The Third Dimension of Eye Care: A Comprehensive Review of 3D Printing in Ophthalmology
Abstract
1. Introduction
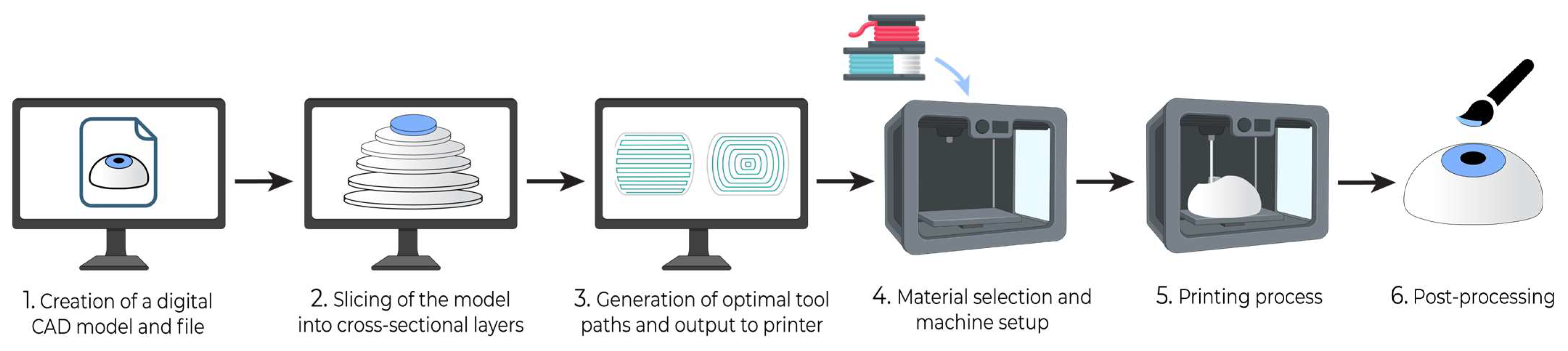
2. Build Instructions for 3D Printing
- Production of a computer-aided design (CAD) model: Each 3D printing project begins with a digital model of the intended product. This model is typically created using computer-aided design (CAD) software or by generating a 3D representation based on CT or MRI scans of an existing object. The geometric data of the 3D model can be stored in a standardized .STL or .OBJ file format (Figure 1).
- Slicing and preparation of print file: Slicing software is then utilized to divide the 3D model into thin horizontal cross-sections, creating a digital representation of each layer to be printed. This step enables the specification of layer height, infill density, and print speed. If the design contains layers that overhang previous layers, it may require the use of support structures, which can be inserted by slicing software to maintain structural integrity against gravity.
- Toolpath Generation and Output: Based on the shape of successive layers, slicing software will generate toolpaths for the 3D printer that contain ordered coordinate directions to create the surface geometry, interior fill pattern, and support structures. Toolpaths are converted into control code specific to the 3D printer that guides the printing process.
- Material Selection and Machine Setup: The printer will be loaded with material and prepared for the physical build process. With the material diversity afforded by 3D printing, the appropriate material should be selected for the desired functional properties of the product.
- 3D Printing Process: The printer will then work layer by layer to additively manufacture the product. The 3D printer largely automates this step, and many have integrated control units that monitor the printing process and alert the user if an issue requiring intervention occurs. Once printing is completed, further steps may include product separation from the build platform and safe handling precautions.
- Postprocessing: After printing, the 3D model may require postprocessing, such as polishing, further curing, chemical treatment, coloring, and the removal of support structures, depending on the target function and appearance requirements. Support structures may be removed manually or via dissolution with a targeted solvent.
3. Three-Dimensional Printing Techniques
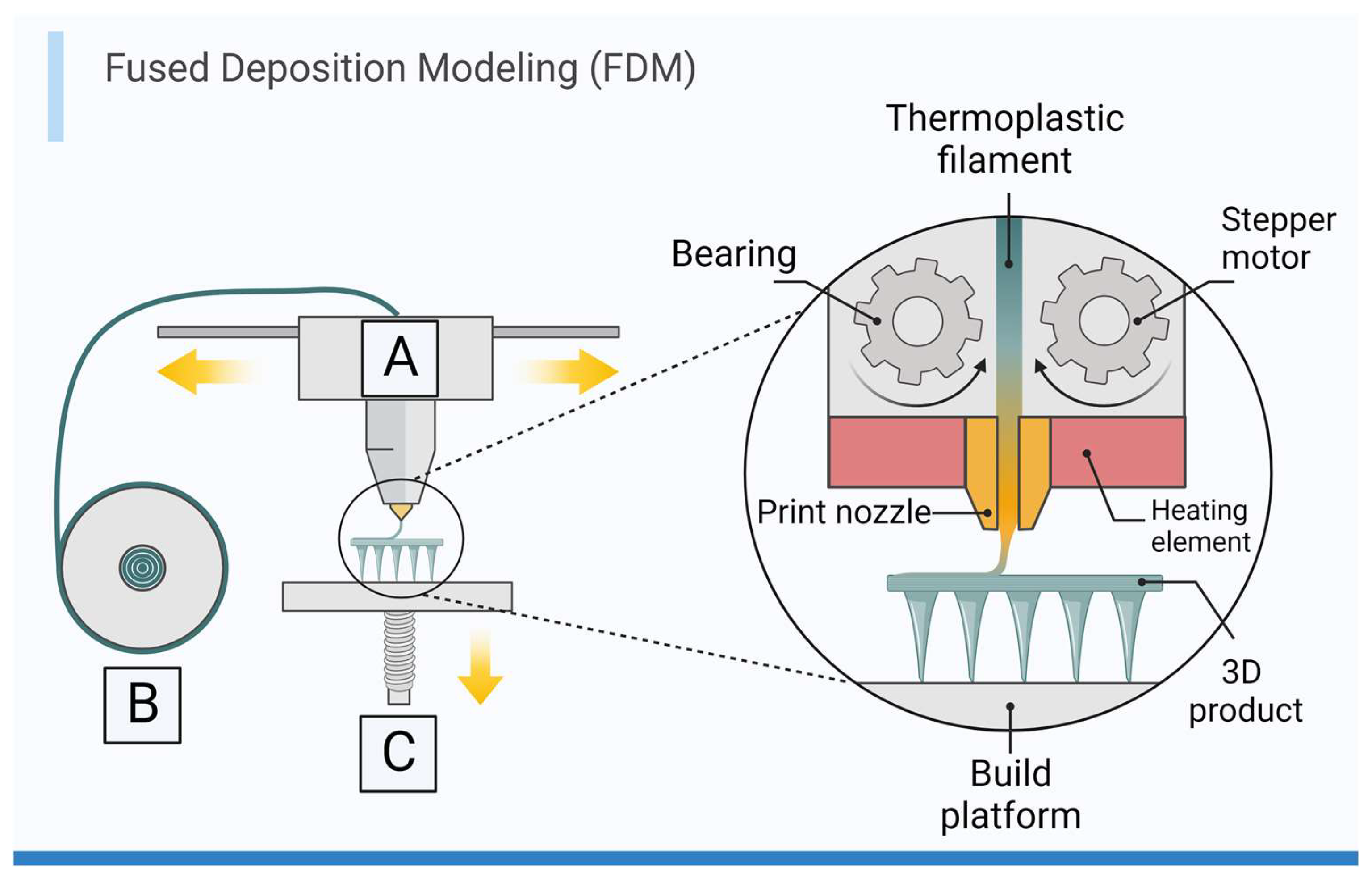
3.1. Extrusion-Based Printing
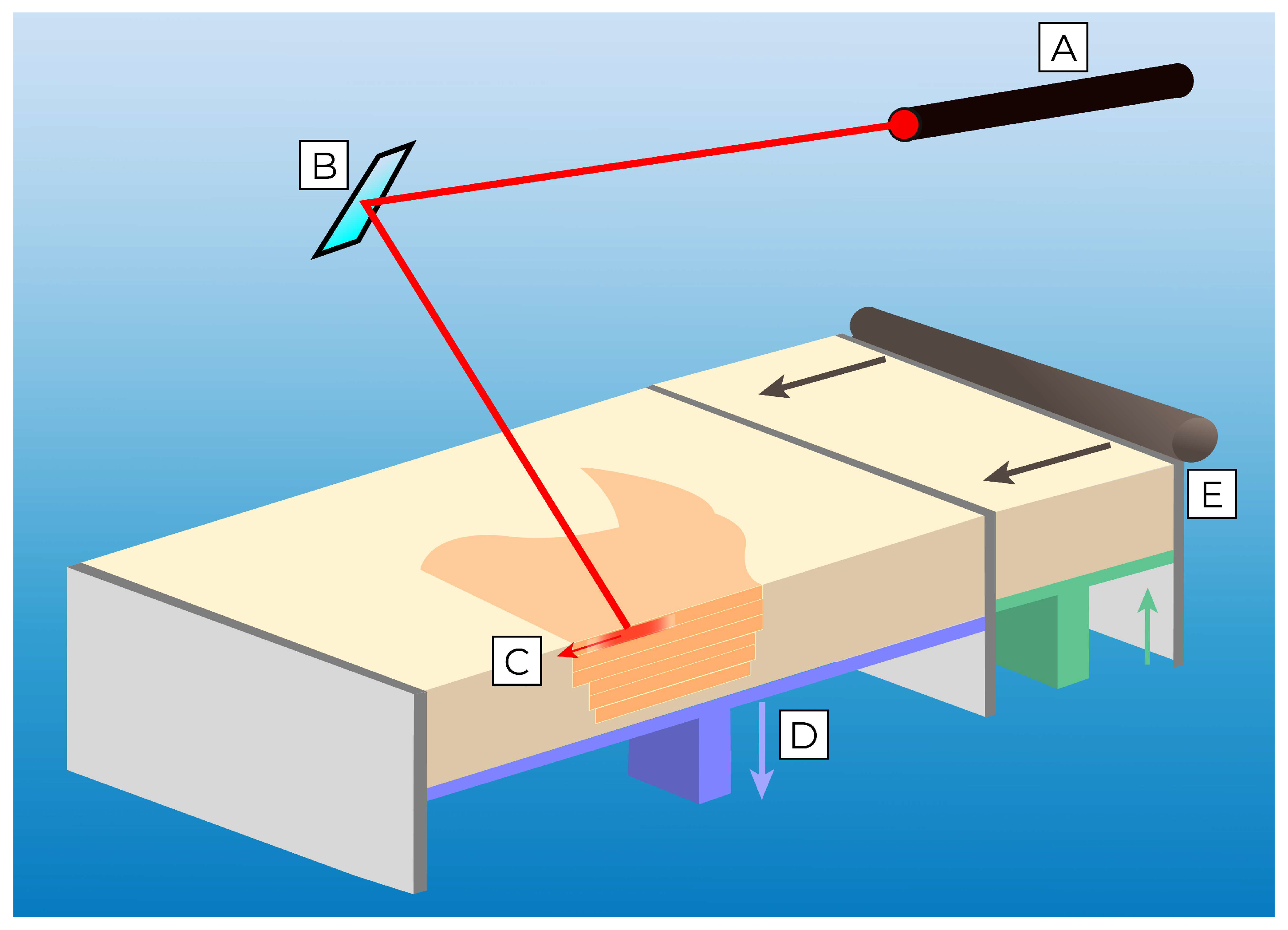
3.2. Powder Bed Fusion Printing
3.3. Binder Jetting
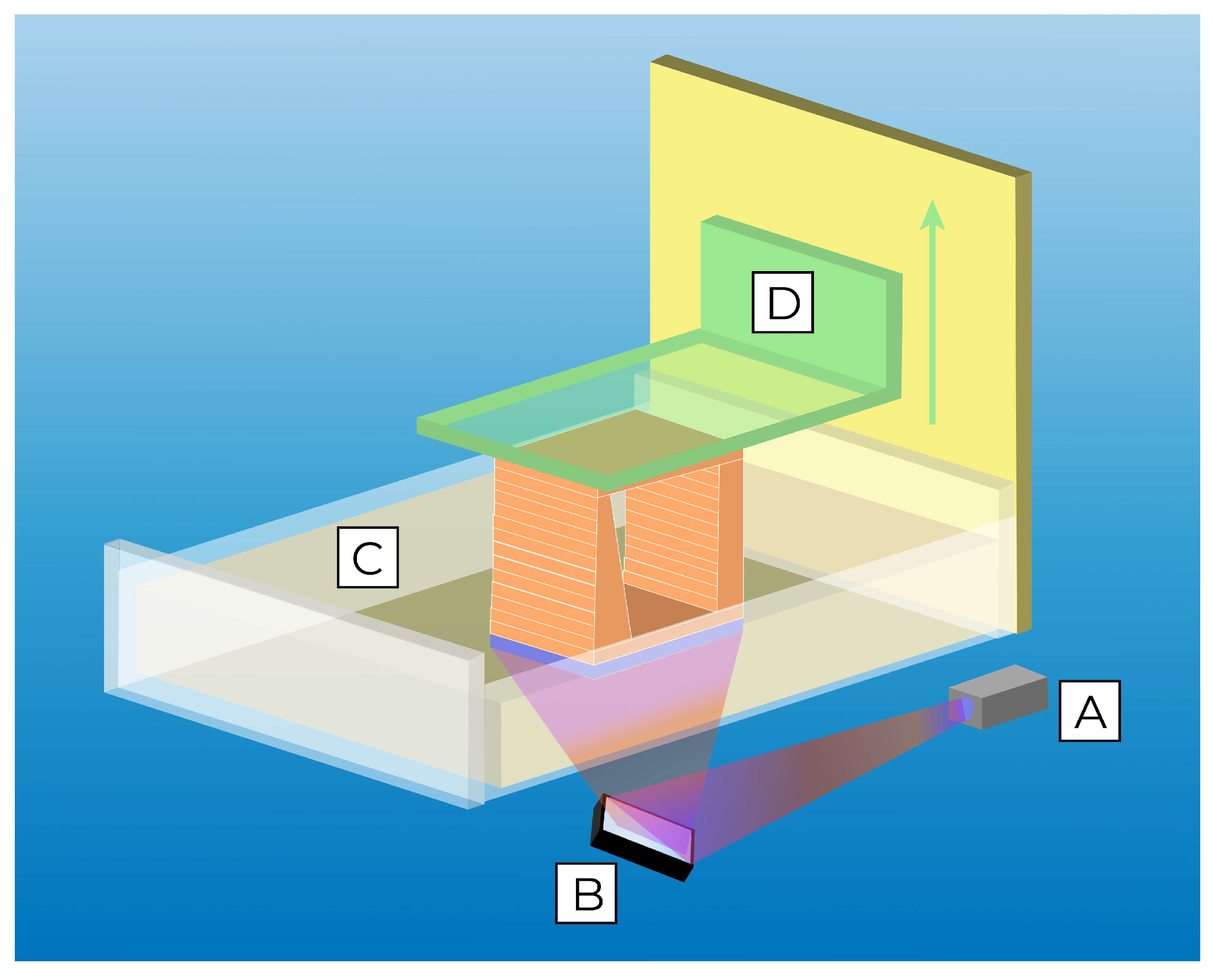
3.4. Vat-Polymerization Printing
3.5. Material Jetting
4. Applications of 3D Printing in Ophthalmology
4.1. Three-Dimensional Printing in Ophthalmic Implants and Prosthetics
4.2. Educational and Anatomical Models
4.3. Surgical Planning and Training
4.4. Drug-Delivery Systems and 4D Printing
4.4.1. Drug-Eluting Implants
4.4.2. Drug-Eluting Contact Lenses
4.5. Four-Dimensional Orbital Implants
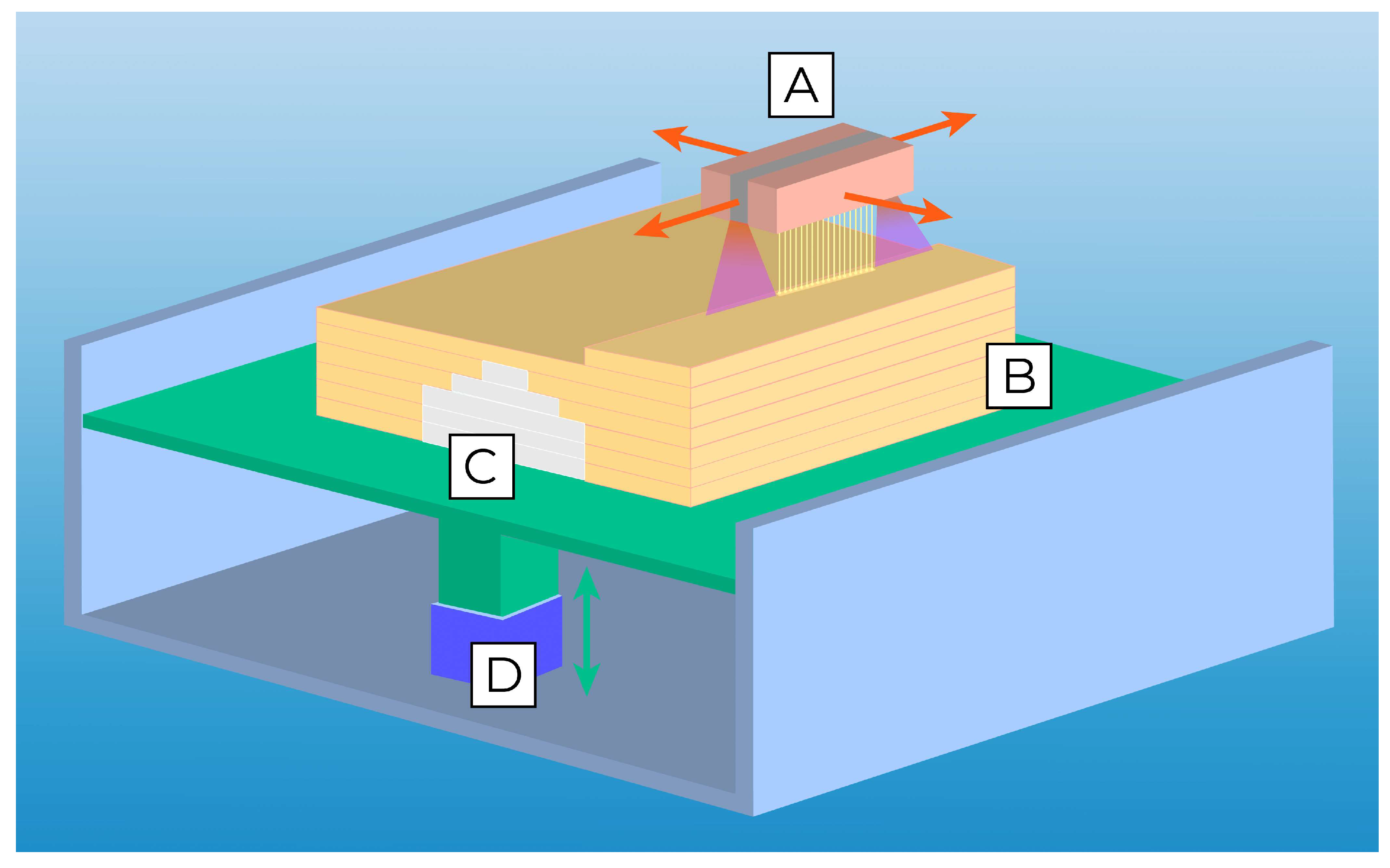
4.6. Adaptive Optics
- (1)
- A wavefront sensor to qualify and quantify the optical aberrations in the light reflected by the eye;
- (2)
- A deformable mirror to correct the identified abnormalities;
- (3)
- A control system to calculate the necessary correction amount and to provide feedback, and;
- (4)
- A processing device to create an image based on the corrected waveform.
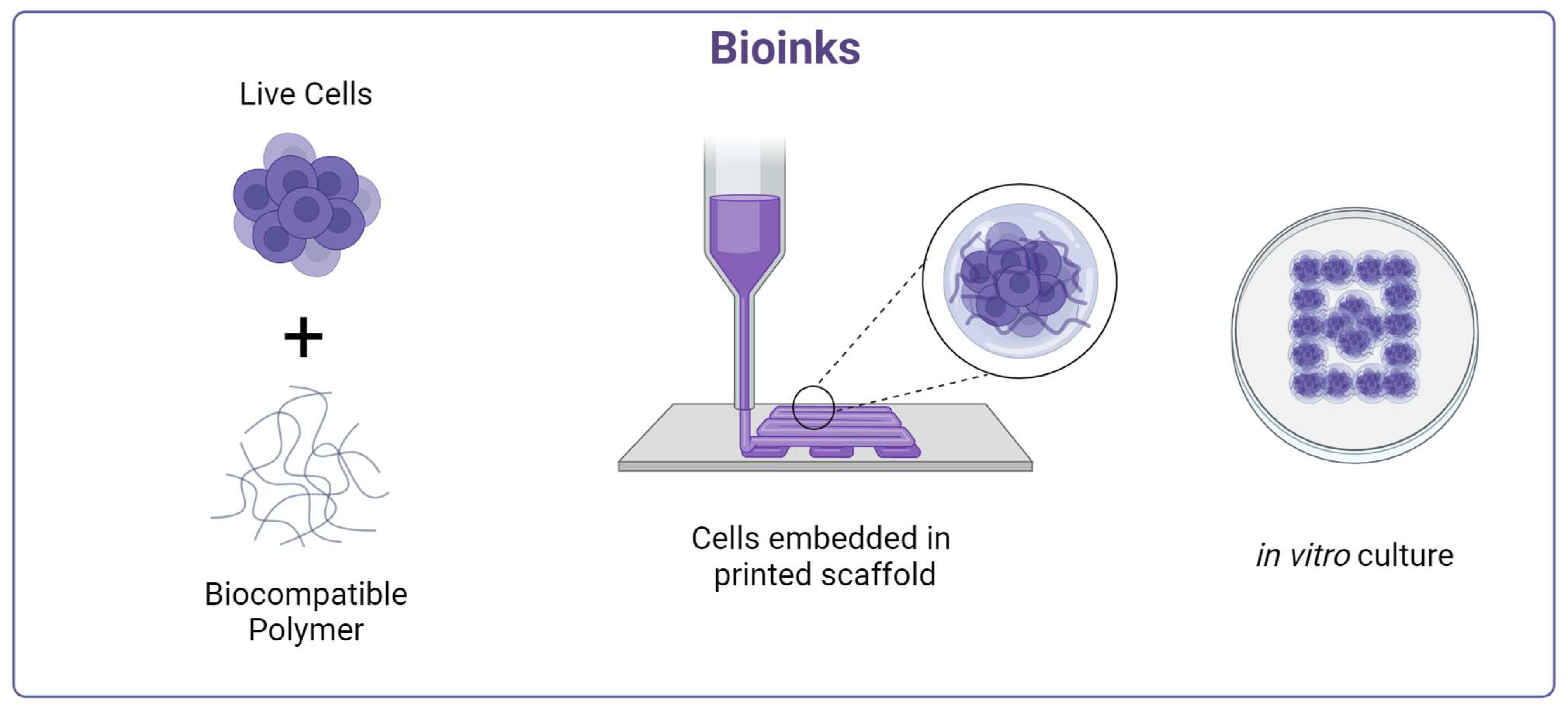
5. Bioprinting
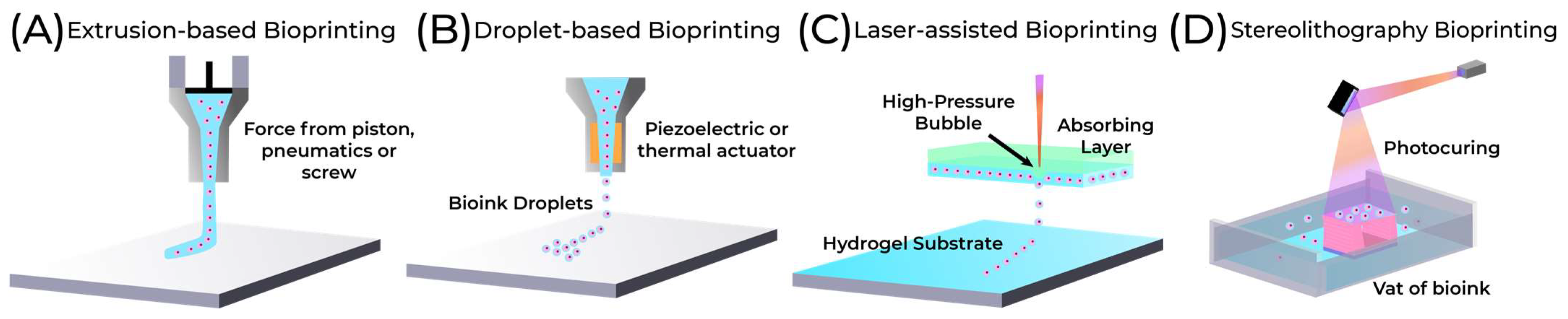
5.1. Extrusion-Based Bioprinting
5.2. Droplet-Based Bioprinting
5.3. Laser-Assisted Bioprinting
5.4. Stereolithography Bioprinting
| Extrusion | Droplet | Laser-Assisted | Stereolithography | |
|---|---|---|---|---|
| Advantages [9,96,102,105] | Biomaterial flexibility High printable cell densities | Ability to print low-viscosity bioinks Fast printing speed High resolution | High resolution Capable of printing bioinks in liquid or solid phase | Fast printing time High resolution Nozzle free, no shear stress High cell viability with visible light |
| Limitations [102,105,106] | Requires viscous bioinks | Limited capability for vertical structures Low cell densities | High cost Risk of thermal damage to cells | Risk of damage to cells if using UV Requires photopolymer bioink |
| Resolution [100,104,106] | Medium (100 μm) | High (50 μm) | Highest (~10 μm) | High (50 μm) |
| Print Speed [105,107,108] | Slow | Fast | Medium | Fast |
| Supported Viscosities [93,108,109,110] | 30 mPa/s to above 6 × 107 mPa/s | 3.5 to 12 mPa/s | 1 to 300 mPa/s | 250–1 × 104 mPa/s |
| Cell density [93] | High | Low | Medium | Medium |
| Cell Viability [104,111,112,113,114] | <90% | 80–95% | <85% | 85%–>90% |
6. Applications of Bioprinting in Ophthalmology
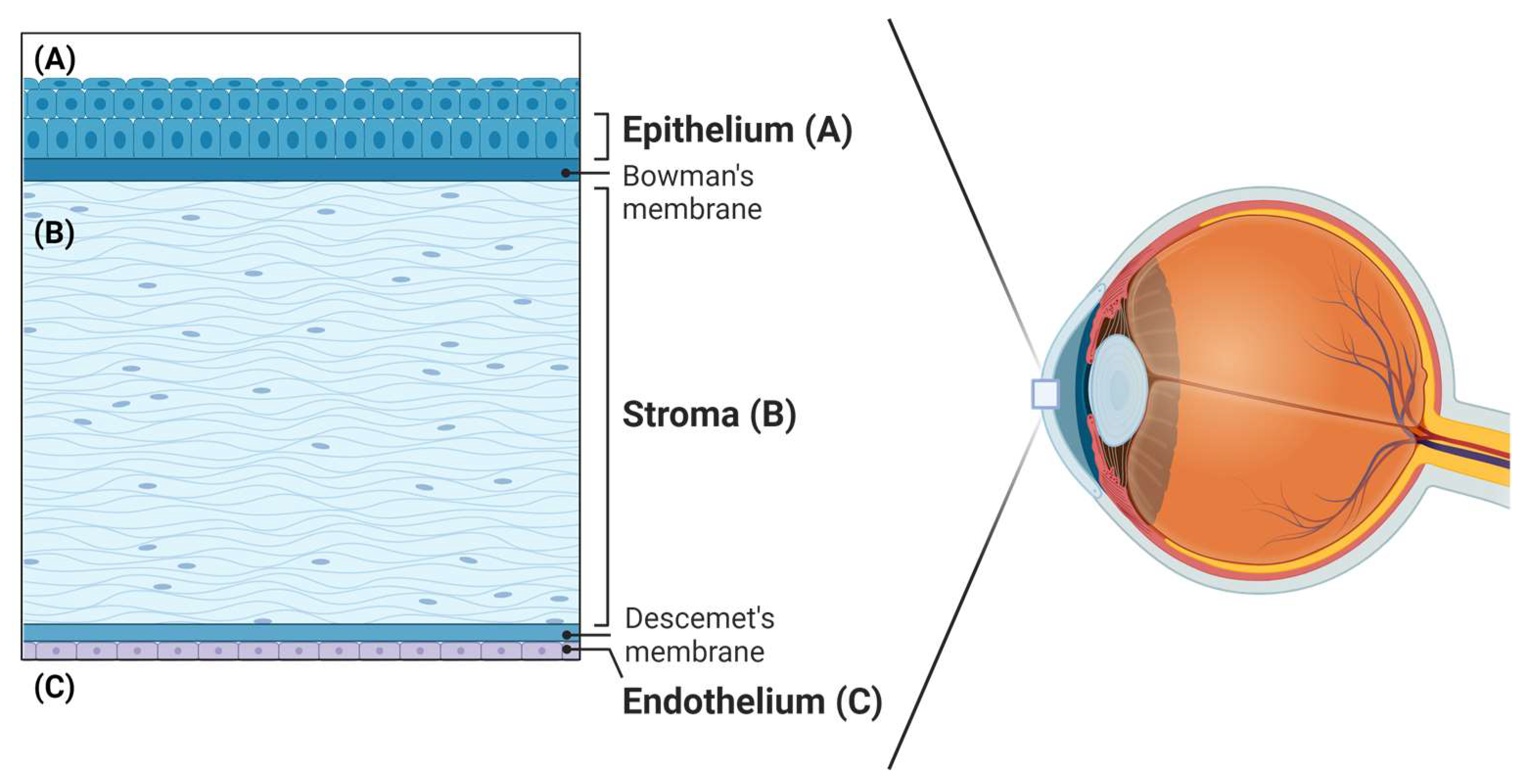
6.1. Cornea
6.2. Retina
- The cell viability after printing
- The bioprinted scaffold’s structure
- The cells’ orientation inside the scaffold
- The cells’ arrangement in various layers
6.3. Conjunctiva
7. Limitations of Ocular 3D Printing and Next Steps
7.1. Bioprinting Challenges
7.2. Material Properties
7.3. Time and Cost
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kholgh Eshkalak, S.; Rezvani Ghomi, E.; Dai, Y.; Choudhury, D.; Ramakrishna, S. The role of three-dimensional printing in healthcare and medicine. Mater. Des. 2020, 194, 108940. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Ye, Z.; Dun, A.; Jiang, H.; Nie, C.; Zhao, S.; Wang, T.; Zhai, J. The role of 3D printed models in the teaching of human anatomy: A systematic review and meta-analysis. BMC Med. Educ. 2020, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, J.P.; Tatum, P.S.; Gada, S.; Wyn, M.; Ho, V.B.; Liacouras, P. Using 3D Printing (Additive Manufacturing) to Produce Low-Cost Simulation Models for Medical Training. Mil. Med. 2018, 183 (Suppl. S1), 73–77. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.B.; Sung, R.; Weinberg, C.; Korelitz, T.; Andrews, R. Three-Dimensional Modeling May Improve Surgical Education and Clinical Practice. Surg. Innov. 2016, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Famery, N.; Abdelmassih, Y.; El-Khoury, S.; Guindolet, D.; Cochereau, I.; Gabison, E.E. Artificial chamber and 3D printed iris: A new wet lab model for teaching Descemet’s membrane endothelial keratoplasty. Acta Ophthalmol. 2019, 97, e179–e183. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ji, Z.; Yan, W.; Zhao, H.; Huang, W.; Liu, H. Application of Bioprinting in Ophthalmology. Int. J. Bioprint. 2022, 8, 552. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Villate-Beitia, I.; Gallego, I.; Lafuente-Merchan, M.; Puras, G.; Saenz-del-Burgo, L.; Pedraz, J.L. Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics 2021, 13, 308. [Google Scholar] [CrossRef]
- Awad, R.H.; Habash, S.A.; Hansen, C.J. Chapter 2—3D Printing Methods. In 3D Printing Applications in Cardiovascular Medicine; Al’Aref, S.J., Mosadegh, B., Dunham, S., Min, J.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 11–32. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J.; Kotta, S. 3D Printing in medicine: Technology overview and drug delivery applications. Ann. 3D Print. Med. 2021, 4, 100037. [Google Scholar] [CrossRef]
- Furdová, A.; Sramka, M.; Thurzo, A.; Furdová, A. Early experiences of planning stereotactic radiosurgery using 3D printed models of eyes with uveal melanomas. Clin. Ophthalmol. 2017, 11, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Ruiters, S.; Mombaerts, I. Applications of three-dimensional printing in orbital diseases and disorders. Curr. Opin. Ophthalmol. 2019, 30, 372. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.S.; Morris, J.M.; Foley, T.A.; Williamson, E.E.; Leng, S.; McGee, K.P.; Kuhlmann, J.L.; Nesberg, L.E.; Vrtiska, T.J. Three-dimensional Physical Modeling: Applications and Experience at Mayo Clinic. RadioGraphics 2015, 35, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.K.S.; Bell, S.; da Cruz, L.; Dick, A.D.; Sagoo, M.S. Applications of three-dimensional printing in ophthalmology. Surv. Ophthalmol. 2022, 67, 1287–1310. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D bioprinting processes: A perspective on classification and terminology. Int. J. Bioprint. 2018, 4, 151. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Bhanvadia, A.A.; Farley, R.T.; Noh, Y.; Nishida, T. High-resolution stereolithography using a static liquid constrained interface. Commun. Mater. 2021, 2, 41. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Chen, J.V.; Dang, A.B.C.; Dang, A. Comparing cost and print time estimates for six commercially-available 3D printers obtained through slicing software for clinically relevant anatomical models. 3D Print. Med. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Jaksa, L.; Pahr, D.; Kronreif, G.; Lorenz, A. Development of a Multi-Material 3D Printer for Functional Anatomic Models. Int. J. Bioprint. 2021, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Puls, N.; Carluccio, D.; Batstone, M.D.; Novak, J.I. The rise of additive manufacturing for ocular and orbital prostheses: A systematic literature review. Ann. 3D Print. Med. 2021, 4, 100036. [Google Scholar] [CrossRef]
- Chalasani, R.; Poole-Warren, L.; Conway, R.M.; Ben-Nissan, B. Porous Orbital Implants in Enucleation: A Systematic Review. Surv. Ophthalmol. 2007, 52, 145–155. [Google Scholar] [CrossRef]
- Chen, X.Y.; Yang, X.; Fan, X.L. The Evolution of Orbital Implants and Current Breakthroughs in Material Design, Selection, Characterization, and Clinical Use. Front. Bioeng. Biotechnol. 2022, 9, 800998. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Shin, H.J. Comparison of Orbital Reconstructive Effect between Customized Orbital Implants Using Three-Dimensional Printed Templates and Conventional Manual-Bending Implants in Blowout Fracture Surgery. Appl. Sci. 2023, 13, 9012. [Google Scholar] [CrossRef]
- Kang, S.; Kwon, J.; Ahn, C.J.; Esmaeli, B.; Kim, G.B.; Kim, N.; Sa, H.-S. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye 2018, 32, 1864–1870. [Google Scholar] [CrossRef]
- Kormann, R.B.; Mörschbächer, R.; Moreira, H.; Akaishi, P. A three-dimensional printed photopolymer resin implant for orbital rehabilitation for evisceration. Arq. Bras. Oftalmol. 2019, 82, 471–475. [Google Scholar] [CrossRef]
- Raizada, K.; Rani, D. Ocular prosthesis. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2007, 30, 152–162. [Google Scholar] [CrossRef]
- Ko, J.; Kim, S.H.; Baek, S.W.; Chae, M.K.; Yoon, J.S. Semi-automated fabrication of customized ocular prosthesis with three–dimensional printing and sublimation transfer printing technology. Sci. Rep. 2019, 9, 2968. [Google Scholar] [CrossRef]
- Alam, M.S.; Sugavaneswaran, M.; Arumaikkannu, G.; Mukherjee, B. An innovative method of ocular prosthesis fabrication by bio-CAD and rapid 3-D printing technology: A pilot study. Orbit 2017, 36, 223–227. [Google Scholar] [CrossRef]
- Ruiters, S.; Sun, Y.; de Jong, S.; Politis, C.; Mombaerts, I. Computer-aided design and three-dimensional printing in the manufacturing of an ocular prosthesis. Br. J. Ophthalmol. 2016, 100, 879–881. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, S.H.; Ko, J.; Baek, S.W.; Park, Y.K.; Kim, Y.J.; Yoon, J.S. A Pilot Clinical Study of Ocular Prosthesis Fabricated by Three-dimensional Printing and Sublimation Technique. Korean J. Ophthalmol. 2021, 35, 37–43. [Google Scholar] [CrossRef]
- Sun, M.G.; Rojdamrongratana, D.; Rosenblatt, M.I.; Aakalu, V.K.; Yu, C.Q. 3D printing for low cost, rapid prototyping of eyelid crutches. Orbit 2019, 38, 342–346. [Google Scholar] [CrossRef]
- Baba, T.; Ohno-Matsui, K.; Futagami, S.; Yoshida, T.; Yasuzumi, K.; Kojima, A.; Tokoro, T.; Mochizuki, M. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am. J. Ophthalmol. 2003, 135, 338–342. [Google Scholar] [CrossRef]
- Gaucher, D.; Haouchine, B.; Tadayoni, R.; Massin, P.; Erginay, A.; Benhamou, N.; Gaudric, A. Long-term Follow-up of High Myopic Foveoschisis: Natural Course and Surgical Outcome. Am. J. Ophthalmol. 2007, 143, 455–462.e1. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Vidakis, N.; Petousis, M.; Maniadi, A. Individualized Ophthalmic Exoplants by Means of Reverse Engineering and 3D Printing Technologies for Treating High Myopia Complications with Macular Buckles. Biomimetics 2020, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Vehmeijer, M.; van Eijnatten, M.; Liberton, N.; Wolff, J. A Novel Method of Orbital Floor Reconstruction Using Virtual Planning, 3-Dimensional Printing, and Autologous Bone. J. Oral Maxillofac. Surg. 2016, 74, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.W.; Paxton, L.; Dawes, K.; Burlak, K.; Quayle, M.; McMenamin, P.G. 3D printed reproductions of orbital dissections: A novel mode of visualising anatomy for trainees in ophthalmology or optometry. Br. J. Ophthalmol. 2015, 99, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, M.; Liu, Y.; Dai, R.; Zhang, M.; Zhong, Y.; Chen, Y. Application of a 3D-printed eye model for teaching direct ophthalmoscopy to undergraduates. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Jiang, H.; Bai, S.; Wang, T.; Yang, D.; Hou, H.; Zhang, Y.; Yi, S. Meta-analyzing the efficacy of 3D printed models in anatomy education. Front. Bioeng. Biotechnol. 2023, 11, 1117555. [Google Scholar] [CrossRef]
- Michaels, R.; Witsberger, C.A.; Powell, A.R.; Koka, K.; Cohen, K.; Nourmohammadi, Z.; Green, G.E.; Zopf, D.A. 3D printing in surgical simulation: Emphasized importance in the COVID-19 pandemic era. J. 3D Print. Med. 2021, 5, 5–9. [Google Scholar] [CrossRef]
- Ardila, C.M.; González-Arroyave, D.; Zuluaga-Gómez, M. Efficacy of three-dimensional models for medical education: A systematic scoping review of randomized clinical trials. Heliyon 2023, 9, e13395. [Google Scholar] [CrossRef]
- Maloca, P.M.; Tufail, A.; Hasler, P.W.; Rothenbuehler, S.; Egan, C.; Ramos de Carvalho, J.E.; Spaide, R.F. 3D printing of the choroidal vessels and tumours based on optical coherence tomography. Acta Ophthalmol. 2019, 97, e313–e316. [Google Scholar] [CrossRef]
- Masada, K.M.; Cristino, D.M.; Dear, K.A.; Hast, M.W.; Mehta, S. 3-D Printed Fracture Models Improve Resident Performance and Clinical Outcomes in Operative Fracture Management. J. Surg. Educ. 2023, 80, 1020–1027. [Google Scholar] [CrossRef]
- Fan, B.; Chen, H.; Sun, Y.-J.; Wang, B.-F.; Che, L.; Liu, S.-Y.; Li, G.-Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komuński, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefańczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio-Maxillofac. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Luo, T.-Y.; Ma, J.; Dong, Z.; Cao, G.; Xu, J.-K.; Liu, B.-Y.; Zhang, Q.-R.; Zhang, S.-L. Application of Three-Dimensional Printing Technology in the Orbital Blowout Fracture Reconstruction. J. Craniofac. Surg. 2019, 30, 1825–1828. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. Application of the three-dimensionally printed biodegradable polycaprolactone (PCL) mesh in repair of orbital wall fractures. J. Cranio-Maxillofacial Surg. 2019, 47, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D printing in Ophthalmology: From medical implants to personalised medicine. Int. J. Pharm. 2022, 625, 122094. [Google Scholar] [CrossRef]
- Willemen, N.G.A.; Morsink, M.A.J.; Veerman, D.; da Silva, C.F.; Cardoso, J.C.; Souto, E.B.; Severino, P. From oral formulations to drug-eluting implants: Using 3D and 4D printing to develop drug delivery systems and personalized medicine. Bio-Des. Manuf. 2022, 5, 85–106. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R.; Mohammadi, A.; Asghartabar Kashi, P. Multimaterial 3D printing of self-assembling smart thermo-responsive polymers into 4D printed objects: A review. Addit. Manuf. 2023, 71, 103598. [Google Scholar] [CrossRef]
- Singh, T.R.R.; Mcmillan, H.; Mooney, K.; Alkilani, A.Z.; Donnelly, R.F. 6—Microneedles for Drug Delivery and Monitoring. In Microfluidic Devices for Biomedical Applications; Li, X., Zhou, Y., Eds.; Woodhead Publishing: Delhi, India, 2013; pp. 185–230. [Google Scholar] [CrossRef]
- Han, D.; Morde, R.S.; Mariani, S.; La Mattina, A.A.; Vignali, E.; Yang, C.; Barillaro, G.; Lee, H. 4D Printing of a Bioinspired Microneedle Array with Backward-Facing Barbs for Enhanced Tissue Adhesion. Adv. Funct. Mater. 2020, 30, 1909197. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef] [PubMed]
- Gadziński, P.; Froelich, A.; Wojtyłko, M.; Białek, A.; Krysztofiak, J.; Osmałek, T. Microneedle-based ocular drug delivery systems—Recent advances and challenges. Beilstein J. Nanotechnol. 2022, 13, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Tătaru, C.P.; Purcărea, V.L. Antiglaucoma pharmacotherapy. J. Med. Life 2012, 5, 247–251. [Google Scholar] [PubMed]
- Sapowadia, A.; Ghanbariamin, D.; Zhou, L.; Zhou, Q.; Schmidt, T.; Tamayol, A.; Chen, Y. Biomaterial Drug Delivery Systems for Prominent Ocular Diseases. Pharmaceutics 2023, 15, 1959. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Bhavanasi, S.; Quadir, M.; Singh, K.; Ghosh, G.; Vasamreddy, K.; Ghosh, A.; Siahaan, T.J.; Banerjee, S.; Banerjee, S.K. Protein PEGylation for cancer therapy: Bench to bedside. J. Cell Commun. Signal. 2019, 13, 319–330. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.-H.; Cho, D.-W. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020, 116, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, H.; Karimi, S.; Ahmadieh, H.; Azarmina, M.; Abrishami, M.; Ahoor, H.; Alizadeh, Y.; Behboudi, H.; Daftarian, N.; Dehghan, M.H.; et al. Intravitreal Injection of Anti-vascular Endothelial Growth Factor Agents for Ocular Vascular Diseases: Clinical Practice Guideline. J. Ophthalmic Vis. Res. 2018, 13, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Reibaldi, M.; Fallico, M.; Avitabile, T.; Marolo, P.; Parisi, G.; Cennamo, G.; Furino, C.; Lucenteforte, E.; Virgili, G. Frequency of Intravitreal Anti-VEGF Injections and Risk of Death: A Systematic Review with Meta-analysis. Ophthalmol. Retina 2022, 6, 369–376. [Google Scholar] [CrossRef]
- Reitan, G.; Kjellevold Haugen, I.B.; Andersen, K.; Bragadottir, R.; Bindesbøll, C. Through the Eyes of Patients: Understanding Treatment Burden of Intravitreal Anti-VEGF Injections for nAMD Patients in Norway. Clin. Ophthalmol. 2023, 17, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Jørstad, Ø.K.; Steffensen, L.A.; Eriksen, K.; Bragadóttir, R.; Moe, M.C. Thirteen years of intravitreal anti-vascular endothelial growth factor therapy: The promises and burdens of a paradigm shift told from the perspective of the largest retina service in Norway. Acta Ophthalmol. 2020, 98, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, I.S.; Haugli Bråten, R.; Jørstad, Ø.K.; Moe, M.C.; Sæther, E.M. Intravitreal therapy for retinal diseases in Norway 2011–2015. Acta Ophthalmol. 2020, 98, 279–285. [Google Scholar] [CrossRef]
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Drug delivery to the retina: Challenges and opportunities. Expert. Opin. Biol. Ther. 2003, 3, 45–56. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef]
- Mutlu, Z.; Shams Es-haghi, S.; Cakmak, M. Recent Trends in Advanced Contact Lenses. Adv. Healthc. Mater. 2019, 8, 1801390. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, X.; Qi, P. Therapeutic contact lenses for ophthalmic drug delivery: Major challenges. J. Biomater. Sci. Polym. Ed. 2020, 31, 549–560. [Google Scholar] [CrossRef]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Bal-Öztürk, A.; Özcan-Bülbül, E.; Gültekin, H.E.; Cecen, B.; Demir, E.; Zarepour, A.; Cetinel, S.; Zarrabi, A. Application of Convergent Science and Technology toward Ocular Disease Treatment. Pharmaceuticals 2023, 16, 445. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Lanier, O.L.; Christopher, K.G.; Macoon, R.M.; Yu, Y.; Sekar, P.; Chauhan, A. Commercialization challenges for drug eluting contact lenses. Expert. Opin. Drug Deliv. 2020, 17, 1133–1149. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, G.-H.; Mun, J.; Cheong, S.; Choi, I.; Kim, H.; Hahn, S.K. Smart contact lens systems for ocular drug delivery and therapy. Adv. Drug Deliv. Rev. 2023, 196, 114817. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, B.; Zhang, F.; Liu, Y.; Sun, J.; Zhang, S.; Zhao, Y.; Yuan, H.; Leng, J. 4D printed orbital stent for the treatment of enophthalmic invagination. Biomaterials 2022, 291, 121886. [Google Scholar] [CrossRef]
- Saravanan, A.; Patel, B.C. Enophthalmos. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK563300/ (accessed on 13 November 2023).
- Liu, Y.; Zhang, W.; Zhang, F.; Lan, X.; Leng, J.; Liu, S.; Jia, X.; Cotton, C.; Sun, B.; Gu, B.; et al. Shape memory behavior and recovery force of 4D printed laminated Miura-origami structures subjected to compressive loading. Compos. Part B Eng. 2018, 153, 233–242. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, F.; Leng, J.; Liu, Y. Personalized 4D printing of bioinspired tracheal scaffold concept based on magnetic stimulated shape memory composites. Compos. Sci. Technol. 2019, 184, 107866. [Google Scholar] [CrossRef]
- Wang, W.; Du, L.; Xie, Y.; Zhang, F.; Li, P.; Xie, F.; Wan, X.; Pei, Q.; Leng, J.; Wang, N. Bioinspired four-dimensional polymeric aerogel with programmable temporal-spatial multiscale structure and functionality. Compos. Sci. Technol. 2021, 206, 108677. [Google Scholar] [CrossRef]
- Yoo, D. New paradigms in hierarchical porous scaffold design for tissue engineering. Mater. Sci. Eng. C 2013, 33, 1759–1772. [Google Scholar] [CrossRef]
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. Biochim. Biophys. Acta BBA—Gen. Subj. 2018, 1862, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Akyol, E.; Hagag, A.M.; Sivaprasad, S.; Lotery, A.J. Adaptive optics: Principles and applications in ophthalmology. Eye 2021, 35, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, A.; Tripathy, K. Adaptive Optics. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK589704/ (accessed on 13 November 2023).
- Gill, J.S.; Moosajee, M.; Dubis, A.M. Cellular imaging of inherited retinal diseases using adaptive optics. Eye 2019, 33, 1683–1698. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A 1997, 14, 2884–2892. [Google Scholar] [CrossRef]
- López-Valdeolivas, M.; Liu, D.; Broer, D.J.; Sánchez-Somolinos, C. 4D Printed Actuators with Soft-Robotic Functions. Macromol. Rapid Commun. 2018, 39, 1700710. [Google Scholar] [CrossRef]
- Hammer, D.X.; Kedia, N.; Liu, Z.; Tam, J.; Agrawal, A. Phantom-Based Model Eyes for Adaptive Optics Performance Assessment. In Applied Industrial Optics 2019; OSA Technical Digest; Optica Publishing Group: Washington, DC, USA, 2019; p. W2A.2. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.S.; Kim, Y.M.; Song, S.C. Thermo-Responsive Nanocomposite Bioink with Growth-Factor Holding and its Application to Bone Regeneration. Small 2023, 19, 2203464. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- You, S.; Xiang, Y.; Hwang, H.H.; Berry, D.B.; Kiratitanaporn, W.; Guan, J.; Yao, E.; Tang, M.; Zhong, Z.; Ma, X.; et al. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 2023, 9, eade7923. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef]
- Guillotin, B.; Catros, S.; Guillemot, F. Laser Assisted Bio-Printing (LAB) of Cells and Bio-Materials Based on Laser Induced Forward Transfer (LIFT). In Laser Technology in Biomimetics: Basics and Applications; Schmidt, V., Belegratis, M.R., Eds.; Biological and Medical Physics, Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 193–209. [Google Scholar] [CrossRef]
- Catros, S.; Guillotin, B.; Bačáková, M.; Fricain, J.C.; Guillemot, F. Effect of laser energy, substrate film thickness and bioink viscosity on viability of endothelial cells printed by Laser-Assisted Bioprinting. Appl. Surf. Sci. 2011, 257, 5142–5147. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.L.; Lee, S.K. Ultraviolet radiation: DNA damage, repair, and human disorders. Mol. Cell Toxicol. 2017, 13, 21–28. [Google Scholar] [CrossRef]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting Toward Organ Fabrication: Challenges and Future Trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; Vlierberghe, S.V.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. Complex 3D bioprinting methods. APL Bioeng. 2021, 5, 011508. [Google Scholar] [CrossRef]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B.; Lopez, J.; Faucon, M.; Pippenger, B.; Bareille, R.; Rémy, M.; Bellance, S.; et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010, 6, 2494–2500. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write Bioprinting Three-Dimensional Biohybrid Systems for Future Regenerative Therapies. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, A.; Chen, X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Hopp, B.; Smausz, T.; Szabó, G.; Kolozsvari, L.; Nogradi, A.; Kafetzopoulos, D.; Fotakis, C. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 2012, 51, 014302. [Google Scholar] [CrossRef]
- Dai, X.; Liu, L.; Ouyang, J.; Li, X.; Zhang, X.; Lan, Q.; Xu, T. Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci. Rep. 2017, 7, 1457. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.-M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Tidke, S.C.; Tidake, P. A Review of Corneal Blindness: Causes and Management. Cureus 2022, 14, e30097. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- AlMutlak, M.; Li, J.Y.; Bin Helayel, H.; Fairaq, R. Future of Corneal Donation and Transplantation: Insights from COVID-19 Pandemic. Cornea 2020, 40, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef] [PubMed]
- Espana, E.M.; Birk, D.E. Composition, Structure and Function of the Corneal Stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3D bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys. 2019, 71, 68–78. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Park, J.; Lee, K.-P.; Lee, S.; Lee, D.-M.; Kim, K.H.; Kim, H.K.; Cho, D.-W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.-F.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. A 2019, 107, 1945–1953. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.J.; Park, S.H.; Kim, J.C. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv. Healthc. Mater. 2018, 7, 1800398. [Google Scholar] [CrossRef]
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef]
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK545310/ (accessed on 18 November 2023).
- Wilkinson-Berka, J.L. Diabetes and retinal vascular disorders: Role of the renin–angiotensin system. Expert. Rev. Mol. Med. 2004, 6, 1–18. [Google Scholar] [CrossRef]
- Berson, D.M. Phototransduction in ganglion-cell photoreceptors. Pflüg Arch—Eur. J. Physiol. 2007, 454, 849–855. [Google Scholar] [CrossRef]
- Masland, R.H. The tasks of amacrine cells. Vis. Neurosci. 2012, 29, 3–9. [Google Scholar] [CrossRef]
- Euler, T.; Haverkamp, S.; Schubert, T.; Baden, T. Retinal bipolar cells: Elementary building blocks of vision. Nat. Rev. Neurosci. 2014, 15, 507–519. [Google Scholar] [CrossRef]
- Murenu, E.; Gerhardt, M.J.; Biel, M.; Michalakis, S. More than meets the eye: The role of microglia in healthy and diseased retina. Front. Immunol. 2022, 13, 1006897. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef] [PubMed]
- Chiba, C. The retinal pigment epithelium: An important player of retinal disorders and regeneration. Exp. Eye Res. 2014, 123, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Foulds, W.S.; Ling, E.A. Hypoxia-ischemia and retinal ganglion cell damage. Clin. Ophthalmol. 2008, 2, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Chen, P.P. Glaucoma. Am. Fam. Physician 2016, 93, 668–674. [Google Scholar] [PubMed]
- Tsang, S.H.; Sharma, T. Retinitis Pigmentosa (Non-syndromic). In Atlas of Inherited Retinal Diseases; Tsang, S.H., Sharma, T., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 125–130. [Google Scholar] [CrossRef]
- Worthington, K.S.; Wiley, L.A.; Kaalberg, E.E.; Collins, M.M.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater. 2017, 55, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Hertz, J.; Qu, B.; Hu, Y.; Patel, R.D.; Valenzuela, D.A.; Goldberg, J.L. Survival and Integration of Developing and Progenitor-Derived Retinal Ganglion Cells following Transplantation. Cell Transplant. 2014, 23, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Bhatia, B.; Jayaram, H.; Becker, S.; Jones, M.F.; Cottrill, P.B.; Khaw, P.T.; Salt, T.E.; Limb, G.A. Human Müller Glia with Stem Cell Characteristics Differentiate into Retinal Ganglion Cell (RGC) Precursors In Vitro and Partially Restore RGC Function In Vivo Following Transplantation. Stem Cells Transl. Med. 2012, 1, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.C.; Hippert, C.; Duran, Y.; West, E.L.; Bainbridge, J.W.B.; Warre-Cornish, K.; Luhmann, U.F.O.; Lakowski, J.; Sowden, J.C.; Ali, R.R.; et al. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 2013, 110, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Ballios, B.G.; Cooke, M.J.; Donaldson, L.; Coles, B.L.K.; Morshead, C.M.; van der Kooy, D.; Shoichet, M.S. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Rep. 2015, 4, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Hynes, S.R.; Lavik, E.B. A tissue-engineered approach towards retinal repair: Scaffolds for cell transplantation to the subretinal space. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Tao, S.L.; Young, M.J. Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering. Polymers 2011, 3, 899–914. [Google Scholar] [CrossRef]
- Treharne, A.J.; Grossel, M.C.; Lotery, A.J.; Thomson, H.A. The chemistry of retinal transplantation: The influence of polymer scaffold properties on retinal cell adhesion and control. Br. J. Ophthalmol. 2011, 95, 768–773. [Google Scholar] [CrossRef]
- Shi, P.; Edgar, T.Y.S.; Yeong, W.Y.; Laude, A. Hybrid three-dimensional (3D) bioprinting of retina equivalent for ocular research. Int. J. Bioprint. 1970, 3, 138–146. [Google Scholar] [CrossRef]
- Lorber, B.; Hsiao, W.K.; Hutchings, I.M.; Martin, K.R. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2013, 6, 015001. [Google Scholar] [CrossRef]
- Kador, K.E.; Grogan, S.P.; Dorthé, E.W.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; D’lima, D.D. Control of Retinal Ganglion Cell Positioning and Neurite Growth: Combining 3D Printing with Radial Electrospun Scaffolds. Tissue Eng. Part A. 2016, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Tan, Y.S.E.; Yeong, W.Y.; Li, H.Y.; Laude, A. A bilayer photoreceptor-retinal tissue model with gradient cell density design: A study of microvalve-based bioprinting. J. Tissue Eng. Regen. Med. 2018, 12, 1297–1306. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Zhu, W.; Zhong, Z.; Moran, A.; Wang, W.; Zhang, K.; Chen, S. 3D bioprinting of hydrogels for retina cell culturing. Bioprinting Amst. Neth. 2018, 11, e00029. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, E.; Forster, V.; Picaud, S.; Karamali, F.; Nasr-Esfahani, M.H.; Marquette, C. Tissue engineering of retina through high resolution 3-dimensional inkjet bioprinting. Biofabrication 2020, 12, 025006. [Google Scholar] [CrossRef]
- Taurone, S.; Spoletini, M.; Ralli, M.; Gobbi, P.; Artico, M.; Imre, L.; Czakò, C.; Kovàcs, I.; Greco, A.; Micera, A. Ocular mucous membrane pemphigoid: A review. Immunol. Res. 2019, 67, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Shumway, C.L.; Motlagh, M.; Wade, M. Anatomy, Head and Neck, Eye Conjunctiva. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK519502/ (accessed on 18 November 2023).
- Elkhenany, H.; El-Derby, A.; Abd Elkodous, M.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the amniotic membrane in tissue engineering and regeneration: The hundred-year challenge. Stem Cell Res. Ther. 2022, 13, 8. [Google Scholar] [CrossRef]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef]
- Ashena, Z.; Holmes, C.; Nanavaty, M.A. Pericardium Patch Graft for Severe Corneal Wound Burn. J. Curr. Ophthalmol. 2021, 33, 342–344. [Google Scholar] [CrossRef]
- Witt, J.; Mertsch, S.; Borrelli, M.; Dietrich, J.; Geerling, G.; Schrader, S.; Spaniol, K. Decellularised conjunctiva for ocular surface reconstruction. Acta Biomater. 2018, 67, 259–269. [Google Scholar] [CrossRef]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef]
- Dehghani, S.; Rasoulianboroujeni, M.; Ghasemi, H.; Keshel, S.H.; Nozarian, Z.; Hashemian, M.N.; Zarei-Ghanavati, M.; Latifi, G.; Ghaffari, R.; Cui, Z.; et al. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: An in vitro & in vivo study. Biomaterials 2018, 174, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Deng, X.; Wang, P.; Yu, C.; Kiratitanaporn, W.; Wu, X.; Schimelman, J.; Tang, M.; Balayan, A.; Yao, E.; et al. Rapid bioprinting of conjunctival stem cell micro-constructs for subconjunctival ocular injection. Biomaterials 2021, 267, 120462. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, J.; Tian, J.; Deng, X.; Balayan, A.; Sun, Y.; Xiang, Y.; Guan, J.; Schimelman, J.; Hwang, H.; et al. Rapid 3D bioprinting of a multicellular model recapitulating pterygium microenvironment. Biomaterials 2022, 282, 121391. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.; Girolamo, N.D.; Wakefield, D.; Coroneo, M.T. The Pathogenesis of Pterygium: Current Concepts and Their Therapeutic Implications. Ocul. Surf. 2008, 6, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Schneider, U.; Naumann, G.O.H. Count and density of human retinal photoreceptors. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 230, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3D bioprinting. Prog. Nat. Sci. Mater. Int. 2020, 30, 618–634. [Google Scholar] [CrossRef]
- Ballard, D.H.; Mills, P.; Duszak, R.; Weisman, J.A.; Rybicki, F.J.; Woodard, P.K. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad. Radiol. 2020, 27, 1103–1113. [Google Scholar] [CrossRef]

| Printer | FDM | SLS | DMLS | Binder Jetting | SLA | Material Jetting |
|---|---|---|---|---|---|---|
| Technique | Extrusion-based | Powder bed fusion | Powder bed fusion | Powder binding | Vat-polymerization | Inkjet droplet deposition |
| Materials [2,3,11] | Thermoplastic, composites | Thermoplastic, ceramics, composites | Metal alloys | Thermoplastic, Metals, Ceramics | Photopolymer resin | Photopolymers, waxes |
| Base Technology [2,3] | 3D articulating print head depositing heated filament | Laser (e.g., CO2) reflected by mirrors onto powder to sinter it solid | High-power laser (e.g., YAG fiber) that sinters metal powder solid | Print head deposits binder to adhere powder layer by layer | Selective solidification of resin by ultraviolet (UV) source | Deposition of photopolymer droplets that are UV flash-cured |
| Machine Cost [11] | Low-Medium | Medium-High | High | Medium | Low-High | Medium-High |
| Material Cost [11] | Low-Medium | Low | High | Low-Medium | Medium-High | High |
| Typical Resolution [3,11] | 100–150 μm | 50–100 μm | 50–100 μm | 100 μm | 25–75 μm | 25–40 μm |
| Benefits [2,3,12] | 1. Good structural strength 2. Inexpensive 3. Capable of multi-material printing 4. Large scalable build volume 5. Widespread and accessible | 1. Good accuracy and detail 2. High strength and durability 3. Suitable for complex parts with internal geometries 4. No support structures required 5. Material variety | 1. High accuracy and precision 2. Suitable for complex parts with internal geometries 3. High strength and durability 4. No support structures required | 1. Cost-effective compared to SLS and DMLS 2. Suitable for complex parts with internal geometries 3. Fast print speed 4. Multiple color printing 5. Material variety | 1. Excellent resolution 2. Best surface finish (smooth) 3. Suitable for complex parts requiring fine detail 4. Print uniformity and isotropy 5. Fast print speed | 1. High repeatability and precision 2. Controllable transparency and color 3. Excellent resolution 4. Capable of multi-material printing |
| Drawbacks [2,3,12] | 1. Slow printing time 2. Rough surface finish with anisotropy 3. Requires support structures 4. Lower dimensional accuracy | 1. Expensive 2. Rough surface finish 3. Requires postprocessing to separate part from powder | 1. Very expensive 2. Often requires postprocessing and surface finishing 3. Slow printing time | 1. Inferior strength compared to SLS and DMLS 2. Relatively lower resolution 3. Rough surface finish | 1. Moderate strength 2. Long-term stability reduced by UV sensitivity of resin material 3. Relatively high cost | 1. Relatively weak strength prints 2. Lower temperature resistance 3. Requires support structures and postprocessing |
| Applications in Ophthalmology [4,13,14,15,16] | Anatomical Models, Prostheses, Surgical Planning Models | Anatomical Models, Prostheses, Surgical Instruments, Implants | Surgical Instruments, Surgical Guides, Implants | Anatomical Models, Surgical Guides and Preplanning Models, Prostheses | Surgical guides, Anophthalmic Socket Conformers, Prostheses | Anatomical Models, Surgical Guides and Preplanning Models, Prostheses |
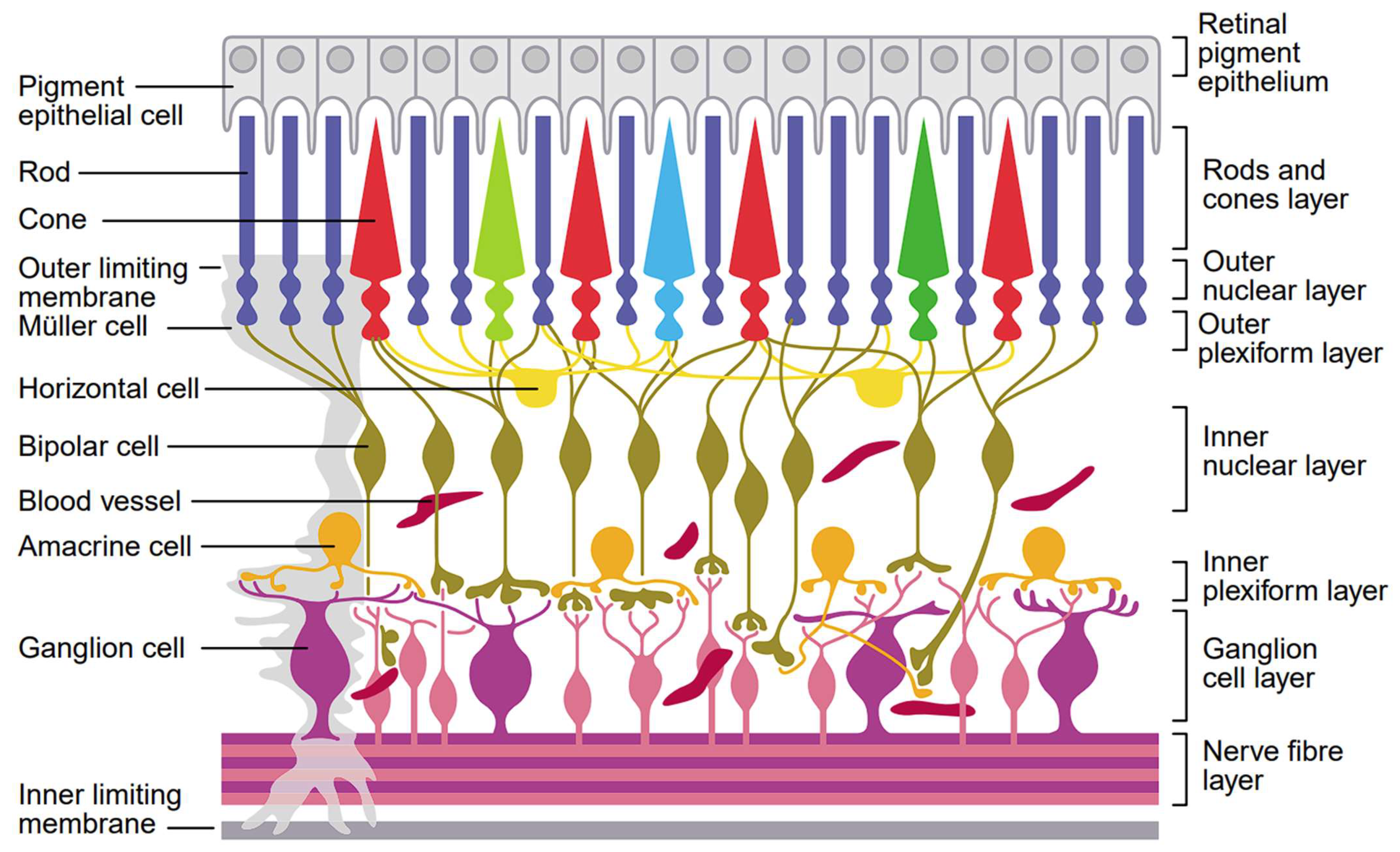
| Basic Cell Types | Cell Lines | Main Function [129,131,132,133,134,135] | |
|---|---|---|---|
| Photoreceptor cells | Rods |
| |
| Cones |
| ||
| Neuronal cells | Retinal ganglion cells (RGCs) |
| |
| Amacrine cells |
| ||
| Bipolar cells |
| ||
| Horizontal cells |
| ||
| Glial cells | Microglia |
| |
| Macroglia | Astrocytes |
| |
| Müller cells |
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, N.; Gagnon, M.; Wu, K.Y. The Third Dimension of Eye Care: A Comprehensive Review of 3D Printing in Ophthalmology. Hardware 2024, 2, 1-32. https://doi.org/10.3390/hardware2010001
Lin N, Gagnon M, Wu KY. The Third Dimension of Eye Care: A Comprehensive Review of 3D Printing in Ophthalmology. Hardware. 2024; 2(1):1-32. https://doi.org/10.3390/hardware2010001
Chicago/Turabian StyleLin, Neil, Maryse Gagnon, and Kevin Y. Wu. 2024. "The Third Dimension of Eye Care: A Comprehensive Review of 3D Printing in Ophthalmology" Hardware 2, no. 1: 1-32. https://doi.org/10.3390/hardware2010001
APA StyleLin, N., Gagnon, M., & Wu, K. Y. (2024). The Third Dimension of Eye Care: A Comprehensive Review of 3D Printing in Ophthalmology. Hardware, 2(1), 1-32. https://doi.org/10.3390/hardware2010001







