Abstract
In this review paper, we will evaluate LRP4, a low-density lipoprotein receptor-related protein, and its many roles involving myasthenia gravis (MG), Wnt signaling, bone formation and craniofacial development. In MG, LRP4 is critical to the formation of the neuromuscular junction (NMJ) and the key function is to allow for controlled muscle contraction. LRP4 works in combination with agrin and MuSK to form the functional complex. In Wnt signaling, LRP4 was recently identified as a critical player in the pathway for both bone and tooth development and function. Its ability to act as an inhibitor sheds new light on bone formation and resorption. LRP4 binds sclerostin to LRP5 and LRP6, facilitating inhibitory effects important for bone homeostasis and remodeling. In this review paper, we will summarize the known roles of LRP4 as well as explore future directions for research surrounding LRP4 functionality.
1. Introduction
LRP4 is a low-density lipoprotein receptor-related protein involved in cell signaling in multiple tissues throughout the body. As part of the LDLR superfamily, LRP4 has been implicated in lipid metabolism [1,2], neuromuscular junction formation [3,4] and the regulation of bone mass [5]. Low-density lipoprotein receptor-related protein (LRP4, also MEF7) mutations have been linked to diseases such as Cenani–Lenz syndrome [6] (OMIM 212780), myasthenia gravis [7] (OMIM 254200) and sclerosteosis [8] (OMIM 614305). First identified for its role in neuromuscular junctions in 2006, LRP4 serves as a scaffolding protein in NMJs through critical interactions with Agrin–MuSK, and as a scaffold for the inhibition of Wnt signaling in bone [4,9]. Mutations in LRP4 also impact limb and kidney development, tooth development (Wnt and BMP signaling) and syndactyly [4,10,11,12,13]
The LDLR family is a class of cell-surface proteins involved in ligand binding, endocytosis, cholesterol homeostasis and other mechanisms [14,15]. Composed of many LRP proteins, including LRP1, LRP5, LRP6, LRP4, LRP8 and many others [16], LDLR protein family members contribute to a variety of signaling pathways and cellular mechanisms. In this review, we will analyze the current literature regarding the structure of LRP4 and the role of LRP4 in neuromuscular junctions and bone and craniofacial development.
2. Structure of LRP4 Protein
LRP4 is a membrane-anchored protein with a large extracellular domain consisting of eight ligand binding repeats, EGF repeats, and four β propeller domains (Figure 1). A transmembrane domain and short NPxY intracellular domain anchor the LRP4 protein and are necessary for endocytosis [17]. Unlike other LRP protein family members, the intracellular domain does not appear critical for LRP4 signaling function [18], although anchoring to the membrane is necessary for NMJ function [16].
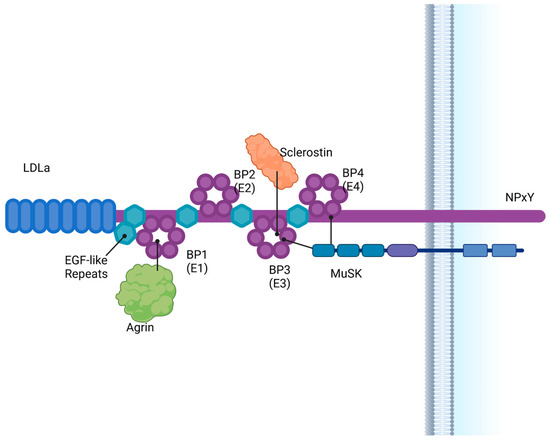
Figure 1.
LRP4 is a large intracellular membrane protein which consists of multiple EGF-like repeats, an LDLa repeat region, 4 beta-propeller domains and a short intracellular NPxY region. LRP4 interacts with ligands including Agrin, MuSK and sclerostin. BP = β propeller. Created in BioRender. Bullock, W. (2025). https://BioRender.com/k49w553 (accessed on 22 December 2024).
2.1. β-Propeller Domains
LRP4 is one of the large class LRP family members containing four YWTD β-propellers [19]. Numerous ligands are known to bind to the four β propeller domains of LRP4, which contain the binding pockets for Agrin, MuSK (muscle-specific kinase) and sclerostin [20]. Evidence suggests binding of Dkk1, Wise and APP in addition to Sost and Agrin [21,22,23]. Mutations in the N-terminal domain, specifically β propeller 1, prevent LRP4–Agrin binding and function, while mutations in the third and fourth β propellers decrease Agrin–MuSK binding affinity [24]. Mutations in the third β propeller have been tied to patients with sclerosteosis (high bone mass) [8,21,25], and recently a patient with sclerosteosis has been identified with a mutation in the first β propeller [16]. Mutations in patients with isolated syndactyly were linked to the fourth β propeller [16,24,26,27], indicating a link not only to bone homeostasis but also bone formation and development.
2.2. Intracellular/Transmembrane Domains
The intracellular domain is not necessary for Agrin/MuSK or Wnt signaling, but is implicated in limb development and syndactyly [18] suggesting membrane anchoring is important for LRP4 function. Modified LRP4 in mice with deletion of the LRP4 ECD showed substantial defects in limb patterning, whereas only mild limb patterning defects were observed in mice with an LRP4 ICD deletion. Mutations altering LRP4 trafficking to the membrane have been linked in Cenani–Lenz as well [27], suggesting that LRP4 serves primarily as an anchor protein in both Agrin–MuSK and Wnt signaling pathways.
3. LRP4 Is Critical for Agrin–MuSK Signaling in the Neuromuscular Junction
3.1. Myasthenia Gravis
Myasthenia gravis (MG) is a neuromuscular autoimmune disease defined by the production of autoantibodies [28]. Clinically, MG presents with a painless, fluctuant muscle weakness that progressively increases with repetitive muscle action. The amount of muscle weakness the body experiences is dependent on how much exertion the patient is putting on the muscle group in use. The muscle weakness patients experience can only decrease once the muscle is allowed to return to rest. MG affects both women and men, but characteristically each sex experiences an increase in symptoms in different decades of their lives. For women, MG occurs more frequently and onset begins in the second and third decades of life. In men, the onset of MG is in their fourth decade of life, with a second peak occurring in their sixth and seventh decades. The most affected muscle group are the ocular muscles. These muscles control eye movement which includes the extraocular muscles, intraocular muscles, and the muscles that control the movement of the upper eyelids. The importance of the ocular muscles can be broken down further, as the extraocular muscle group can be further categorized into an additional seven muscles, all supporting the movement and alignment of the eyes. Though this is most common, any type of voluntary muscle can become affected by MG. With disease progression found typically within the first 2 years, patients can also develop bulbar symptoms leading to facial, axial and limb weakness [28].
3.2. Neuromuscular Transmission
As previously mentioned, the body uses autoantibodies produced by MG against molecules involved in neuromuscular transmission (NMT). These molecules are Agrin, LRP4 and MuSK, which have been further described in a previous study [28]. The NMT pathway is responsible for the generation of an action potential leading to a final product of a muscle contraction. First, the neuron action potential causes depolarization of neuron terminals, allowing for the release of acetylcholine (ACh) into the synaptic cleft [29]. Next, ACh combines with receptor sites in the postjunctional membrane (PJM), causing depolarization of the PJM. Once the PJM is depolarized, the propagated muscle action potential is able to undergo excitation–contraction coupling, allowing for muscle tension development [29]. The impulse created by the NMT is transferred to the neuromuscular junction (NMJ), where the NMJ is then able to send the impulse from the nerve ending to the muscle. We look to the NMJ formation pathway to understand where MG originates and how LRP4 is connected to the development of this disease [30].
3.3. Agrin, LRP4 and MuSK
The NMJ is important as its primary role is to transfer signals from motor neurons to skeletal muscle fibers to allow for controlled muscle contraction [31]. In order for the NMJ to form, communication between the presynaptic motor neurons and postsynaptic muscle fibers is required (Figure 2). This communication is possible because of a nerve-derived organizer, Agrin, which allows for postsynaptic differentiation at the NMJ [30]. Agrin is a large glycoprotein that is able to regulate synaptic differentiation by activating mouse genome informatics (via MuSK) [4]. Neural Agrin is a specific form of Agrin produced by neurons and is responsible for developing and stabilizing the synapses at the NMJ. The pathway requires neural Agrin binding to LRP4, which then activates MuSK [32]. MuSK, a receptor tyrosine kinase selectively expressed by skeletal muscle, is essential for Agrin-induced clustering [31].
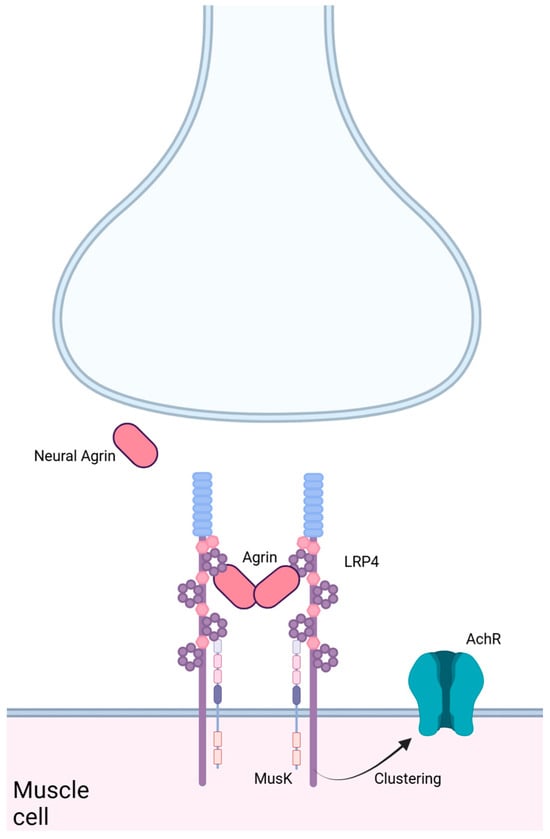
Figure 2.
LRP4 in the neuromuscular junction. LRP4 binds Agrin from the neural synapse and MuSK on the muscle cell surface, leading to clustering of the AchR. Created in BioRender. Bullock, W. (2025). https://BioRender.com/s61v264 (accessed on 22 December 2024).
Agrin and MuSK do not directly interact, which makes LRP4 crucial to the formation of the NMJ. Without LRP4 facilitation, Agrin and MuSK do not participate in NMJ signaling, thus eliminating controlled muscle contractions. In patients with MG, the body develops antibodies against the molecules Agrin, LRP4 and MuSK [31]. This indicates the importance of the Agrin-LRP4-MuSK signaling pathway because when just one of these molecules is removed, the NMJ cannot be formed [33]. Not only is LRP4 important for connecting Agrin and MuSK, but it has been suggested that LRP4 also regulates the stability of synaptic Agrin [31]. Synaptic Agrin can be derived from either neurons or muscle cells and plays an important role in postsynaptic differentiation and organization at the synapse. Barik et al. described an experiment in which LRP4 expression was eliminated in adult mice by generating inducible LRP4 mutant mice. The loss of LRP4 in the mice resulted in a loss of synaptic Agrin as well. These findings suggest that LRP4 is critical for maintaining the NMJ, in addition to its role in allowing neural Agrin to interact with MuSK. With these findings, we can conclude that without LRP4, the NMJ would not function properly. A non-functioning NMJ would prevent the transfer of signals from motor neurons to skeletal muscle fibers, therefore preventing a controlled muscle contraction, causing voluntary muscles to experience weakness with repetitive use.
4. LRP4 Is Necessary for Inhibition of Wnt Signaling in Bone
The Wnt signaling pathway has an extensive presence throughout developmental and regenerative cellular functions and is commonly studied within the context of bone mechanotransduction. Wnt signaling regulates bone modeling during embryogenesis as well as bone remodeling, which occurs throughout an individual’s lifespan [21,34]. This pathway’s critical role in skeletogenesis and the maintenance of bone structure and integrity renders it worthy of careful investigation. When dysregulated, the Wnt signaling pathway has been linked to a variety of degenerative and congenital conditions, such as osteoporosis [35]. Recent studies have discovered that LRP4 acts to facilitate the inhibition of the canonical Wnt pathway via interactions with sclerostin (SOST) and other antagonists [22]. Therefore, outlining the involvement of LRP4 in this cascade is valuable and could provide insights into various aspects of bone development and homeostasis.
4.1. Wnt Signaling
Wnt proteins are a class of 19 lipid-modified glycoproteins that play roles in various functions including development, tumorigenesis, bone mechanotransduction and vascularization/cardiovascular disease [36]. The unique functions of these ligands are further diversified as they interact with and activate the ten recognized Frizzled (Fz) receptors present in the Wnt signaling pathway [37,38]. The most commonly recognized Wnt cascade is the Wnt/β-catenin, or canonical, pathway, but several non-canonical pathways, such as the planar cell polarity cascade or the calcium-dependent pathway, also play a critical role in various cellular functions [36]. Several members of the LDLR superfamily possess critical roles in the functionality of the canonical Wnt signaling cascade, namely LRP5/6, as well as LRP4. Upon binding of Wnt ligands, LRP5/6 interact with and activate the transmembrane protein, Frizzled (Fz), which recruits a complex of intracellular proteins, glycogen synthase kinase 3-β (GSK-3), Axin, Dishevelled (Dsh) and Adenomatous Polyuposis Coli (APC) [34]. The association of this complex inhibits the formation of the β-catenin destruction complex consisting of APC, Axin, CK1-α and GSK-3β, which facilitates the sequestration, phosphorylation and ubiquitination of β-catenin [39]. The absence of this complex allows for the cytoplasmic accumulation of β-catenin and its eventual translocation into the nucleus. Upon its translocation, β-catenin binds to T cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factors to displace the bound transcriptional corepressors, thus permitting the translation of genes of interest [40]. In the absence of Wnt proteins, the β-catenin destruction complex degrades any β-catenin present in the cytosol and thus prevents the dissociation of transcriptional corepressors and gene transcription (Figure 3).
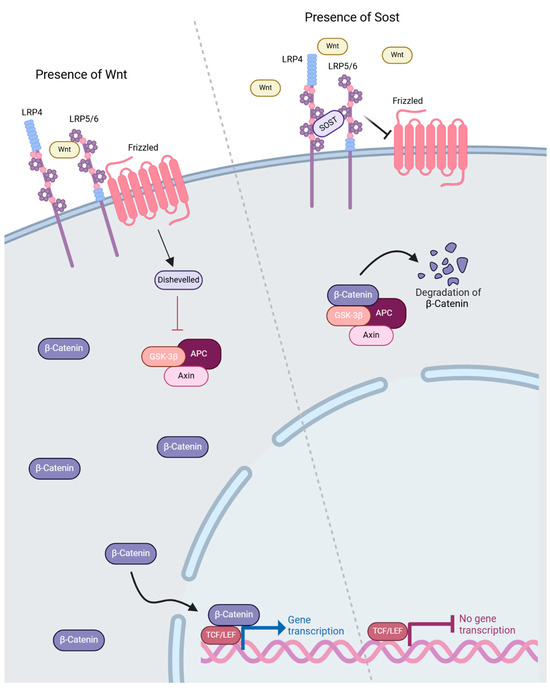
Figure 3.
LRP4 involvement in Wnt signaling. LRP4 facilitates binding of sclerostin by serving as an anchoring protein in bone cells and facilitates sclerostin–LRP5/6 interaction. This interaction inhibits canonical Wnt signaling via the inhibition of soluble Wnt binding to LRP5/6 and prevents Frizzled activation. Subsequent activity of the GSK3/Axin/APC complex results in the ubiquitination and degradation of -catenin [21]. Created in BioRender. Bullock, W. (2025). https://BioRender.com/b58u961 (accessed on 22 December 2024).
4.2. LRP4 Antagonists in Wnt Signaling
In recent years, LRP4 has emerged as a critical player in the regulation and function of the Wnt signaling pathway. Identified as a co-regulator and inhibitor of the cascade, LRP4 facilitates the binding of sclerostin (SOST) to LRP5/6 in order to decrease Wnt activity [21]. Several studies have observed that the overexpression of LRP4 in cultured cells results in a decrease in canonical Wnt activity, confirming that the role of LRP4 can be inhibitory in this context [6,23,41,42]. These inhibitory functions have not only been linked to interactions with sclerostin but also with other Wnt antagonists such as Wise (SOSTdc1/ectodin), Agrin and dickkopf-1 (DKK1) [21,22,23]. Various studies have explored the impact of LRP4 mutations on Wnt inhibition as well as disease-specific mutations that could provide information on additional functions of LRP4 [27].
Sclerostin (SOST), a glycoprotein secreted by osteocytes, is an inhibitor of the Wnt pathway and thus of osteoblastogenesis [43]. The current understanding of the function of LRP4 in Wnt inhibition and sclerosteosis is limited; however, the site of interaction between LRP4 and sclerostin has been identified within the central domain of the third β-propellor (3βCD) [8,21,25,27]. This binding pocket allows LRP4 to facilitate the binding of sclerostin to LRP5/6, deeming LRP4 a critical player in the regulation and inhibition of the Wnt pathway. Sclerostin-mediated regulation of the Wnt pathway is critical for bone homeostasis and remodeling, because it limits osteocyte and osteoblast activity [44]. Sclerosteosis, a skeletal hyperostosis disorder, is linked to loss-of-function mutations in SOST and causes distinct osteosclerosis in the skull, mandible and tubular bones [16,27,45,46,47]. Mutations in the third β-propeller domain of LRP4 (R1170W and W1186S) have been linked to decreased sclerostin inhibition of the Wnt pathway and have also been identified in patients with sclerosteosis [8,27]. Syndactyly, a rarer feature of sclerosteosis, is also seen in patients with Cenani–Lenz syndrome (CLS) and congenital myasthenia, indicating that there may be a connection between these mutations and the presentation of syndactyly [6,21,24,27].
Another antagonist of the Wnt pathway that has been discovered to interact with LRP4 is Wise (Sostdc1, ectodin), a secreted cysteine knot-containing protein [48]. Wise is expressed throughout development and has been recognized as both an inhibitor and contextually dependent activator of Wnt signaling by means of interactions with LRP4 and LRP6 [49,50]. Although the specific details of the interactions between Wise and LRP4 remain elusive, it has been made clear in various studies that the co-expression of LRP4 and Wise modulate Wnt signaling and play a significant role in dental and craniofacial development [23,41,49].
4.3. LRP4 in Craniofacial Development
LRP4 and Wise are co-expressed during tooth development and throughout the organogenesis of many craniofacial features [13,41]. In LRP4 and Wise mutant mice, malformed palatal rugae were detected, an observation that was linked to the likely involvement of Wise and LRP4 in regulating reaction–diffusion mechanisms through Shh and Fgf signaling [51]. Supernumerary incisors and molars, fused molars, abnormal cusp formation and calcified tissues within the pulp cavity were also observed in Wise/LRP4 mutant mice, indicating that tooth development can be interrupted by Wise through Wnt inhibition [10,52,53]. Sostdc1 knockout mice also presented with enlarged enamel knots, extra teeth in the vestigial premolar primordium and incisor region, and unique molar morphology, indicating that Wise plays a specific role in molar development [46,52,54,55].
Sclerostin and LRP4 are also highly correlated with craniofacial and dental development by modulating Wnt signaling. Two of the defining features of sclerosteosis include distinct mandibular overgrowth and midfacial hypoplasia, indicating that the sclerostin/LRP4 relationship may contribute to mandibular shape [10,56]. Sost knockout mice were observed to have an increased mandible length/maxilla length ratio as well as an increased mandibular body height and width [56]. These occurrences indicate that the LRP4/sclerostin relationship is highly impactful on craniofacial morphology and integrity, and offers exciting potential for future studies to discover targeted treatments in this area.
5. Future Directions
Given the critical role of LRP4 in neuromuscular junctions, bone development and maintenance, craniofacial and dental development, and limb patterning (Figure 4), it warrants further investigation as a key signaling molecule. LRP4 serves as a crucial anchor protein to facilitate Agrin–MuSK and Wnt signaling.
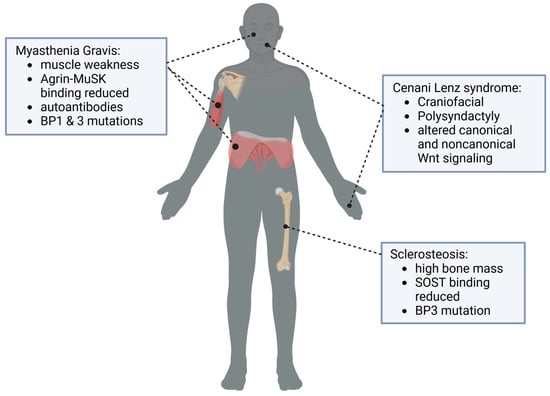
Figure 4.
LRP4 is critical for multiple body systems, including neuromuscular junction formation and Agrin/MuSK signaling, maintenance of bone mass, limb development and craniofacial development. Created in BioRender. Bullock, W. (2025). https://BioRender.com/y58o297 (accessed on 22 December 2024).
Recently, additional studies have identified a potential role of LRP4 in adipocyte and neural development [1], indicating a further role of LRP4 that has not been explored previously. Deletion of adipocyte-specific LRP4 in mice reduced white adipocyte size, although not total fat pad mass or Sostdc1 or Dkk1 levels, suggesting the LRP4-Sost interaction may play a crucial role in adipocyte size. Additionally, mice with a deletion of LRP4 in white adipocytes had improved glucose metabolism. These additional studies have indicated the role of LRP4 in lipid metabolism and adipocyte function [1], which should be further explored to provide further insight into the multi-faceted role of LRP4 in health and disease.
Patients with MG can also have higher levels of anti-LRP4 antibodies, indicating that LRP4 may serve as a novel target for the treatment of MG and potentially ALS [57]. Understanding the role and development of these autoantibodies can guide future efforts in the treatment of MG and ALS. LRP4 plays a critical role in many body systems and the development of disease. Further understanding LRP4 function in neuromuscular junctions, bone and craniofacial development, and adipogenesis holds promise for future treatments.
Author Contributions
Conceptualization, W.B.; writing—original draft preparation, W.B., A.D. and T.B.; writing—review and editing, W.B., A.D. and T.B; visualization, A.D.; supervision, W.B.; funding acquisition, W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Thanks go to the Eckerd College Natural Sciences Collegium for supporting the authors through the Natural Sciences Summer Research Program, and to Eckerd College for their support through the Ford Scholars Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, S.P.; Da, H.; Li, Z.; Kushwaha, P.; Beil, C.; Mei, L.; Xiong, W.-C.; Wolfgang, M.J.; Clemens, T.L.; Riddle, R.C. Lrp4 Expression by Adipocytes and Osteoblasts Differentially Impacts Sclerostin’s Endocrine Effects on Body Composition and Glucose Metabolism. J. Biol. Chem. 2019, 294, 6899–6911. [Google Scholar] [CrossRef]
- Jiang, H.; Li, D.; Wang, L.; Zhang, N.; Yu, S.; Zhang, H.; Liu, J.; Ma, D.; Lu, A.; Sheng, H.; et al. Sclerostin Loop3-LRP4 Interaction Required by Sclerostin for Lipid and Glucose Metabolism Impairment in Adipocyte. Metabolism 2023, 142, 155432. [Google Scholar] [CrossRef]
- Beffert, U.; Stolt, P.C.; Herz, J. Functions of Lipoprotein Receptors in Neurons. J. Lipid Res. 2004, 45, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Weatherbee, S.D.; Anderson, K.V.; Niswander, L.A. LDL-Receptor-Related Protein 4 Is Crucial for Formation of the Neuromuscular Junction. Development 2006, 133, 4993–5000. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, W.-C.; Mei, L. LRP4 in Neuromuscular Junction and Bone Development and Diseases. Bone 2015, 80, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pawlik, B.; Elcioglu, N.; Aglan, M.; Kayserili, H.; Yigit, G.; Percin, F.; Goodman, F.; Nürnberg, G.; Cenani, A.; et al. LRP4 Mutations Alter Wnt/β-Catenin Signaling and Cause Limb and Kidney Malformations in Cenani-Lenz Syndrome. Am. J. Hum. Genet. 2010, 86, 696–706. [Google Scholar] [CrossRef] [PubMed]
- CELESIA, G.G. Myasthenia Gravis in Two Siblings. Arch. Neurol. 1965, 12, 206–210. [Google Scholar] [CrossRef]
- Leupin, O.; Piters, E.; Halleux, C.; Hu, S.; Kramer, I.; Morvan, F.; Bouwmeester, T.; Schirle, M.; Bueno-Lozano, M.; Ramos Fuentes, F.J.; et al. Bone Overgrowth-Associated Mutations in the LRP4 Gene Impair Sclerostin Facilitator Function. J. Biol. Chem. 2011, 286, 19489–19500. [Google Scholar] [CrossRef]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 Is a Receptor for Agrin and Forms a Complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Porntaveetus, T.; Ota, M.S.; Herz, J.; Sharpe, P.T. Lrp4: A Novel Modulator of Extracellular Signaling in Craniofacial Organogenesis. Am. J. Med. Genet. Part A 2010, 152A, 2974. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Dieckmann, M.; Herz, J.; Niemeier, A. Lrp4, a Novel Receptor for Dickkopf 1 and Sclerostin, Is Expressed by Osteoblasts and Regulates Bone Growth and Turnover In Vivo. PLoS ONE 2009, 4, e7930. [Google Scholar] [CrossRef]
- Karner, C.M.; Dietrich, M.F.; Johnson, E.B.; Kappesser, N.; Tennert, C.; Percin, F.; Wollnik, B.; Carroll, T.J.; Herz, J. Lrp4 Regulates Initiation of Ureteric Budding and Is Crucial for Kidney Formation—A Mouse Model for Cenani-Lenz Syndrome. PLoS ONE 2010, 5, e10418. [Google Scholar] [CrossRef]
- Laurikkala, J.; Kassai, Y.; Pakkasjärvi, L.; Thesleff, I.; Itoh, N. Identification of a Secreted BMP Antagonist, Ectodin, Integrating BMP, FGF, and SHH Signals from the Tooth Enamel Knot. Dev. Biol. 2003, 264, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Go, G.; Mani, A. Low-Density Lipoprotein Receptor (LDLR) Family Orchestrates Cholesterol Homeostasis. Yale J. Biol. Med. 2012, 85, 19. [Google Scholar]
- Chen, B.-H.; Lin, Z.-Y.; Zeng, X.-X.; Jiang, Y.-H.; Geng, F. LRP4-Related Signalling Pathways and Their Regulatory Role in Neurological Diseases. Brain Res. 2024, 1825, 148705. [Google Scholar] [CrossRef]
- Huybrechts, Y.; Boudin, E.; Hendrickx, G.; Steenackers, E.; Hamdy, N.; Mortier, G.; Martínez Díaz-Guerra, G.; Bracamonte, M.S.; Appelman-Dijkstra, N.M.; Van Hul, W. Identification of Compound Heterozygous Variants in LRP4 Demonstrates That a Pathogenic Variant Outside the Third β-Propeller Domain Can Cause Sclerosteosis. Genes 2022, 13, 80. [Google Scholar] [CrossRef]
- Chen, W.J.; Goldstein, J.L.; Brown, M.S. NPXY, a Sequence Often Found in Cytoplasmic Tails, Is Required for Coated Pit-Mediated Internalization of the Low Density Lipoprotein Receptor. J. Biol. Chem. 1990, 265, 3116–3123. [Google Scholar] [CrossRef]
- Pohlkamp, T.; Durakoglugil, M.; Lane-Donovan, C.; Xian, X.; Johnson, E.B.; Hammer, R.E.; Herz, J. Lrp4 Domains Differentially Regulate Limb/Brain Development and Synaptic Plasticity. PLoS ONE 2015, 10, e0116701. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Dagil, R.; Kragelund, B.B. New Horizons for Lipoprotein Receptors: Communication by β-Propellers. J. Lipid Res. 2013, 54, 2763–2774. [Google Scholar] [CrossRef]
- Zhang, W.; Coldefy, A.-S.; Hubbard, S.R.; Burden, S.J. Agrin Binds to the N-Terminal Region of Lrp4 Protein and Stimulates Association between Lrp4 and the First Immunoglobulin-like Domain in Muscle-Specific Kinase (MuSK). J. Biol. Chem. 2011, 286, 40624–40630. [Google Scholar] [CrossRef] [PubMed]
- Bullock, W.A.; Hoggatt, A.M.; Horan, D.J.; Elmendorf, A.J.; Sato, A.Y.; Bellido, T.; Loots, G.G.; Pavalko, F.M.; Robling, A.G. Lrp4 Mediates Bone Homeostasis and Mechanotransduction through Interaction with Sclerostin In Vivo. iScience 2019, 20, 205–215. [Google Scholar] [CrossRef]
- Xiong, L.; Jung, J.-U.; Wu, H.; Xia, W.-F.; Pan, J.-X.; Shen, C.; Mei, L.; Xiong, W.-C. Lrp4 in Osteoblasts Suppresses Bone Formation and Promotes Osteoclastogenesis and Bone Resorption. Proc. Natl. Acad. Sci. USA 2015, 112, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Sims, C.; Murray, M.J.; Kuhlmann, P.K.; Fuentes-Antrás, J.; Weatherbee, S.D.; Krumlauf, R. Multiple Modes of Lrp4 Function in Modulation of Wnt/β-Catenin Signaling during Tooth Development. Development 2017, 144, 2824–2836. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, B.; Cabrera-Serrano, M.; Nakata, T.; Milone, M.; Asai, N.; Ito, K.; Ito, M.; Masuda, A.; Ito, Y.; Engel, A.G.; et al. LRP4 Third β-Propeller Domain Mutations Cause Novel Congenital Myasthenia by Compromising Agrin-Mediated MuSK Signaling in a Position-Specific Manner. Hum. Mol. Genet. 2014, 23, 1856–1868. [Google Scholar] [CrossRef]
- Boudin, E.; Yorgan, T.; Fijalkowski, I.; Sonntag, S.; Steenackers, E.; Hendrickx, G.; Peeters, S.; De Maré, A.; Vervaet, B.; Verhulst, A.; et al. The Lrp4 R1170Q Homozygous Knock-In Mouse Recapitulates the Bone Phenotype of Sclerosteosis in Humans: LRP4 R1170Q HOMOZYGOUS KI MOUSE RECAPITULATES HUMAN SCLEROSTEOSIS. J. Bone Miner. Res. 2017, 32, 1739–1749. [Google Scholar] [CrossRef]
- Sukenik Halevy, R.; Chien, H.-C.; Heinz, B.; Bamshad, M.J.; Nickerson, D.A.; University of Washington Center for Mendelian Genomics; Kircher, M.; Ahituv, N. Mutations in the Fourth β-Propeller Domain of LRP4 Are Associated with Isolated Syndactyly with Fusion of the Third and Fourth Fingers. Hum. Mutat. 2018, 39, 811–815. [Google Scholar] [CrossRef]
- Fijalkowski, I.; Geets, E.; Steenackers, E.; Van Hoof, V.; Ramos, F.J.; Mortier, G.; Fortuna, A.M.; Van Hul, W.; Boudin, E. A Novel Domain-Specific Mutation in a Sclerosteosis Patient Suggests a Role of LRP4 as an Anchor for Sclerostin in Human Bone. J. Bone Miner. Res. 2016, 31, 874–881. [Google Scholar] [CrossRef]
- Catalin, J.; Silviana, J. Barsan Claudia Clinical Presentation of Myasthenia Gravis. In Thymus; Rezaei, N., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 8; ISBN 978-1-78985-134-2. [Google Scholar]
- Fagerlund, M.J.; Eriksson, L.I. Current Concepts in Neuromuscular Transmission. Br. J. Anaesth. 2009, 103, 108–114. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, S.; Wang, Q.; Suzuki, T.; Xiong, W.C.; Mei, L. LRP4 Serves as a Coreceptor of Agrin. Neuron 2008, 60, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Lu, Y.; Sathyamurthy, A.; Bowman, A.; Shen, C.; Li, L.; Xiong, W.; Mei, L. LRP4 Is Critical for Neuromuscular Junction Maintenance. J. Neurosci. 2014, 34, 13892–13905. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.R.; Canciani, A.; Forneris, F. Dissecting the Extracellular Complexity of Neuromuscular Junction Organizers. Front. Mol. Biosci. 2020, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xing, G.; Xiong, W.; Mei, L. Agrin and LRP4 Antibodies as New Biomarkers of Myasthenia Gravis. Ann. N. Y. Acad. Sci. 2018, 1413, 126–135. [Google Scholar] [CrossRef]
- Wang, Y. Wnt and the Wnt Signaling Pathway in Bone Development and Disease. Front. Biosci. 2014, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/β-Catenin Signaling Is Required for Normal Bone Homeostasis. Mol. Cell. Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef]
- Kornsuthisopon, C.; Photichailert, S.; Nowwarote, N.; Tompkins, K.A.; Osathanon, T. Wnt Signaling in Dental Pulp Homeostasis and Dentin Regeneration. Arch. Oral Biol. 2022, 134, 105322. [Google Scholar] [CrossRef]
- Dijksterhuis, J.P.; Petersen, J.; Schulte, G. WNT/Frizzled Signalling: Receptor–Ligand Selectivity with Focus on FZD-G Protein Signalling and Its Physiological Relevance: IUPHAR Review 3. Br. J. Pharmacol. 2014, 171, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bakeri, H.; Li, T.; Swaroop, A. Regulation of Retinal Progenitor Expansion by Frizzled Receptors: Implications for Microphthalmia and Retinal Coloboma. Hum. Mol. Genet. 2012, 21, 1848–1860. [Google Scholar] [CrossRef]
- Parker, T.W.; Neufeld, K.L. APC Controls Wnt-Induced β-Catenin Destruction Complex Recruitment in Human Colonocytes. Sci. Rep. 2020, 10, 2957. [Google Scholar] [CrossRef]
- Krishnan, V. Regulation of Bone Mass by Wnt Signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef]
- Ohazama, A.; Johnson, E.B.; Ota, M.S.; Choi, H.J.; Porntaveetus, T.; Oommen, S.; Itoh, N.; Eto, K.; Gritli-Linde, A.; Herz, J.; et al. Lrp4 Modulates Extracellular Integration of Cell Signaling Pathways in Development. PLoS ONE 2008, 3, e4092. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.B.; Steffen, D.J.; Lynch, K.W.; Herz, J. Defective Splicing of Megf7/Lrp4, a Regulator of Distal Limb Development, in Autosomal Recessive Mulefoot Disease. Genomics 2006, 88, 600–609. [Google Scholar] [CrossRef][Green Version]
- Silverman, S.L. Sclerostin. J. Osteoporos. 2010, 2010, 941419. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin Mediates Bone Response to Mechanical Unloading Through Antagonizing Wnt/Β-Catenin Signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ominsky, M.S.; Niu, Q.-T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Collette, N.M.; Genetos, D.C.; Economides, A.N.; Xie, L.; Shahnazari, M.; Yao, W.; Lane, N.E.; Harland, R.M.; Loots, G.G. Targeted Deletion of Sost Distal Enhancer Increases Bone Formation and Bone Mass. Proc. Natl. Acad. Sci. USA 2012, 109, 14092–14097. [Google Scholar] [CrossRef]
- Balemans, W. Increased Bone Density in Sclerosteosis Is Due to the Deficiency of a Novel Secreted Protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, R.F.L.; Yeo, W.; Gautier, J.; Jahoda, C.A.B.; Christiano, A.M. The WNT Signalling Modulator, Wise, Is Expressed in an Interaction-Dependent Manner During Hair-Follicle Cycling. J. Investig. Dermatol. 2004, 123, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Itasaki, N.; Jones, C.M.; Mercurio, S.; Rowe, A.; Domingos, P.M.; Smith, J.C.; Krumlauf, R. Wise, a Context-Dependent Activator and Inhibitor of Wnt Signalling. Development 2003, 130, 4295–4305. [Google Scholar] [CrossRef] [PubMed]
- Lintern, K.B.; Guidato, S.; Rowe, A.; Saldanha, J.W.; Itasaki, N. Characterization of Wise Protein and Its Molecular Mechanism to Interact with Both Wnt and BMP Signals. J. Biol. Chem. 2009, 284, 23159–23168. [Google Scholar] [CrossRef]
- Kawasaki, M.; Kawasaki, K.; Meguro, F.; Yamada, A.; Ishikawa, R.; Porntaveetus, T.; Blackburn, J.; Otsuka-Tanaka, Y.; Saito, N.; Ota, M.S.; et al. Lrp4/Wise Regulates Palatal Rugae Development through Turing-Type Reaction-Diffusion Mechanisms. PLoS ONE 2018, 13, e0204126. [Google Scholar] [CrossRef]
- Kassai, Y.; Munne, P.; Hotta, Y.; Penttilä, E.; Kavanagh, K.; Ohbayashi, N.; Takada, S.; Thesleff, I.; Jernvall, J.; Itoh, N. Regulation of Mammalian Tooth Cusp Patterning by Ectodin. Science 2005, 309, 2067–2070. [Google Scholar] [CrossRef]
- Ahn, Y.; Sanderson, B.W.; Klein, O.D.; Krumlauf, R. Inhibition of Wnt Signaling by Wise (Sostdc1) and Negative Feedback from Shh Controls Tooth Number and Patterning. Development 2010, 137, 3221–3231. [Google Scholar] [CrossRef]
- Munne, P.M.; Tummers, M.; Järvinen, E.; Thesleff, I.; Jernvall, J. Tinkering with the Inductive Mesenchyme: Sostdc1 Uncovers the Role of Dental Mesenchyme in Limiting Tooth Induction. Development 2009, 136, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Murashima-Suginami, A.; Takahashi, K.; Sakata, T.; Tsukamoto, H.; Sugai, M.; Yanagita, M.; Shimizu, A.; Sakurai, T.; Slavkin, H.C.; Bessho, K. Enhanced BMP Signaling Results in Supernumerary Tooth Formation in USAG-1 Deficient Mouse. Biochem. Biophys. Res. Commun. 2008, 369, 1012–1016. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, X.; Pilawski, I.; Liu, X.; Delgado-Calle, J.; Bellido, T.; Turkkahraman, H.; Helms, J.A. Molecular Basis for Craniofacial Phenotypes Caused by Sclerostin Deletion. J. Dent. Res. 2020, 100, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tzartos, J.S.; Zisimopoulou, P.; Rentzos, M.; Karandreas, N.; Zouvelou, V.; Evangelakou, P.; Tsonis, A.; Thomaidis, T.; Lauria, G.; Andreetta, F.; et al. LRP 4 Antibodies in Serum and CSF from Amyotrophic Lateral Sclerosis Patients. Ann. Clin. Transl. Neurol. 2014, 1, 80–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).