Risk Factors for Emergency Room Visits in Patients with Digestive Bleeding Associated with Direct-Acting Anticoagulants
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Li, X.; Tse, V.C.; Au-Doung, L.W.; Wong, I.C.K.; Chan, E.W. The impact of ischaemic stroke on atrial fibrillation-related healthcare cost: A systematic review. Europace 2017, 19, 937–947. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.; Bellino, L.; Consoli, D.; Mori, F.; Zaninelli, A.; Baldereschi, M.; Cattarinussi, A.; D’alfonso, M.G.; Gradia, C.; Sgherzi, B.; et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: The FAI Project. Europace 2019, 21, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Schulman, S.; Dowlatshahi, D.; Holbrook, A.M.; Simpson, C.S.; Shepherd, L.E.; Wells, P.S.; Giulivi, A.; Gomes, T.; Mamdani, M.; et al. Bleeding Effected by Direct Oral Anticoagulants (BLED-AC) Study Group. Direct Oral Anticoagulant- or Warfarin-Related Major Bleeding: Characteristics, Reversal Strategies, and Outcomes From a Multicenter Observational Study. Chest 2017, 152, 81–91. [Google Scholar] [CrossRef]
- Piccini, J.P.; Hammill, B.G.; Sinner, M.F.; Hernandez, A.F.; Walkey, A.J.; Benjamin, E.J.; Curtis, L.H.; Heckbert, S.R. Clinical course of atrial fibrillation in older adults: The importance of cardiovascular events beyond stroke. Eur. Heart J. 2014, 35, 250–256. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESCGuidelines for the diagnosis management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Yamashita, T.; Suzuki, S.; Inoue, H.; Akao, M.; Atarashi, H.; Ikeda, T.; Okumura, K.; Koretsune, Y.; Shimizu, W.; Tsutsui, H.; et al. Two-year outcomes of more than 30,000 elderly patients with atrial fibrillation: Results from the All Nippon AF In the Elderly (ANAFIE) Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 202–213. [Google Scholar] [CrossRef]
- Diener, H.-C.; Aisenberg, J.; Ansell, J.; Atar, D.; Breithardt, G.; Eikelboom, J.; Ezekowitz, M.D.; Granger, C.B.; Halperin, J.L.; Hohnloser, S.H.; et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: Part 2. Eur. Heart J. 2017, 38, 860–868. [Google Scholar]
- Ntaios, G.; Papavasileiou, V.; Makaritsis, K.; Vemmos, K.; Michel, P.; Lip, G.Y.H. Real-World Setting Comparison of Nonvitamin-K Antagonist Oral Anticoagulants Versus Vitamin-K Antagonists for Stroke Prevention in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Stroke 2017, 48, 2494–2503. [Google Scholar] [CrossRef]
- Pollack, C.V.; Peacock, W.F.; Bernstein, R.A.; Clark, C.L.; Douketis, J.; Fermann, G.J.; Frost, A.; Jahromi, B.; Johnson, C.; Merli, G.; et al. The safety of oral anticoagulants registry (SOAR): A national, ED-based study of the evaluation and management of bleeding and bleeding concerns due to the use of oral anticoagulants. Am. J. Emerg. Med. 2019, 38, 1163–1170. [Google Scholar] [CrossRef]
- Marmo, R.; Del Piano, M.; Rotondano, G.; Koch, M.; Bianco, M.A.; Zambelli, A.; Di Matteo, G.; Grossi, E.; Cipolletta, L. Mortality from nonulcer bleeding is similar to that of ulcer bleeding in high-risk patients with nonvariceal hemorrhage: A prospective database study in Italy. Gastrointest. Endosc. 2012, 75, 263–272.e1. [Google Scholar] [CrossRef]
- Ruiz, J.; López-Vinardell, L.; Juanes, A.; Riera-Magallon, A.; Puig, M.; Mangues, M.A. Risk factors for emergency department revisit in elderly patients with gastrointestinal bleeding secondary to anticoagulant therapy. Eur. J. Hosp. Pharm. 2022, 29, 271–274. [Google Scholar] [CrossRef]
- Meeker, E.; Dennehy, C.E.; Weber, E.J.; Kayser, S.R. Emergency department management of patients on warfarin therapy. Ann. Emerg. Med. 2011, 58, 192–199. [Google Scholar] [CrossRef]
- Garwood, C.L.; Corbett, T.L. Use of anticoagulation in elderly patients with atrial fibrillation who are at risk for falls. Ann. Pharmacother. 2008, 42, 523–532. [Google Scholar] [CrossRef]
- Alalwan, A.; Voils, S.; Hartzema, A. Trends in the Utilization of Warfarin and Non-Vitamin K Oral Anticoagulants in Elderly Patients with Atrial Fibrillation. Value Health 2016, 19, A42. [Google Scholar] [CrossRef]
- Torn, M.; Bollen, W.L.; van der Meer, F.J.; van der Wall, E.E.; Rosendaal, F.R. Risks of oral anticoagulant therapy with increasing age. Arch. Intern. Med. 2005, 165, 1527–1532. [Google Scholar] [CrossRef]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Xu, Y.; Siegal, D.M. Anticoagulant-associated gastrointestinal bleeding: Framework for decisions about whether, when and how to resume anticoagulants. J. Thromb. Haemost. 2021, 19, 2383–2393. [Google Scholar] [CrossRef]
- Gomes, T.; Mamdani, M.M.; Holbrook, A.M.; Paterson, J.M.; Hellings, C.; Juurlink, D.N. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ Can. Med. Assoc. J. 2013, 185, E121–E127. [Google Scholar] [CrossRef]
- Laursen, S.B.; Oakland, K.; Laine, L.; Bieber, V.; Marmo, R.; Redondo-Cerezo, E.; Dalton, H.R.; Ngu, J.; Schultz, M.; Soncini, M.; et al. ABC score: A new risk score that accurately predicts mortality in acute upper and lower gastrointestinal bleeding: An international multicentre study. Gut 2021, 70, 707–716. [Google Scholar] [CrossRef]
- Desai, J.; Kolb, J.M.; Weitz, J.I.; Aisenberg, J. Gastrointestinal bleeding with the new oral anticoagulants--defining the issues and the management strategies. Thromb. Haemost. 2013, 110, 205–212. [Google Scholar] [CrossRef]
- Chen, W.-C.; Chen, Y.-H.; Hsu, P.-I.; Tsay, F.-W.; Chan, H.-H.; Cheng, J.-S.; Lai, K.-H. Gastrointestinal hemorrhage in warfarin anticoagulated patients: Incidence, risk factor, management, and outcome. Biomed. Res. Int. 2014, 2014, 463767. [Google Scholar] [CrossRef]
- Gallagher, A.M.; van Staa, T.P.; Murray-Thomas, T.; Schoof, N.; Clemens, A.; Ackermann, D.; Bartels, D.B. Population-based cohort study of warfarin-treated patients with atrial fibrillation: Incidence of cardiovascular and bleeding outcomes. BMJ Open 2014, 4, e003839. [Google Scholar] [CrossRef]
- Leonard, C.E.; Brensinger, C.M.; Bilker, W.B.; Kimmel, S.E.; Han, X.; Nam, Y.H.; Gagne, J.J.; Mangaali, M.J.; Hennessy, S. Gastrointestinal bleeding and intracranial hemorrhage in concomitant users of warfarin and antihyperlipidemics. Int. J. Cardiol. 2017, 228, 761–770. [Google Scholar] [CrossRef]
- Zagoridis, K.; Karatisidis, L.; Mprotsis, T.; Pentidou, A.; Bezirgianidou, Z.; Misidou, C.; Spanoudakis, E. Apixaban reduces the risk of major and clinically relevant non-major bleeding compared to warfarin in patients with end stage renal disease; a systematic review and meta-analysis of ten studies. Thromb. Res. 2023, 231, 17–24. [Google Scholar] [CrossRef]
- Mamas, M.A.; Batson, S.; Pollock, K.G.; Grundy, S.; Matthew, A.; Chapman, C.; Manuel, J.A.; Farooqui, U.; Mitchell, S.A. Meta-Analysis Comparing Apixaban Versus Rivaroxaban for Management of Patients With Nonvalvular Atrial Fibrillation. Am. J. Cardiol. 2022, 166, 58–64. [Google Scholar] [CrossRef]
- Witt, D.M. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 2016, 620–624. [Google Scholar] [CrossRef]
- Sengupta, N.; Marshall, A.L.; Jones, B.A.; Ham, S.; Tapper, E.B. Rebleeding vs Thromboembolism After Hospitalization for Gastrointestinal Bleeding in Patients on Direct Oral Anticoagulants. Clin. Gastroenterol. Hepatol. 2018, 16, 1893–1900.e2. [Google Scholar] [CrossRef]
- Witt, D.M.; Delate, T.; Garcia, D.A.; Clark, N.P.; Hylek, E.M.; Ageno, W.; Dentali, F.; Crowther, M.A. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch. Intern. Med. 2012, 172, 1484–1491. [Google Scholar] [CrossRef]
- Sengupta, N.; Feuerstein, J.D.; Patwardhan, V.R.; Tapper, E.B.; Ketwaroo, G.A.; Thaker, A.M.; Leffler, D.A. The Risks of Thromboembolism Vs. Recurrent Gastrointestinal Bleeding after Interruption of Systemic Anticoagulation in Hospitalized Inpatients With Gastrointestinal Bleeding: A Prospective Study. Am. J. Gastroenterol. 2015, 110, 328. [Google Scholar] [CrossRef]
- Gulløv, A.L.; Koefoed, B.G.; Petersen, P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: The AFASAK 2 study. Atrial Fibrillation Aspirin and Anticoagulation. Arch. Intern. Med. 1999, 159, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.; Mittal, C.; Patsias, I.; Garikapati, K.; Kuchipudi, A.; Cheema, G.; Elbatta, M.; Alirhayim, Z.; Khalid, F. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am. J. Cardiol. 2014, 113, 662–668. [Google Scholar] [CrossRef]
- Wang, W.; Lessard, D.; Kiefe, C.I.; Goldberg, R.J.; Parish, D.; Helm, R.; Trymbulak, K.; Mehawej, J.; Abu, H.; Bamgbade, B.A.; et al. Differential effect of anticoagulation according to cognitive function and frailty in older patients with atrial fibrillation. J. Am. Geriatr. Soc. 2023, 71, 394–403. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.S.; Yu, H.T.; Kim, T.H.; Jang, E.; Sung, J.H.; Pak, H.-N.; Lee, M.-Y.; Lee, M.-H.; Lip, G.Y.H.; et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: Data from a population-based cohort. Eur. Heart J. 2019, 40, 2313–2323. [Google Scholar] [CrossRef]
- Rydén, L.; Zettergren, A.; Seidu, N.M.; Guo, X.; Kern, S.; Blennow, K.; Zetterberg, H.; Sacuiu, S.; Skoog, I. Atrial fibrillation increases the risk of dementia amongst older adults even in the absence of stroke. J. Intern. Med. 2019, 286, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Fogg, C.; Meredith, P.; Culliford, D.; Bridges, J.; Spice, C.; Griffiths, P. Cognitive impairment is independently associated with mortality, extended hospital stays and early readmission of older people with emergency hospital admissions: A retrospective cohort study. Int. J. Nurs. Stud. 2019, 96, 1–8. [Google Scholar] [CrossRef]
- Nagata, K.; Inoue, H.; Yamashita, T.; Akao, M.; Atarashi, H.; Ikeda, T.; Koretsune, Y.; Okumura, K.; Shimizu, W.; Suzuki, S.; et al. Impact of cognitive impairment on clinical outcomes in elderly p1atients with atrial fibrillation: ANAFIE Registry. BMJ Neurol. Open 2023, 5, e000370. [Google Scholar] [CrossRef]
- Anderson, R.E.; Birge, S.J. Cognitive Dysfunction, Medication Management, and the Risk of Readmission in Hospital Inpatients. J. Am. Geriatr. Soc. 2016, 64, 1464–1468. [Google Scholar] [CrossRef]
- Wang, P.J.; Lu, Y.; Mahaffey, K.W.; Lin, A.; Morin, D.P.; Sears, S.F.; Chung, M.K.; Russo, A.M.; Lin, B.; Piccini, J.; et al. Randomized Clinical Trial to Evaluate an Atrial Fibrillation Stroke Prevention Shared Decision-Making Pathway. J. Am. Heart Assoc. 2023, 12, e028562. [Google Scholar] [CrossRef]
- Flack, K.F.; Desai, J.; Kolb, J.M.; Chatterjee, P.; Wallentin, L.C.; Ezekowitz, M.; Yusuf, S.; Connolly, S.; Reilly, P.; Brueckmann, M.; et al. Major Gastrointestinal Bleeding Often Is Caused by Occult Malignancy in Patients Receiving Warfarin or Dabigatran to Prevent Stroke and Systemic Embolism From Atrial Fibrillation. Clin. Gastroenterol. Hepatol. 2017, 15, 682–690. [Google Scholar] [CrossRef]
- Yang, E.; Chang, M.A.; Savides, T.J. New Techniques to Control Gastrointestinal Bleeding. Gastroenterol. Hepatol. 2019, 15, 471–479. [Google Scholar]
- Ballestri, S.; Romagnoli, E.; Arioli, D.; Coluccio, V.; Marrazzo, A.; Athanasiou, A.; Di Girolamo, M.; Cappi, C.; Marietta, M.; Capitelli, M. Risk and Management of Bleeding Complications with Direct Oral Anticoagulants in Patients with Atrial Fibrillation and Venous Thromboembolism: A Narrative Review. Adv. Ther. 2023, 40, 41–66. [Google Scholar] [CrossRef]
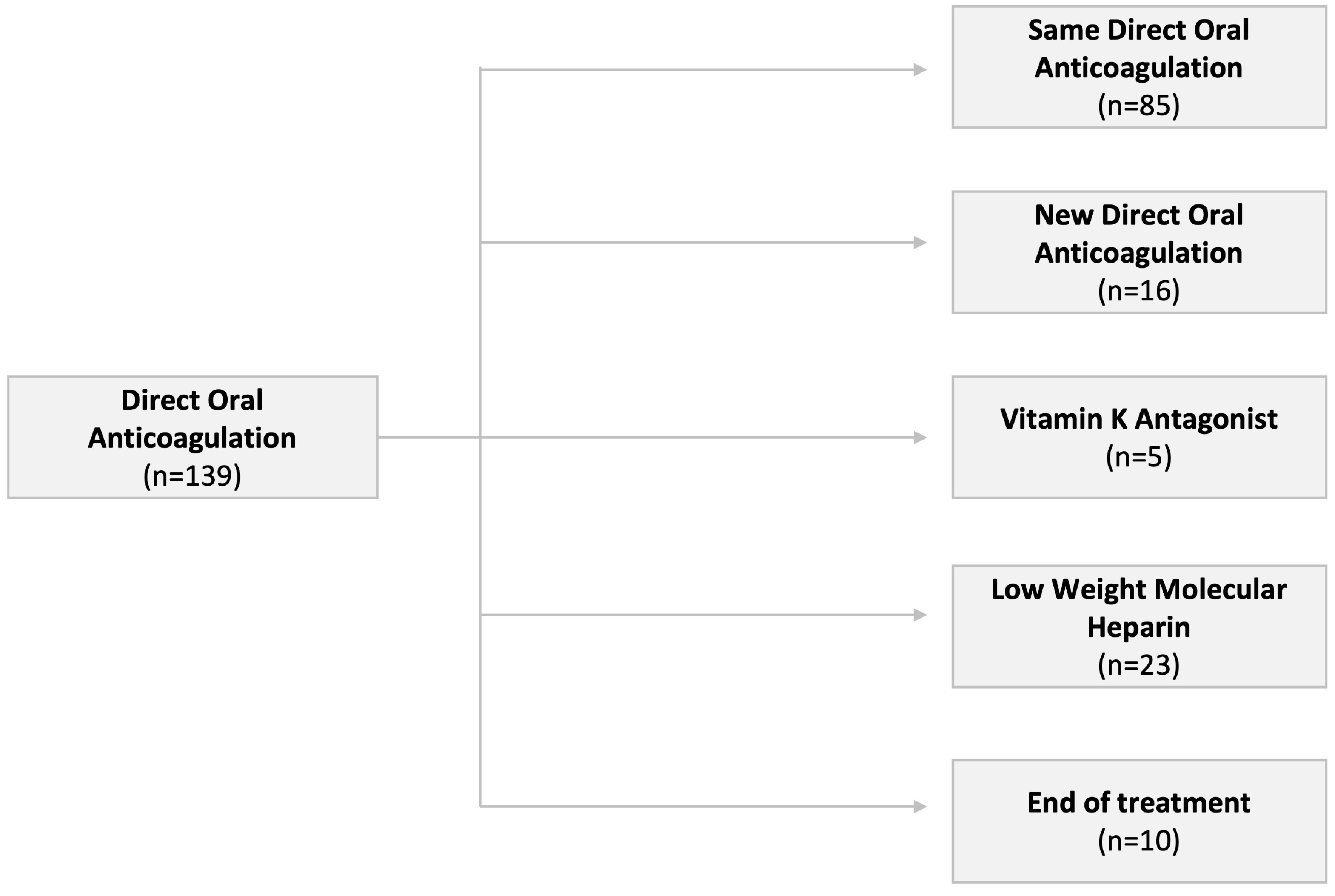
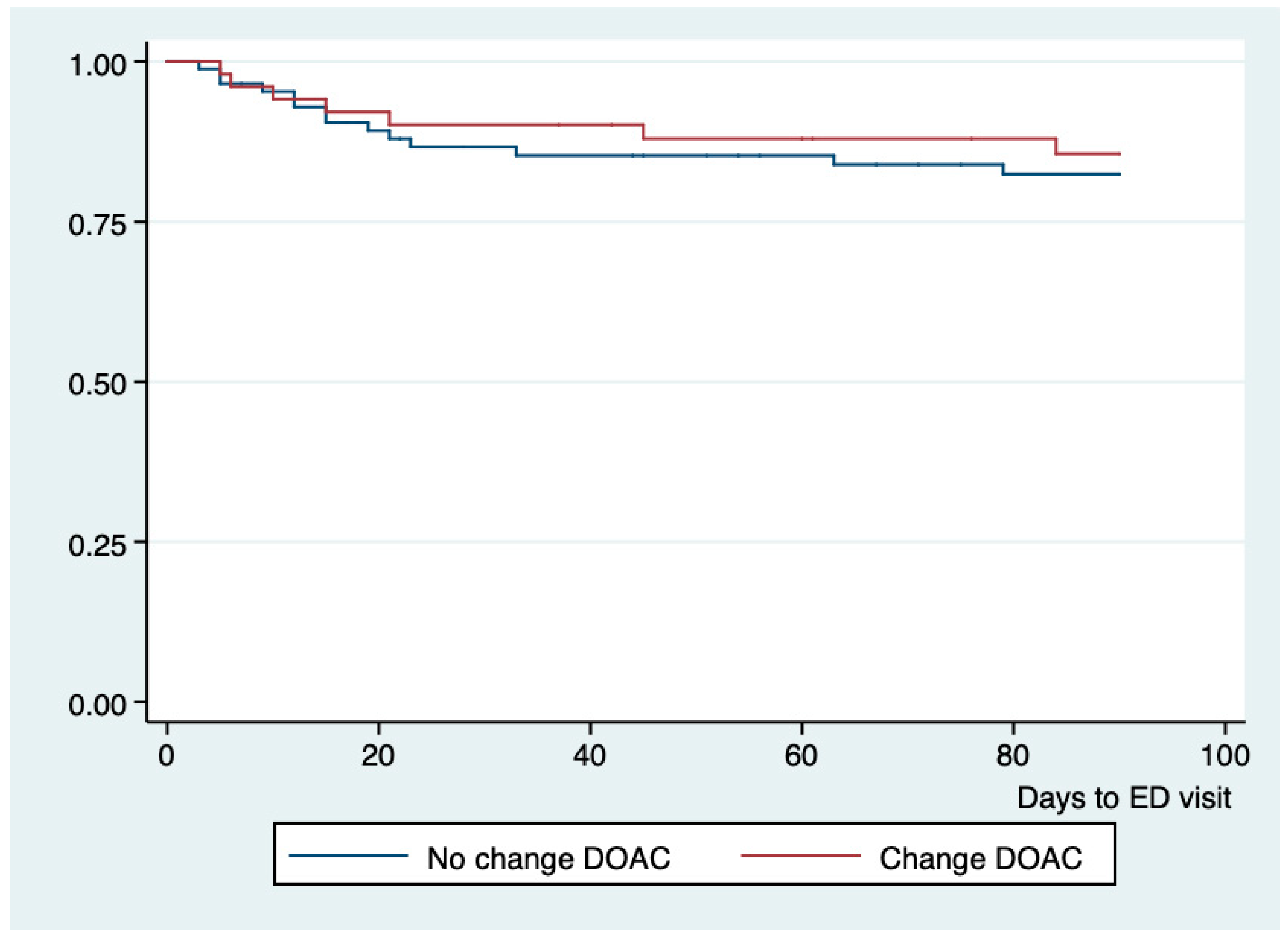
| Total | No Change (n = 85) | Change (n = 44) | End of Treatment (n = 10) | p | |
|---|---|---|---|---|---|
| Age (Mean; SD) | 84.6 (7.5) | 85.1 (6.1) | 83.6 (6.1) | 89.6 (7.1) | 0.265 |
| Female (%) | 85 (61.1) | 50 (58.8) | 28 (63.6) | 7 (70.0) | 0.408 |
| Comorbidities (%) | |||||
| Hypertension | 117 (84.1) | 71 (81.6) | 38 (86.4) | 8 (80.0) | 0.284 |
| Heart failure | 54 (38.8) | 34 (39.1) | 17 (31.5) | 3 (30.0) | 0.942 |
| Diabetes | 36 (25.9) | 19 (22.3) | 16 (33.3) | 1 (10.0) | 0.158 |
| COPD | 19 (13.8) | 13 (14.9) | 5 (11.6) | 1 (10.0) | 0.553 |
| Renal Chronic Failure III–V | 44 (31.6) | 24 (27.6) | 17 (38.6) | 2 (20.0) | 0.182 |
| Cognitive disorder | 37 (26.6) | 24 (27.6) | 11 (25.0) | 3 (30.0) | 0.353 |
| Severe CCI (>4 points) | 107 (77.0) | 66 (77.6) | 35 (79.6) | 8 (80.0) | 0.827 |
| Major bleeding (%) | 49 (35.2) | 25 (29.4) | 20 (45.4) | 4 (40.0) | 0.027 |
| Destination at discharge (%) | 0.197 | ||||
| Home | 102 (73.4) | 58 (68.2) | 38 (86.3) | 6 (60.0) | |
| Nursing home | 7 (5.0) | 4 (4.7) | 1 (2.2) | 2 (20.0) | |
| Long-term hospitalization | 30 (21.6) | 23 (27.1) | 5 (11.4) | 2 (20.0) | |
| Nº Drugs at discharge (Mean; SD) | 9.8 (3.4) | 9.7 (3.2) | 10.1 (3.3) | 10 (4.2) | 0.663 |
| Severe polypharmacy (>10 drugs) (%) | 61 (43.8) | 41 (47.1) | 17 (38.6) | 3 (30.0) | 0.319 |
| Antiplatelets (%) | 22 (15.9) | 18 (20.7) | 3 (7.0) | 1 (10.0) | 0.047 |
| Gastroprotection (%) | 62 (44.6) | 33 (37.9) | 23 (52.2) | 6 (60.0) | 0.041 |
| Univariate Analysis OR (IC95%) | p-Value | |
|---|---|---|
| Change in treatment | 0.63 (0.21–1.81) | 0.485 |
| End of treatment | 1.41 (0.28–4.749) | 0.655 |
| Age > 80 years | 1.44 (0.45–4.64) | 0.555 |
| Female | 0.82 (0.32–2.10) | 0.683 |
| Hypertension | 0.76 (0.23–2.54) | 0.662 |
| Chronic Heart Failure | 1.21 (0.47–3.12) | 0.683 |
| Diabetes | 1.17 (0.45–3.29) | 0.762 |
| COPD | 1.15 (0.39–2.13) | 0.645 |
| Renal Chronic Failure III–V | 1.09 (0.40–2.63) | 0.858 |
| Cognitive disorder | 2.96 (1.87–7.12) | <0.001 |
| Severe CCI | 3.23 (0.71–10.7) | 0.128 |
| Major bleeding | 0.58 (0.22–2.11) | 0.201 |
| Destination at discharge | 1.21 (0.29–4.67) | 0.863 |
| Severe polypharmacy (>10 drugs) | 1.16 (0.46–2.97) | 0.751 |
| Antiplatelets | 1.29 (0.39–4.29) | 0.764 |
| Gastroprotection | 1.81 (0.60–4.83) | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Ramos, J.; Pérez-Méndez, M.C.; Socias-Cañellas, C.M.; Lozano-Polo, L.; Plaza-Diaz, A.; Puig-Campmany, M.; Juanes-Borrego, A.M. Risk Factors for Emergency Room Visits in Patients with Digestive Bleeding Associated with Direct-Acting Anticoagulants. Emerg. Care Med. 2024, 1, 199-209. https://doi.org/10.3390/ecm1030021
Ruiz-Ramos J, Pérez-Méndez MC, Socias-Cañellas CM, Lozano-Polo L, Plaza-Diaz A, Puig-Campmany M, Juanes-Borrego AM. Risk Factors for Emergency Room Visits in Patients with Digestive Bleeding Associated with Direct-Acting Anticoagulants. Emergency Care and Medicine. 2024; 1(3):199-209. https://doi.org/10.3390/ecm1030021
Chicago/Turabian StyleRuiz-Ramos, Jesús, María Carmenza Pérez-Méndez, Catalina Maria Socias-Cañellas, Laura Lozano-Polo, Adrián Plaza-Diaz, Mireia Puig-Campmany, and Ana María Juanes-Borrego. 2024. "Risk Factors for Emergency Room Visits in Patients with Digestive Bleeding Associated with Direct-Acting Anticoagulants" Emergency Care and Medicine 1, no. 3: 199-209. https://doi.org/10.3390/ecm1030021
APA StyleRuiz-Ramos, J., Pérez-Méndez, M. C., Socias-Cañellas, C. M., Lozano-Polo, L., Plaza-Diaz, A., Puig-Campmany, M., & Juanes-Borrego, A. M. (2024). Risk Factors for Emergency Room Visits in Patients with Digestive Bleeding Associated with Direct-Acting Anticoagulants. Emergency Care and Medicine, 1(3), 199-209. https://doi.org/10.3390/ecm1030021







