1. Introduction
Propofol is a preferred sedative in the intensive care unit (ICU) [
1]. Formulated in a 10% fat emulsion, propofol use has been linked to adverse events, including infection, hypertriglyceridemia, hypertriglyceridemia-related pancreatitis, and propofol-related infusion syndrome (PRIS) [
2]. Hypertriglyceridemia occurs in up to 45% of patients on propofol, with higher doses and longer durations of use being a risk factor [
2,
3,
4]. Although not formally addressed in international guidelines, monitoring serum triglyceride (TG) levels every 48–72 h is recommended, and if significant hypertriglyceridemia (e.g., ≥5.65 mmol/L) occurs, alternative sedative strategies are recommended to avoid development of pancreatitis or other adverse effects of propofol use, such as PRIS [
2,
3].
Insulin has been used to reduce serum TG in the setting of hypertriglyceridemia-induced pancreatitis [
5,
6,
7,
8]. Insulin acts via membrane bound receptors on target tissues to produce a number of effects [
9]. Specifically, in the liver, insulin stimulates glycogen synthesis and promotes the synthesis of fatty acids, which are then released into circulation as lipoproteins. One of these lipoproteins, lipase, that is produced is the enzyme needed for the hydrolysis of triglycerides. Case reports and observational data indicate intensive insulin therapy, either as monotherapy or in combination with heparin, results in significant reductions in serum TG levels in patients with hypertriglyceridemia [
5,
6,
7,
8,
10,
11]. Although limited by quality of trials included, a meta-analysis of three randomized controlled trials for evaluating the use of intensive insulin therapy for the treatment of severe acute pancreatitis found intensive insulin therapy was associated with a shorter length of hospitalization without serious adverse effects [
12].
The impact of concomitant insulin infusion and propofol infusions on serum TG concentration in critically ill patients without hypertriglyceridemia pancreatitis is unknown. This relationship is important to elucidate since propofol-induced hypertriglyceridemia may lead to propofol cessation and the initiation of less preferred sedation strategies, such as benzodiazepines [
1].
The purpose of this retrospective cohort study is to characterize the relationship between serum TG levels in critically ill patients who received concomitant intravenous insulin and propofol infusions and compared to those who received propofol alone. By extrapolating mechanistic data for insulin in the treatment of non-propofol related hypertriglyceridemia, we hypothesize that critically ill patients receiving concomitant insulin and propofol infusions for at least 24 h will have a lower serum TG as compared to those receiving propofol alone.
2. Materials and Methods
This is a single-center, retrospective cohort study that included mechanically ventilated adult patients admitted to the critical care medicine service at a tertiary care academic medical center. Patients who received at least 48 h of propofol therapy with and without a minimum of 24 h of concomitant insulin infusions between 1 January 2013 and 1 September 2018 were included. The critical care primary medicine service admits patients to a closed, 26-bed medical ICU with two attending physician rounding teams and 24 h ICU pharmacist coverage. ICU pharmacists have autonomy to order lab values for the purposes of drug therapy monitoring per site protocol. TG values are typically ordered every 48–72 h for patients on propofol infusion; however, the frequency of TG monitoring is not protocolized. The study was reviewed and approved by the site’s Institutional Review Board, and the study conforms to the Australian National Statement on Ethical Conduct in Human Research/the Declaration of Helsinki.
Inclusion criteria included patients ≥ 18 years old who were admitted to the critical care medicine service, mechanically ventilated, received at least 48 h of continuous propofol infusions, and had at least two serum TG levels obtained while on propofol therapy. Concomitant propofol and insulin infusions for a minimum of 24 h was required for inclusion in the propofol and insulin group. We excluded patients who had a baseline serum TG concentration of 5.65 mmol/L or greater and/or patients concomitantly receiving other lipid-containing products (e.g., intravenous lipid infusions).
The primary outcome was median change in the serum TG concentration in critically ill patients receiving concomitant propofol and insulin infusions, as compared to those receiving propofol alone. Secondary outcomes included median change in TG per day of propofol, clinician response to hypertriglyceridemia (≥2.26 mmol/L) [
13] in patients receiving a propofol infusion, and the incidence of hypoglycemia and diagnosis of pancreatitis during ICU stay. Clinician responses were defined as (1) reduced dose of propofol, (2) cessation of propofol and initiation of another sedative, or (3) cessation of propofol without initiation of another sedative.
Demographic data collected included age, weight, admitting diagnosis, baseline sequential organ failure assessment (SOFA) score, history of hyperlipidemia or diabetes mellitus, and prescribed lipid lowering agent or insulin prior to admission. Biochemical data included serum amylase, lipase, TG concentration, hemoglobin A1c and baseline blood glucose values. All TG values obtained during the study period were recorded. Clinical data included duration, total dose, and infusion rates of both propofol and insulin. Insulin infusions initiated for glycemic control and were dosed per the best practices to maintain blood sugar values between 1.7–2.04 mmol/L [
14]. Daily quantity of long-acting and short-acting subcutaneous insulin while on propofol was collected in both groups. It is worth noting that only long-acting insulin products are allowed concurrently with insulin infusions at our institution. Safety outcomes included number of hypoglycemic events, defined as serum blood glucose < 0.79 mmol/L, and acute pancreatitis, defined as two of the following three conditions: elevation of pancreatic enzymes (serum amylase ≥ 125 IU/L and/or serum lipase ≥ 60 IU/L), epigastric pain, and abdominal computed tomography scan consistent with acute pancreatitis [
15].
An estimated 32 patients total, 16 in each group, were needed to detect a mean decrease in TG level of 1.13 mmol/L at an alpha of 0.05 and a power of 80% between patients on propofol with an insulin infusion compared to those on propofol alone. A difference of 1.13 mmol/L was used based on previously documented impact of 24 h of an IV insulin infusion on TG levels [
8]. Additionally, a decrease in TG value by 1.13 mmol/L was deemed clinically significant to allow for the safe continuation of propofol. Baseline patient characteristics were summarized using the median and interquartile range (IQR) for continuous and ordinal data, and frequency and percentage for categorical variables. The frequency of missing values was reported for each variable. Continuous variables were evaluated using a Mann–Whitney U test, and a chi-squared test was used for nominal data. Pearson correlation was used to evaluate the relationship between age and change in TG value. In the event of missing data, cases were dropped from a particular analysis if they had data points missing for the variables in that analysis. These cases were included in other analyses where data points were not missing. A two-way ANOVA was performed to evaluate the impact of age and sex on TG levels. Changes in TG levels over the course of propofol treatment were visualized using a box and whisker plot. A multiple mixed effects linear model was used to model triglyceride levels including a random patient-specific intercept and time-dependent covariates for duration on propofol and duration on insulin. An autoregressive correlation structure (lag 1) was specified for within-patient repeated observations. All statistics were calculated using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
3. Results
Two hundred and ninety-four consecutive patients were screened for inclusion. Of these, 207 (70.4%) did not have two recorded TG levels while on propofol therapy, 49 (16.7%) were on propofol for <48 h, 5 (1.7%) were receiving concomitant lipid-containing products, and 1 patient was <18 years old. A total of 32 patients, 16 propofol only and 16 in the propofol and insulin group, were included. Baseline demographic data are summarized in
Table 1. Baseline median blood glucose levels were lower in the propofol only group compared with the propofol and insulin group (1.70 (IQR 1.25–2.43) mmol/L vs. 2.77 (IQR 1.85–4.20) mmol/L,
p = 0.017). Patients in the propofol only group had lower median (IQR) baseline hemoglobin A1c (HbA1c) values, obtained within 3 months of admission, compared with the propofol and insulin group (5.7 (4.9–7.5)% vs. 7.2 (6.6–9.4)%,
p = 0.031), however, this value was not available for most patients. There were no other differences in baseline demographics between groups. Seven patients (43.8%) in the propofol-only group had a diagnosis of diabetes prior to admission compared with eleven (68.8%) in the propofol and insulin group. More patients in the propofol and insulin group were prescribed insulin prior to ICU admission, as compared with the propofol only group (62.5% vs. 43.8% in the propofol and insulin and propofol only groups, respectively). These differences were not statistically significant.
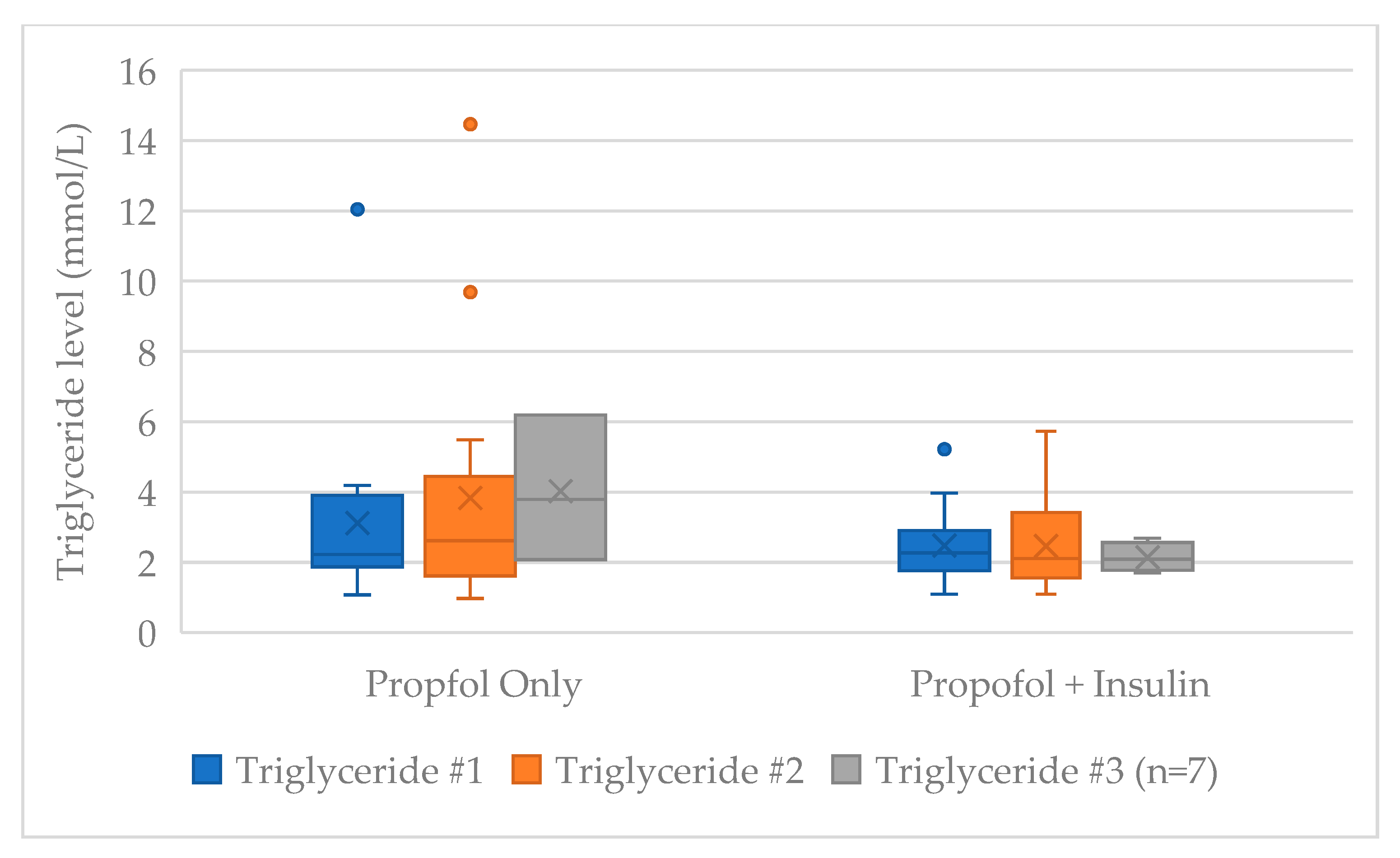
There was no difference between the first (TG #1), second (TG #2), or third (TG #3) serum TG values obtained between those receiving propofol only compared with propofol and insulin (
Table 2). The median (IQR) change between the first and second TG values was +0.27 (−0.31–1.16) mmol/L in the propofol only group and −0.07 (−1.08–0.30) mmol/L in the propofol and insulin group (
p = 0.071). Seven of thirty-two patients had an additional TG level obtained. The median (IQR) change in serum TG level from first to last drawn TG while on propofol was +0.35 (−0.31–1.33) mmol/L in the propofol only group and −0.07 (−1.08–0.42) mmol/L in the propofol and insulin group (
p = 0.051). Propofol duration and dosing at time of triglyceride value recording did not differ between groups (
Table 2). The median change in TG value was similar between males and females in both groups (
p = 0.38), and age was not correlated with change in TG value (r(32) = −0.13 (
p = 0.54)). Two-way ANOVA identified that age impacted the first TG value (F(21, 32) = 9.1,
p= 0.01), but not the second TG value or the change in TG value (F(21,32) = 2.14,
p = 0.2 & F(21, 21) = 0.44,
p = 0.92, respectively). The first and second TG values as well as the change in TG value were not impacted by sex (
p = 0.10,
p = 0.63,
p = 0.72, respectively).
A multiple mixed-effects linear model indicated that each additional day on propofol was associated with an estimated 0.21 mmol/L (95% confidence interval (CI) 0.004 to 0.41,
p = 0.046) increase in TG, and each additional day of insulin was associated with a 0.14 mmol/L (95% CI −0.63 to 0.35,
p = 0.571) decrease in TG (
Table 3). The median (IQR) quantity of subcutaneous insulin received while on propofol did not differ between groups [43.5 (4.3–125.8) units vs. 40.5 (7.0–176.5) units in the propofol only and propofol and insulin groups, respectively (
p = 0.926)]. The median (IQR) insulin infusion rate in the propofol and insulin group was 4.0 (2.7–6.0) units/h with a median duration of 3.3 (2.1–4.6) days.
Propofol was discontinued in six (19%) patients due to hypertriglyceridemia. The median (IQR) TG value prompting discontinuation of propofol was 3.92 (2.61–8.26) mmol/L. Three patients were changed to midazolam infusions, and three were changed to dexmedetomidine infusions. Median SOFA score on the day propofol was discontinued due to high TG values was 9.7 in patients transitioned to midazolam infusions compared with 6.7 in those transitioned to dexmedetomidine. Patients transitioned from propofol to midazolam had higher average respiratory component scores (3.7) compared to those transitioned to dexmedetomidine (2.3). All 3 patients transitioned to midazolam had a diagnosis of acute respiratory distress syndrome (ARDS). Four of the six patients who had propofol discontinued due to TG values were in the propofol and insulin group. however, the insulin infusion was discontinued prior to the propofol in three of these patients. One patient was receiving an insulin infusion at the time propofol was discontinued due to high TG values (
Figure 1). When evaluating only the patients in whom propofol was discontinued due to high TG values, the median TG value at the first recording was higher in the propofol only group (8.12 mmol/L,
n = 2) compared with the propofol and insulin group (3.29 mmol/L,
n = 4). Additionally, the median change between the first and second measured TG value was +1.86 mmol/L in the propofol only group, compared with −0.62 mmol/L in the propofol and insulin group.
In the seven patients that had a third TG value recorded, the median total propofol duration was 124 h (n = 3) in the propofol only group and 100.7 h (n = 4) in the propofol and insulin group. Median total propofol dose (23.4 g vs. 31.2 g), propofol infusion rate (31.6 vs. 49.7 mcg/kg/min), and max rate (55.0 vs. 80.0 mcg/kg/min) was numerically lower in the three patients in the propofol only group compared with the four patients in the propofol and insulin group. Statistics were not run on the patient subset given the small sample size. No patient in either group developed acute pancreatitis or hypoglycemia.
4. Discussion
To our knowledge, this is the first report describing the relationship between concomitant propofol and insulin infusion on serum TG concentrations. Our study found no difference between first and last median serum TG values in the propofol only group compared with the propofol and insulin group. The quantity of subcutaneous insulin received while on propofol did not differ between groups. Therefore, we surmise that the observed differences in serum TG values between groups was related to the insulin infusion. Each additional day of propofol was associated with an estimated 0.21 mmol/L increase in TG while each day of insulin was associated with a 0.14 mmol/L decrease in TG value. This increase in daily TG associated with propofol use was statistically significant, whereas the decrease in daily TG associated with insulin use did not reach statistical significance. Propofol was discontinued due to high TG values in six patients. In all six instances, clinicians responded by substituting another sedative in lieu of propofol.
The change in serum TG, although not statistically significant between groups, was numerically lower in the propofol and insulin group. This may be relevant in a patient’s clinical course. Lowering TG values while on propofol therapy may allow for continued use of propofol, especially in situations where sedation strategies are limited. Increases in TG values are correlated with the dose and duration of propofol infusions [
3]. Therefore, the impact of insulin infusions on TG is more likely to be significant in patients requiring higher doses and/or longer infusions of propofol. In our study, we started to observe this when looking at the differences in the second and third TG values obtained. The second TG values obtained on propofol therapy were higher in the propofol only group compared with the propofol and insulin group. The median duration and dose of propofol at the time of the second TG recording was not different between groups. When factoring in the seven patients that had a third serum TG value, the median change in TG value between the first and last measured TG values was greater than the difference between the first and second TG values obtained (
Table 2). Although the total duration of propofol did not differ between groups in those patients who had greater than two TG values obtained, the total dose, median rate, and max rate of the propofol infusion was higher in the four patients in the propofol and insulin group compared with the three patients in the propofol only group.
Six patients had propofol discontinued due to high TG values. One of the six patients was receiving an insulin infusion at the time propofol was discontinued. Statistical analysis was not performed on this outcome due to the small sample size, but nonetheless, this finding may be clinically relevant. When longer durations of sedation are needed, options are limited. Current practice guidelines recommend dexmedetomidine or propofol over benzodiazepine therapy given increased duration of mechanical ventilation, longer hospitalizations, and development of delirium associated with benzodiazepine use [
1]. Dexmedetomidine does not provide adequate sedation for all patients. A lack of efficacy was noted in one out of every eight to ten patients in the MIDEX and PRODEX trials [
16]. Failure rates of dexmedetomidine have been noted to be as high as 21% and 50% in other studies [
17,
18]. Additionally, dexmedetomidine is not able to induce the deeper levels of sedation required for some disease states or if paralysis is indicated. If dexmedetomidine is ineffective or not indicated, sedation strategies are limited and include using benzodiazepines or other less-studied therapies, such as ketamine. Our study found a 0.14 mmol/L decrease in serum TG value for each day of insulin therapy, as compared to an 0.21 mmol/L increase in serum TG value with each day of propofol alone. Although the decrease in TG associated with insulin did not reach statistical significance and should be interpreted with caution, for patients with limited sedation strategies, use of insulin infusions may allow for longer duration of propofol use, especially when concomitant hyperglycemia is present. Dose lowering strategies, such as spontaneous awakening trails and analgosedation, should also be implemented when indicated to limit propofol dose and therefore minimize TG accumulation.
The observed change in serum TG values may also be related to insulin doses received by patients in this study. The median dose of the insulin infusion received in our study was 4.0 units/h (0.04 units/kg/h). Insulin infusion dose for the treatment of hypertriglyceridemia-induced pancreatitis varies, but much higher insulin doses are commonly used, ranging from 0.1 to 1 unit/kg/h [
7,
11,
19]. These higher-dose insulin infusions often necessitate concomitant dextrose infusions to avoid hypoglycemia. In addition, the insulin dose for hypertriglyceridemia-induced pancreatitis is fairly constant and adjusted based on serum TG values, as compared to serum glucose values. Patients in our study receiving an insulin infusion underwent hourly blood glucose checks and the insulin dose was titrated to a blood sugar goal between 1.70 and 2.03 mmol/L. It is unclear whether a higher insulin infusion dose would be associated with lower than observed serum TG values. No episodes of hypoglycemia were observed in our study. The optimal dosing of insulin infusions for management of propofol-induced hypertriglyceridemia has not been elucidated, but likely depends on the TG value and the goal reduction. When factoring in the results of the mixed linear effects model, the insulin dosing used in this study accounted for a daily decrease in TG values of 0.14 mmol/L, which is not enough to offset the daily increase of 0.21 mmol/L predicted. However, this finding suggests that the use of lower dose insulin infusions may slow the rise of TG values while on propofol.
Limitations
There are a number of limitations to the study. This is a single-center, retrospective analysis, therefore, is subject to selection bias inherent to this study design and the findings are reliant on the accuracy of documentation. Additionally, a lack of a standardized triglyceride monitoring protocol may have impacted our results and further played into the potential for selection bias. However, we inducted consecutive patients meeting inclusion criteria to help minimize bias. Although the sample size requirement was met, the estimated effect size of a difference in TG of 1.13 mmol/L was not observed between the groups. A larger sample size would have helped increase the study power. Additionally, extrapolating changes in serum TG levels in the treatment of hypertriglyceridemia-induced pancreatitis to this patient group may have impacted our ability to find a difference. The reasons for discontinuation of propofol were obtained via chart review and pharmacist documentation notes. It is possible the reason for discontinuation was not documented or may have been multifactorial. This may explain the range of TG values observed that prompted propofol discontinuation. Patients in both groups received subcutaneous insulin. Overall, the dose of subcutaneous insulin did not differ between groups; however, there are reports of using subcutaneous insulin to treat hypertriglyceridemia, and this may have modestly influenced our results [
5]. We acknowledge residual confounders may be present, and we did not account for other causes of hypertriglyceridemia, including alcoholism and diabetic control. However, baseline serum glucose values and hemoglobin A1c were higher in the propofol and insulin infusion group, suggesting glycemic control was worse and we would have expected higher serum TG in this group. Additionally, although practice standard is to avoid obtaining serum TG values from the same or adjacent line through which propofol is infused, given the retrospective nature of the study, we were not able to verify if the samples were obtained correctly. Finally, patients in the propofol and insulin group had higher blood glucose values at baseline, and lower-dose insulin infusions were utilized to manage hyperglycemia compared to doses used in the related literature. The use of insulin infusions for the management of propofol-related hypertriglyceridemia in patients without hyperglycemia was not evaluated; however, an attempt was made to control for the impact of glucose values on serum TG by excluding patients with baseline hypertriglyceridemia. Although this trial serves as a necessary first step in evaluating the impact of insulin on propofol-induced hypertriglyceridemia, a randomized trial is needed to confirm the trends found in this study. Further, subsequent randomized trials would ideally focus on patients with normoglycemic propofol-related hypertriglyceridemia to broaden the clinical application of this treatment strategy.