Abstract
Methaemoglobinaemia (MetHb) is a functional anaemia that can be life-threatening in severe cases. MetHb in adults and older children usually results from exposure to toxins from ingestion or skin exposure, whereas MetHb in infants under six months old usually occurs due to exposure to well water, severe metabolic acidosis from diarrhoea, or, in rare cases, secondary to cow’s milk protein allergy (CMPA). In this case report, a young infant presented acutely with shock secondary to profuse diarrhoea and MetHb requiring intravenous fluids and methylene blue and was subsequently diagnosed with CMPA. The early recognition and prompt treatment of CMPA may prevent the recurrence of MetHb symptoms and excessive diagnostic testing in this vulnerable population.
1. Introduction
MetHb is a functional anaemia in which haemoglobin is unable to reversibly bind oxygen due to the oxidation of ferrous (Fe2+) ions of heme to the ferric (Fe3+) state [1]. The oxygen affinity of any remaining normal ferrous heme is increased, causing a left shift of the oxyhemoglobin dissociation curve and subsequent hypoxia [1]. MetHb can present with a range of symptoms, with patients being asymptomatic in low levels; patients with cyanosis, tachycardia, respiratory depression, and shock at levels over 20%; and even life-threatening symptoms at levels over 40% [2]. MetHb can be caused by exposure to toxins, including nitrates in well water and skin exposure to oxidising agents; acidosis; diet; and genetic enzyme deficiencies such as cytochrome b5 reductase deficiency, cytochrome b5 deficiency, or haemoglobin M [1,2]. MetHb in children over six months old and adults usually results from exposure to toxins from ingestion or skin exposure [1,2], whereas MetHb in infants under six months old usually occurs due to exposure to well water or severe metabolic acidosis from diarrhoea [2], but it has also been reported in cases of CMPA or food protein-induced enterocolitis syndrome (FPIES) [3,4,5].
Cow’s milk protein is a major dietary allergen in infants and young children and affects about 2% of infants under 2 years of age [6]. CMPA may be IgE-mediated, non-IgE-mediated, or mixed in its pathophysiology and can present as acute or chronic syndromes [6,7]. IgE-mediated syndromes include immediate allergic reactions and atopic eczema, whereas non-IgE-mediated syndromes include CMPA, FPIES, and other gastrointestinal syndromes [6,7]. Infants with non-IgE-mediated CMPA may present with vomiting, chronic diarrhoea, malabsorption, failure to thrive [6], and, in rare cases, MetHb [3,4,5,7].
2. Case Presentation
Baby Z, a 5-week-old term male, presented to the Emergency Department (ED) with two days of watery diarrhoea and lethargy. Baby Z was feeding well on mixed breastmilk and formula feeds with an adequate urine output and no fevers or vomiting. Baby Z examined well with a weight of 3.885 kg (birth weight 3.79 kg) in the ED (Figure 1), a blood glucose level of 6.6 mg/L, and a ketone level of 0.3 mmol/L and was discharged from the ED with a diagnosis of infectious diarrhoea.
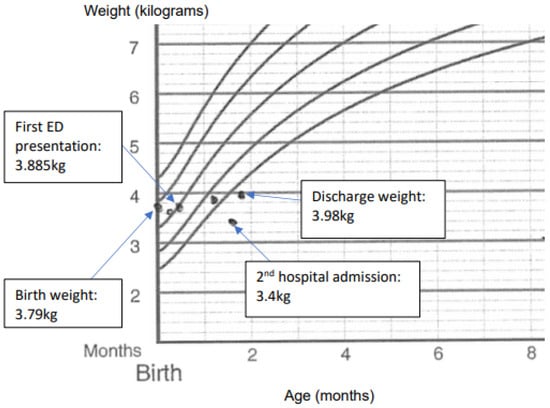
Figure 1.
Baby Z’s weights before and after presentation and admissions.
Baby Z presented the following day to the ED with over 30 episodes of profuse diarrhoea within 24 h and worsening lethargy but no fevers or vomiting. The examination revealed mottled peripheries, sunken eyes and anterior fontanel, and reduced skin turgor. Baby Z had tachycardia at 195 bpm and tachypnoea at 45 bpm with a central capillary refill time of 3 secs, increased work of breathing, and oxygen saturation of 96%. The venous blood gas test showed metabolic acidosis, and his blood was dark brown in colour with an elevated methaemoglobin fraction of 11.9% (normal < 1.5%), and a diagnosis of MetHb was made based on the venous blood gas results (Table 1). The complete blood count showed a white blood cell (WBC) count of 43.67 × 103/mcL, a haemoglobin count of 12.8 g/dL, a platelet count of 639 × 103/mcL, and a C-reactive protein (CRP) count of 20 mg/dL. The renal function test revealed a sodium concentration of 148 mmol/L, a bicarbonate concentration of <5 mmol/L, a urea concentration of 8.2 mmol/L, and a creatinine concentration of 65 umol/L. Baby Z was resuscitated with a total of 20 mL/kg of normal saline and was started empirically on antibiotics for presumed sepsis after a septic screen (including blood, urine, and stool cultures and viral swabs) was performed. Sodium bicarbonate and intravenous (IV) fluids were administered to correct the electrolyte imbalances and fluid deficits (Table 1), and baby Z was admitted to the Paediatric Intensive Care Unit (PICU). Multiple subspecialty teams were consulted in view of baby Z’s MetHb and normal anion gap metabolic acidosis (NAGMA), including the haematology, metabolic, and infectious diseases teams. Further investigations (including a urine metabolic screen, plasma ammonia, and inflammatory markers) were ordered, and a provisional diagnosis of infectious gastroenteritis was made based on elevated inflammatory markers, with MetHb and NAGMA being attributed to dehydration and bicarbonate losses from diarrhoea. Baby Z was transferred to the ward after stabilisation and after blood gases were improved (Table 1) and was started on nasogastric hydrolyte rehydration at a 150% maintenance rate. Antibiotics were ceased after the septic screen returned a negative result, and baby Z was discharged after tolerating oral feeds with only two bowel motions within 24 h with a plan for follow-up in two days in the outpatient clinic.

Table 1.
Blood gas results for baby Z during 1st admission.
Baby Z subsequently presented to the ED within 36 h of discharge with eight episodes of diarrhoea in 12 h. Baby Z was found to be pale with cool peripheries with a weight loss of 265 g from the previous discharge (Figure 1) despite tolerating oral feeds, and he had oxygen saturations of 85%, which did not improve upon supplemental oxygen. The capillary blood gas test showed metabolic acidosis with a methaemoglobin fraction of 26.1% (Table 1). The complete blood count showed a WBC count of 27.72 × 103/mcL, a haemoglobin count of 12.5 g/dL, a platelet count of 718 × 103/mcL, and a CRP count of 7.3 mg/dL. Baby Z was resuscitated with IV fluid boluses with minimal effect and started empirically on antibiotics again for presumed sepsis. After discussion with the Haematology Department, methylene blue was administered for MetHb with good effect. A repeat blood gas test showed a decreased methaemoglobin fraction of 2.2% (Table 1), after which baby Z was admitted to the PICU and continued IV hydration with sodium bicarbonate to correct his metabolic acidosis. The gastroenterology and immunology teams were consulted due to persistent diarrhoea despite having no infectious agent detected on the septic screen again. Additional investigations were ordered to screen for a possible underlying immunodeficiency, such as IPEX-like syndromes, in view of baby Z’s presentation of enteropathy with associated metabolic derangement and failure to thrive in a male infant, with his haemoglobin studies, plasma amino acids, G6PD assay, stool alpha 1 antitrypsin, IgM, liver function, thyroid function, and HIV serology all within normal limits. Lymphocyte subsets showed elevated overall lymphocyte count, CD19 (B cells) percentage, and absolute count (Table 2), and serum immunoglobulins were noted to be elevated (Table 3).

Table 2.
Lymphocyte subsets for baby Z during admission and 2 months post-discharge.

Table 3.
Serum immunoglobulins for baby Z during admission and 2 months post-discharge.
Baby Z was trialled on an extensively hydrolysed formula but had persistent diarrhoea, and after a discussion with the gastroenterology and immunology teams, he was switched to an amino acid formula with good effect. A clinical diagnosis of CMPA was made in view of symptom resolution on an elimination diet, and baby Z was discharged after demonstrating good weight gain (Figure 1). A follow-up appointment with the Immunology team at a month post-discharge revealed no further episodes of diarrhoea on the amino acid formula, with repeat bloods at 2 months post-discharge showing normal serum immunoglobulins (Table 3), and other immunological investigations (including DHR-Oxidative Burst, Nitroblue Tetrazolium (NBT), Lymphocyte Subsets, and T Regulatory-like cells) revealed no underlying immunodeficiency. A diagnosis of non-IgE-mediated CMPA was made at the follow-up at 7 months of age based on clinical improvement, with baby Z thriving on amino acid formula and with no further episodes of profuse diarrhoea, and after a consultation with the immunology team, further testing was not performed due to the clinical resolution of symptoms.
3. Discussion
Cow’s milk protein allergy is common in Australia and New Zealand, with more than 2% (1 in 50) of infants being allergic to cow’s milk protein [8]. Unlike IgE-mediated cow’s milk allergy, where there is a rapid onset of allergic reaction usually within 15 min to 2 h after the ingestion of cow’s milk and with allergen-specific IgE usually testing positive, non-IgE-mediated CMPA usually occurs two or more hours after the consumption of cow’s milk, with symptoms including the exacerbation of eczema, delayed vomiting, and/or diarrhoea, and allergy tests to cow’s milk are usually negative [8].
Methaemoglobinaemia is a potentially life-threatening condition caused by the formation of methaemoglobin [1]. There are rare genetic disorders that can cause congenital methaemoglobinemia, but most cases are acquired from the effects of specific drugs or environmental exposures including dapasone and inhaled nitric oxide [1,9]. Severe MetHb is potentially life-threatening but is usually associated with either the intentional ingestion of nitrites or, in paediatric patients, the ingestion of food and water contaminated with nitrates [1,9]. A recent study involving 310 patients showed a mortality rate of 8.4% in patients with methaemoglobin levels of over 3%, albeit with only one of the fatalities (the intentional ingestion of sodium nitrite for a suicide attempt by a 20-year-old woman) clearly being linked to methemoglobinemia as the primary cause [9].
In this case report, a young infant presented acutely with shock secondary to profuse diarrhoea and MetHb requiring IV fluids and methylene blue and was subsequently diagnosed with CMPA. MetHb has previously been reported in infants with CMPA [3,4,5,9]; however, our search of the literature suggests this is the first case report in Australia. Improvement is usually seen within minutes, and additional doses of methylene blue may be given after 30–60 min if indicated [2,9]. Other possible alternative treatments include ascorbic acid (in cases such as G6PD where methylene blue is contraindicated), hyperbaric oxygen, and exchange transfusion [2].
A recent literature review found 32 cases of MetHb associated with CMPA or FPIES [3], and a case series involving 45 infants with MetHb and diarrhoeal illness found that 18% were eventually diagnosed with CMPA or soy protein intolerance [8]. The proposed mechanisms for MetHb include increased nitrite-induced methaemoglobin conversion secondary to metabolic acidemia [3] or intestinal bacterial overgrowth [3,4] and altered colonic homeostasis in nitrate formation by mucosal inflammatory cells due to decreased epithelial catalase activity [3,4,7].
4. Conclusions
- CMPA is a rare but well-recognised cause of MetHb and should be considered in a shocked or cyanotic infant with unexplained watery diarrhoea.
- MetHb should be treated promptly with the removal of causative agents and fluid resuscitation in mild cases, and treatment with 1–2 mg/kg of IV methylene blue administered over 5–10 min should be carried out in severe cases.
- The early recognition and prompt treatment of CMPA may prevent the recurrence of symptoms, excessive diagnostic testing, and persistent failure to thrive.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent was obtained from legal guardians.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the author on request.
Conflicts of Interest
The author declares no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Informed Consent Statement. This change does not affect the scientific content of the article.
References
- Kaminecki, I.; Huang, D. Methemoglobinemia. Pediatr. Rev. 2021, 42, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; Bianchi, P.; Andolfo, I.; Russo, R.; Barcellini, W.; Fermo, E.; Toldi, G.; Ghirardello, S.; Rees, D.; Van Wijk, R.; et al. Recommendations for diagnosis and treatment of methemoglobinemia. Am. J. Hematol. 2021, 96, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Bahabri, A.; Moradi, J.; Choong, K.; Pai, N.; Bhatt, M. Food Protein Induced Enterocolitis Syndrome Presenting with Life-Threatening Methemoglobinemia: A Case Report and Review of the Literature. Int. J. Clin. Pediatr. 2020, 9, 35–40. [Google Scholar] [CrossRef]
- Murray, K.F.; Christie, D.L. Dietary protein intolerance in infants with transient methemoglobinemia and diarrhea. J. Pediatr. 1993, 122, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.C.; Sánchez, P.C.; Gentles, M.G.; Botia, J.G.; Calzado, A.G.; Abascal, G.B.; Hachero, J.G. Methemoglobinemia, acidemia and diarrhea induced by hypersensitivity to cow’s milk proteins. An. Esp. De Pediatr. 1996, 44, 295–296. [Google Scholar]
- Kemp, A.S.; Hill, D.J.; Allen, K.J.; Anderson, K.; Davidson, G.P.; Day, A.S.; Heine, R.G.; Peake, J.E.; Prescott, S.L.; Shugg, A.W.; et al. Guidelines for the use of infant formulas to treat cows milk protein allergy: An Australian consensus panel opinion. Med. J. Aust. 2008, 188, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.; Nowak-Wegrzyn, A. Educational clinical case series for pediatric allergy and immunology: Allergic proctocolitis, food protein-induced enterocolitis syndrome and allergic eosinophilic gastroenteritis with protein-losing gastroenteropathy as manifestations of non-IgE-mediated cow’s milk allergy. Pediatr. Allergy Immunol. 2007, 18, 360–367. [Google Scholar] [PubMed]
- Cow’s Milk (Dairy) Allergy—Australasian Society of Clinical Immunology and Allergy (ASCIA). Updated 11 June 2024. Available online: https://www.allergy.org.au/patients/food-allergy/cows-milk-dairy-allergy (accessed on 22 July 2024).
- Belzer, A.; Krasowski, M.D. Causes of acquired methemoglobinemia—A retrospective study at a large academic hospital. Toxicol. Rep. 2024, 12, 331–337. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).