Model
4.2. Influencing Factors of In-Hospital Mortality
4.3. Limitations of the Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Avishay, D.M.; Jones, C.R.; Shaikh, J.D.; Kaur, R.; Aljadah, M.; Kichloo, A.; Shiwalkar, N.; Keshavamurthy, S. Sudden cardiac death: Epidemiology, pathogenesis and management. Rev. Cardiovasc. Med. 2021, 22, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bayés de Luna, A.; Elosua, R. Sudden death. Rev. Esp. Cardiol. (Engl. Ed.) 2012, 65, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Pasupula, D.K.; Bhat, A.; Siddappa Malleshappa, S.K.; Munir, M.B.; Barakat, A.; Jain, S.; Wang, N.C.; Saba, S.; Bhonsale, A. Impact of Change in 2010 American Heart Association Cardiopulmonary Resuscitation Guidelines on Survival After Out-of-Hospital Cardiac Arrest in the United States: An Analysis From 2006 to 2015. Circ. Arrhythmia Electrophysiol. 2020, 13, e007843. [Google Scholar] [CrossRef]
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef]
- OngOng, M.E.H.; Shin, S.D.; De Souza, N.N.A.; Tanaka, H.; Nishiuchi, T.; Song, K.J.; Ko, P.C.-I.; Leong, B.S.-H.; Khunkhlai, N.; Naroo, G.Y.; et al. Outcomes for out-of-hospital cardiac arrests across 7 countries in Asia: The Pan Asian Resuscitation Outcomes Study (PAROS). Resuscitation 2015, 96, 100–108. [Google Scholar] [CrossRef]
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong, M.E.H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef]
- Poole, K.; Couper, K.; Smyth, M.A.; Yeung, J.; Perkins, G.D. Mechanical CPR: Who? When? How? Crit. Care 2018, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Oda, Y.; Oshima, C.; Kaneko, T.; Kaneda, K.; Kawamura, Y.; Ogino, Y.; Yamashita, S.; Ichihara, K.; Maekawa, T.; et al. Development of a prompt model for predicting neurological outcomes in patients with return of spontaneous circulation from out-of-hospital cardiac arrest. Acute Med. Surg. 2014, 2, 176–182. [Google Scholar] [CrossRef]
- Alshahrani, M.S.; Aldandan, H.W. Use of sodium bicarbonate in out-of-hospital cardiac arrest: A systematic review and meta-analysis. Int. J. Emerg. Med. 2021, 14, 21. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.H.; Lee, B.K.; Kim, D.K.; Ryu, S.J. Association Between Procalcitonin Level at 72 Hours After Cardiac Arrest and Neurological Outcomes in Cardiac Arrest Survivors. Ther. Hypothermia Temp. Manag. 2023, 13, 23–28. [Google Scholar] [CrossRef]
- Yoon, J.H.; Choi, W.S.; Lim, Y.S.; Jang, J.H. Comparison of Prognostic Performance between Procalcitonin and Procalcitonin-to-Albumin Ratio in Post Cardiac Arrest Syndrome. J. Clin. Med. 2023, 12, 4568. [Google Scholar] [CrossRef]
- Li, Z.; Gao, J.; Wang, J.; Xie, H.; Guan, Y.; Zhuang, X.; Liu, Q.; Fu, L.; Hou, X.; Hei, F. Mortality risk factors in patients receiving ECPR after cardiac arrest: Development and validation of a clinical prognostic prediction model. Am. J. Emerg. Med. 2024, 76, 111–122. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, N.; Zhu, H. The relationship between the level of NMLR on admission and the prognosis of patients after cardiopulmonary resuscitation: A retrospective observational study. Eur. J. Med. Res. 2023, 28, 424. [Google Scholar] [CrossRef] [PubMed]
- Perman, S.M.; Elmer, J.; Maciel, C.B.; Uzendu, A.; May, T.; Mumma, B.E.; Bartos, J.A.; Rodriguez, A.J.; Kurz, M.C.; Panchal, A.R.; et al. 2023 American Heart Association Focused Update on Adult Advanced Cardiovascular Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2024, 149, e254–e273. [Google Scholar] [CrossRef]
- Waldmann, P.; Mészáros, G.; Gredler, B.; Fuerst, C.; Sölkner, J. Evaluation of the lasso and the elastic net in genome-wide association studies. Front. Genet. 2013, 4, 270. [Google Scholar] [CrossRef]
- White, N.; Parsons, R.; Collins, G.; Barnett, A. Evidence of questionable research practices in clinical prediction models. BMC Med. 2023, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Bougouin, W.; Slimani, K.; Renaudier, M.; Binois, Y.; Paul, M.; Dumas, F.; Lamhaut, L.; Loeb, T.; Ortuno, S.; Deye, N.; et al. Sudden Death Expertise Center Investigators. Epinephrine versus norepinephrine in cardiac arrest patients with post-resuscitation shock. Intensive Care Med. 2022, 48, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Smida, T.; Crowe, R.P.; Martin, P.S.; Scheidler, J.F.; Price, B.S.; Bardes, J.M. A retrospective, multi-agency ‘target trial emulation’ for the comparison of post-resuscitation epinephrine to norepinephrine. Resuscitation 2024, 198, 110201. [Google Scholar] [CrossRef]
- Newell, C.; Grier, S.; Soar, J. Airway and ventilation management during cardiopulmonary resuscitation and after successful resuscitation. Crit. Care 2018, 22, 190. [Google Scholar] [CrossRef]
- Sun, Y.; He, Z.; Ren, J.; Wu, Y. Prediction model of in-hospital mortality in intensive care unit patients with cardiac arrest: A retrospective analysis of MIMIC -IV database based on machine learning. BMC Anesthesiol. 2023, 23, 178. [Google Scholar] [CrossRef]
- Sagris, D.; Korompoki, E.; Ntaios, G.; Tzeis, S.; Manios, E.; Kanakakis, J.; Milionis, H.; Papanagiotou, P.; Andrikopoulos, G.; Lip, G.Y.; et al. Sinus rhythm restoration and improved outcomes in patients with acute ischemic stroke and in-hospital paroxysmal atrial fibrillation. Eur. Stroke J. 2022, 7, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ohbe, H.; Kudo, D.; Kimura, Y.; Matsui, H.; Yasunaga, H.; Kushimoto, S. In-hospital mortality of patients admitted to the intermediate care unit in hospitals with and without an intensive care unit: A nationwide inpatient database study. Crit. Care 2025, 29, 34. [Google Scholar] [CrossRef] [PubMed]




| Items | Training Set (N = 208) | Validation Set (N = 87) | ||||
|---|---|---|---|---|---|---|
| Death (N = 119) | Survival (N = 89) | p | Death (N = 49) | Survival (N = 38) | p | |
| CPR Duration (minutes) | 30.0 (15.5, 47.0) | 12.0 (5.00, 20.0) | <0.001 * | 29.0 (19.0, 40.0) | 10.0 (5.00, 19.5) | <0.001 * |
| Gender (n, %) | - | 0.996 | - | - | 0.029 * | |
| Female | 75 (63.0) | 57 (64.0) | 40 (81.6) | 22 (57.9) | ||
| Male | 44 (37.0) | 32 (36.0) | 9 (18.4) | 16 (42.1) | ||
| Age (years) | 68.0 (56.5, 77.0) | 62.0 (56.0, 73.0) | 0.180 | 67.0 (53.0, 75.0) | 65.0 (56.2, 73.8) | 0.990 |
| Hospital Stay (days) | 3.14 (3.89) | 8.91 (9.34) | <0.001 * | 3.27 (4.40) | 6.97 (6.14) | 0.002 * |
| Emergency Department Stay (minutes) | 56.0 (42.5, 77.0) | 65.0 (42.0, 127) | 0.060 | 59.0 (44.0, 86.0) | 86.5 (49.0, 147) | 0.044 * |
| History of Coronary Heart Disease (n, %) | 33 (27.7) | 22 (24.7) | 0.743 | 9 (18.4) | 10 (26.3) | 0.530 |
| Inpatient ward (n, %) | 0.001 * | 0.019 * | ||||
| General ward (n, %) | 1 (0.84) | 10 (11.2) | 1 (2.04) | 7 (18.4) | ||
| ICU ward (n, %) | 118 (99.2) | 79 (88.8) | 48 (98.0) | 31 (81.6) | ||
| History of Diabetes (n, %) | 31 (26.1) | 36 (40.4) | 0.040 * | 10 (20.4) | 5 (13.2) | 0.547 |
| History of Malignant Tumors (n, %) | 24 (20.2) | 7 (7.87) | 0.023 * | 4 (8.16) | 0 (0.00) | 0.128 |
| History of Major Surgery (n, %) | 38 (31.9) | 26 (29.2) | 0.788 | 16 (32.7) | 10 (26.3) | 0.686 |
| History of Stroke(n, %) | 24 (20.2) | 19 (21.3) | 0.972 | 10 (20.4) | 8 (21.1) | 1.000 |
| Chronic Obstructive Pulmonary Disease (n, %) | 9 (7.56) | 2 (2.25) | 0.121 | 4 (8.16) | 2 (5.26) | 0.692 |
| Deep Vein Thrombosis History(n, %) | 2 (1.68) | 2 (2.25) | 1.000 | 2 (4.08) | 0 (0.00) | 0.502 |
| Chronic Kidney Disease Stage 3 or Above (n, %) | 10 (8.40) | 4 (4.49) | 0.405 | 2 (4.08) | 2 (5.26) | 1.000 |
| Other Past Medical Histories (n, %) | 41 (34.5) | 26 (29.2) | 0.516 | 20 (40.8) | 15 (39.5) | 1.000 |
| Pulse (beats/minute) | 82.0 (0.00, 104) | 90.0 (78.0, 107) | 0.006 * | 85.0 (0.00, 104) | 84.0 (75.0, 100) | 0.429 |
| Respiration (breaths/minute) | 16.0 (0.00, 20.0) | 18.0 (16.0, 20.0) | <0.001 * | 18.0 (0.00, 20.0) | 18.0 (16.2, 20.0) | 0.089 |
| Systolic Blood Pressure (mmHg) | 80.0 (50.0, 122) | 120 (97.0, 142) | <0.001 * | 93.0 (62.0, 116) | 124 (107, 139) | <0.001 * |
| Diastolic Blood Pressure (mmHg) | 45.0 (22.5, 66.0) | 70.0 (52.0, 82.0) | <0.001 * | 55.0 (35.0, 70.0) | 74.5 (56.0, 88.2) | 0.001 * |
| Pulse Pressure Difference (mmHg) | 34.0 (16.0, 45.0) | 46.0 (35.0, 64.0) | <0.001 * | 32.0 (15.0, 45.0) | 51.0 (30.0, 59.2) | 0.006 * |
| Blood Glucose on Admission (mmol/L) | 10.5 (7.20, 14.9) | 10.1 (7.70, 14.8) | 0.634 | 10.2 (7.70, 15.5) | 10.2 (6.20, 14.5) | 0.617 |
| Serum Calcium (mmol/L) | 2.07 (0.72) | 2.16 (0.24) | 0.244 | 2.06 (0.41) | 2.11 (0.27) | 0.525 |
| Serum Sodium (mmol/L) | 146 (11.9) | 141 (5.92) | <0.001 * | 144 (8.07) | 143 (5.56) | 0.309 |
| Serum Chloride (mmol/L) | 102 (7.91) | 103 (5.93) | 0.665 | 102 (7.25) | 104 (9.07) | 0.176 |
| Activated Partial Thromboplastin Time (s) | 35.6 (28.2, 62.3) | 28.1 (24.1, 32.0) | <0.001 * | 35.1 (28.8, 54.2) | 28.3 (23.6, 47.1) | 0.041 * |
| Prothrombin Time (s) | 17.5 (9.14) | 13.0 (3.81) | <0.001 * | 18.5 (9.60) | 14.9 (7.44) | 0.054 |
| Fibrinogen (g/L) | 2.72 (1.28) | 2.98 (1.09) | 0.118 | 2.63 (1.45) | 2.38 (0.84) | 0.315 |
| Thrombin Time (s) | 18.8 (17.0, 25.1) | 17.5 (15.5, 19.1) | <0.001 * | 19.0 (17.7, 22.3) | 18.7 (16.4, 20.5) | 0.168 |
| Hemoglobin Concentration (G/L) | 113 (32.0) | 124 (28.8) | 0.017 * | 121 (35.5) | 128 (28.3) | 0.262 |
| White Blood Cell Count (×109/L) | 13.7 (6.24) | 13.0 (5.54) | 0.448 | 12.7 (6.28) | 12.2 (5.41) | 0.643 |
| Neutrophil Count (×109/L) | 10.5 (5.95, 14.2) | 9.90 (6.30, 13.3) | 0.989 | 9.20 (6.50, 11.9) | 10.3 (7.28, 13.6) | 0.171 |
| Platelet Count (×109/L) | 166 (89.3) | 205 (77.8) | 0.001 * | 161 (84.3) | 208 (73.3) | 0.006* |
| Mean Platelet Volume (FL) | 11.7 (14.3) | 11.8 (12.3) | 0.974 | 10.5 (1.23) | 10.4 (1.23) | 0.685 |
| Red Blood Cell Distribution Width—Coefficient of Variation (%) | 14.3 (2.85) | 13.5 (1.47) | 0.005 * | 13.8 (1.83) | 13.6 (1.56) | 0.574 |
| Lymphocyte Count (×109/L) | 2.42 (1.94) | 1.98 (1.89) | 0.105 | 4.66 (18.1) | 1.72 (1.47) | 0.262 |
| Lactate (mmol/L) | 13.4 (7.13) | 6.34 (6.45) | <0.001 * | 13.3 (7.22) | 6.18 (5.79) | <0.001 * |
| Total Bilirubin (μmol/L) | 14.9 (12.8) | 14.8 (12.9) | 0.934 | 28.2 (54.1) | 16.3 (13.8) | 0.145 |
| Direct Bilirubin (μmol/L) | 6.77 (7.53) | 6.32 (7.42) | 0.665 | 14.2 (35.5) | 7.05 (8.73) | 0.179 |
| Albumin (g/L) | 32.6 (8.35) | 37.1 (5.79) | <0.001 * | 33.4 (7.47) | 36.6 (5.30) | 0.021 * |
| Neutrophil-to-Lymphocyte Ratio | 9.37 (12.8) | 10.9 (10.7) | 0.356 | 11.3 (14.9) | 10.9 (10.5) | 0.886 |
| Platelet-to-Lymphocyte Ratio | 143 (172) | 207 (197) | 0.017 * | 186 (201) | 193 (137) | 0.836 |
| Procalcitonin (ng/mL) | 0.40 (0.10, 3.10) | 0.10 (0.00, 1.00) | 0.004 * | 0.40 (0.10, 1.30) | 0.10 (0.10, 2.38) | 0.576 |
| B-Type Natriuretic Peptide (pg./mL) | 323 (83.5, 1354) | 324 (80.2, 1278) | 0.961 | 305 (43.2, 1160) | 156 (51.0, 486) | 0.392 |
| D-Dimer (ug/mL.FEU) | 29.0 (57.2) | 16.2 (40.7) | 0.061 | 17.6 (20.1) | 12.2 (23.3) | 0.257 |
| Oxygen Partial Pressure (mmHg) | 166 (142) | 151 (103) | 0.374 | 208 (162) | 127 (55.8) | 0.002 * |
| Carbon Dioxide Partial Pressure (mmHg) | 55.6 (29.8) | 47.0 (19.9) | 0.014 * | 50.7 (25.5) | 42.5 (19.3) | 0.093 |
| PH Value (mmHg) | 7.09 (0.30) | 7.27 (0.21) | <0.001 * | 7.12 (0.20) | 7.32 (0.21) | <0.001 * |
| Arterial Blood Potassium (mmol/L) | 4.61 (1.72) | 4.18 (1.03) | 0.027 * | 4.53 (1.58) | 3.75 (0.85) | 0.004 * |
| Serum Potassium (mmol/L) | 4.70 (1.57) | 4.17 (0.89) | 0.002 * | 4.86 (1.89) | 4.04 (0.72) | 0.007 * |
| Epinephrine Dose (mg) | 43.9 (59.0) | 15.8 (36.1) | <0.001 * | 31.5 (44.5) | 16.2 (35.6) | 0.079 |
| Norepinephrine Dose (mg) | 41.7 (59.9) | 19.4 (33.1) | 0.001 * | 29.4 (41.9) | 21.4 (35.1) | 0.336 |
| Mannitol Treatment (n, %) | 26 (21.8) | 17 (19.1) | 0.756 | 12 (24.5) | 6 (15.8) | 0.467 |
| 34–36 degrees Celsius Hypothermia treatment (n, %) | 84(70.6) | 44(49.4) | 0.003 * | 36(73.5) | 17(44.7) | 0.012 * |
| Antibiotic Treatment (n, %) | 83 (69.7) | 69 (77.5) | 0.274 | 35 (71.4) | 29 (76.3) | 0.789 |
| The Use of Sodium Bicarbonate (n, %) | 69 (58.0) | 35 (39.3) | 0.012 * | 21 (42.9) | 16 (42.1) | 1.000 |
| Tracheal Intubation and Mechanical Ventilation Assistance (n, %) | 102 (85.7) | 58 (65.2) | 0.001 * | 41 (83.7) | 20 (52.6) | 0.004 * |
| Gastric Protection Treatment (n, %) | 39 (32.8) | 37 (41.6) | 0.247 | 21 (42.9) | 15 (39.5) | 0.922 |
| Sinus Rhythm Recovery After Resuscitation (n, %) | 64 (53.8) | 79 (88.8) | <0.001 * | 31 (63.3) | 33 (86.8) | 0.026 * |
| ST-T Changes After Resuscitation (n, %) | 61 (51.3) | 50 (56.2) | 0.573 | 28 (57.1) | 25 (65.8) | 0.550 |
| Time of Cardiac Arrest Occurrence (hours) | 13.5 (6.31) | 13.2 (6.69) | 0.712 | 13.0 (6.04) | 13.6 (6.16) | 0.662 |
| The Time of Sudden Death Occurrence (n, %) | 0.055 | 0.614 | ||||
| 22:00–6:00 (n, %) | 26 (21.8) | 31 (34.8) | 11 (22.4) | 6 (15.8) | ||
| 6:00–22:00 (n, %) | 93 (78.2) | 58 (65.2) | 38 (77.6) | 32 (84.2) | ||
| Variables | Univariate Regression | Multivariate Regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | 0R | 95% CI | Z | p | B | SE | OR | 95% CI | Z | p | |
| Albumin | 0.087 | 0.022 | 1.09 | 1.04–1.14 | 4.009 | <0.001 * | 0.055 | 0.031 | 1.06 | 0.99–1.12 | 1.781 | 0.075 |
| CPR Duration | −0.074 | 0.012 | 0.93 | 0.91–0.95 | −6.027 | <0.001 * | - | - | - | - | - | - |
| Lactate | −0.156 | 0.026 | 0.86 | 0.81–0.9 | −5.967 | <0.001 * | - | - | - | - | - | - |
| Actual Hospital Stay | 0.166 | 0.033 | 1.18 | 1.11–1.26 | 4.95 | <0.001 * | 0.141 | 0.042 | 1.15 | 1.06–1.25 | 3.349 | 0.001 * |
| In-Hospital Norepinephrine Dose | −0.012 | 0.004 | 0.99 | 0.98–1 | −2.852 | 0.004 * | −0.015 | 0.005 | 0.99 | 0.98–0.99 | −2.840 | 0.005 * |
| Respiratory Rate After Resuscitation | 0.096 | 0.021 | 1.1 | 1.06–1.15 | 4.636 | <0.001 * | 0.091 | 0.028 | 1.10 | 1.04–1.16 | 3.223 | 0.001 * |
| Sinus Rhythm After Resuscitation | 1.915 | 0.383 | 6.79 | 3.2–14.38 | 5.005 | <0.001 * | 1.325 | 0.485 | 3.76 | 1.65–9.73 | 2.732 | 0.006 * |
| Tracheal Intubation and Mechanical Ventilation Assistance | −1.165 | 0.344 | 0.31 | 0.16–0.61 | −3.301 | 0.001 * | −0.756 | 0.537 | 0.47 | 0.16–1.34 | −1.409 | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, Z.; Guo, X.; Liang, Y.; Wang, J.; Li, J.; Yang, X.; Ai, F. Establishing and Validating a Predictive Model for the Risk of In-Hospital Mortality Post-Resuscitation in Patients with Sudden Death, as Well as Conducting Clinical Analysis Research: A Case-Control Study. Emerg. Care Med. 2025, 2, 15. https://doi.org/10.3390/ecm2010015
Li Y, Chen Z, Guo X, Liang Y, Wang J, Li J, Yang X, Ai F. Establishing and Validating a Predictive Model for the Risk of In-Hospital Mortality Post-Resuscitation in Patients with Sudden Death, as Well as Conducting Clinical Analysis Research: A Case-Control Study. Emergency Care and Medicine. 2025; 2(1):15. https://doi.org/10.3390/ecm2010015
Chicago/Turabian StyleLi, Yu, Zhen Chen, Xin Guo, Yifan Liang, Jueyan Wang, Jinglei Li, Xianting Yang, and Fen Ai. 2025. "Establishing and Validating a Predictive Model for the Risk of In-Hospital Mortality Post-Resuscitation in Patients with Sudden Death, as Well as Conducting Clinical Analysis Research: A Case-Control Study" Emergency Care and Medicine 2, no. 1: 15. https://doi.org/10.3390/ecm2010015
APA StyleLi, Y., Chen, Z., Guo, X., Liang, Y., Wang, J., Li, J., Yang, X., & Ai, F. (2025). Establishing and Validating a Predictive Model for the Risk of In-Hospital Mortality Post-Resuscitation in Patients with Sudden Death, as Well as Conducting Clinical Analysis Research: A Case-Control Study. Emergency Care and Medicine, 2(1), 15. https://doi.org/10.3390/ecm2010015





